Abstract
Varicella-zoster virus reactivation leads to herpes zoster – the main complication of which is postherpetic neuralgia (PHN). Rapid antiviral therapy initiated within 72 h of rash onset has been shown to accelerate rash healing, reduce the duration of acute pain and, to some extent, attenuate the development and duration of PHN. Other adjunctive therapies such as analgesics, antidepressants and some anticonvulsants are frequently required in the management of severe PHN. A live, attenuated zoster vaccine has been recently shown to significantly decrease herpes zoster incidence, PHN and the overall burden of illness when administered to adults older than 60 years of age. This new prophylactic modality has been reported to be cost-effective in the Canadian context, especially in the 60- to 75-year-old age group.
Keywords: Antivirals, Postherpetic pain, Prevention, Vaccine, Zoster
Abstract
La réactivation du virus varicelle-zona est à l’origine du zona, dont la principale complication est la névralgie post-zostérienne (NPZ). Un traitement antiviral rapide, débuté dans les 72 heures du déclenchement de l’érythème, s’est révélé capable d’accélérer la guérison de l’érythème, d’abréger la durée de la douleur aiguë et, dans une certaine mesure, d’atténuer le développement et la durée de la NPZ. D’autres traitements d’appoint, comme les analgésiques, les antidépresseurs et certains anticonvulsivants, sont souvent nécessaires pour la prise en charge de la NPZ. Il a récemment été démontré qu’un vaccin vivant atténué contre le virus varicelle-zona pouvait atténuer significativement l’incidence du zona et de la NPZ et alléger le fardeau global de la maladie chez des adultes de plus de 60 ans. Cette nouvelle modalité prophylactique serait rentable dans le contexte canadien, surtout pour la population âgée de 60 à 75 ans.
PATHOGENESIS AND DIAGNOSIS
Varicella-zoster virus (VZV), a member of the Herpesviridae family, infects more than 95% of North Americans. Following an incubation period of 14 to 21 days, the primary infection is varicella (chickenpox). The virus then migrates via retrograde axonal transport to sensory ganglia, where it establishes lifelong latency. VZV reactivations can be asymptomatic or symptomatic leading to the development of zoster, which typically occurs many decades after primary infection. Cell-mediated immunity (CMI) to VZV antigens is more important than humoral response (ie, antibody levels) in preventing the development of zoster (1). Aging and immunosuppressive conditions result in a decline in VZV-specific CMI that predisposes to zoster (please refer to the hypothetical model presented in Figure 1). However, laboratory tests that measure CMI response to VZV antigens (ie, proliferation of peripheral blood mononuclear cells or frequency of interferon-gamma-releasing peripheral blood mononuclear cells) are not available in clinical laboratories, and no threshold value associated with short-term development of zoster has been established (2). A typical zoster rash in an immunocompetent individual involves one or two adjacent dermatomes, and usually lasts seven to 10 days. Thoracic dermatomes are the most frequently affected, accounting for up to 50% of cases, whereas ophthalmic zoster is seen in 1% to 10% of cases. Almost three of four patients report having prodromal pain, which can precede the rash by days to weeks and is termed zoster sine herpete.
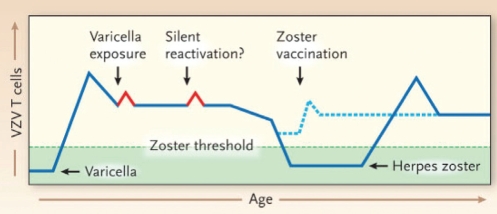
Figure 1).
Cell-mediated immunity and development of herpes zoster (reproduced with permission from reference 1). Varicella is the primary infection caused by varicella-zoster virus (VZV), and its resolution is associated with the induction of VZV-specific memory T cells (blue line). Memory immunity to VZV may be boosted periodically by exposure to varicella or silent reactivation from latency (red peaks). VZV-specific memory T cell levels decline with age. The decline below a threshold (dashed green line) correlates with an increased risk of zoster. The occurrence of zoster, in turn, is associated with an increase in VZV-specific T cell levels. The administration of zoster vaccine to older persons may prevent VZV-specific T cell levels from dropping below the threshold for zoster occurrence (dashed blue line)
Because zoster rash is so typical, diagnosis can be made clinically in most instances. In some cases, zoster rash can be confused with that of herpes simplex virus (HSV) or with other conditions such as impetigo, scabies, folliculitis, contact dermatitis, urticaria or drug eruption. Furthermore, the rash may be atypical in immunocompromised patients due to dissemination or chronicity. A laboratory diagnostic test may be needed when atypical lesions are present or when it is unclear whether VZV or HSV is causal. Swabs and cell scrapings from the base of the lesions are used for direct fluorescent antibody staining, cell culture or polymerase chain reaction. Direct fluorescent antibody staining is rapid, with relatively good sensitivity (90%) when lesions are at the vesicular stage (3). Because of the lability of the virus, cell culture is not sensitive (60% to 75%) and typically takes one week to perform (3). Polymerase chain reaction, which is not available in all clinical laboratories, is the most sensitive diagnostic method to distinguish wild-type VZV from the vaccine Oka strain (3).
COMPLICATIONS
The most frequent complication associated with zoster is pain, which can be roughly divided into three phases: acute pain occurring within 30 days after rash onset, subacute pain (between 30 days and 90 to 120 days) and postherpetic neuralgia (PHN), which is significant pain that persists longer than 90 to 120 days after rash onset (3,4). Besides PHN, zoster can be associated with many other complications (Table 1). Keratitis occurs in approximately two-thirds of patients with herpes zoster (HZ) ophthalmicus (involvement of the ophthalmic division of the trigeminal nerve). Because complications of ophthalmic zoster can be sight threatening, patients with this condition should be immediately referred to an ophthalmologist. The Ramsay Hunt syndrome refers to HZ of the facial nerve, with vesicles on the ear, palate or tongue leading to facial paresis, hearing loss and vertigo. Zoster can also be accompanied by neurological complications including myelitis, aseptic meningitis, acute or chronic encephalitis, and cranial nerve palsies such as Bell’s palsy. A rare but serious complication of HZ ophthalmicus – termed granulomatous arteritis – can occur weeks or months after zoster rash and presents similar to stroke (hemiplegia) contralateral to the original rash. In patients with cellular immunodeficiency such as those with HIV, hematological malignancies, solid tumours, and following stem cell or organ transplantation, there is a possibility of VZV viremia leading to cutaneous dissemination and seeding of the internal organs (lungs, liver, gut and brain). Visceral dissemination is associated with a case fatality rate of 5% to 15%, even with antiviral therapy.
TABLE 1.
Complications associated with herpes zoster
| Cutaneous |
| Disseminated cutaneous zoster* |
| Bacterial superinfection |
| Chronic atypical lesions with hyperkeratosis* |
| Recurrent zoster* |
| Neurological |
| Postherpetic neuralgia |
| Meningitis, myelitis, encephalitis* |
| Cranial nerve palsies including Bell’s palsy and Ramsay Hunt syndrome |
| Granulomatous cerebral angiitis |
| Guillain-Barré syndrome |
| Ophthalmological |
| Keratitis |
| Chorioretinitis, progressive outer retinal necrosis* |
| Uveitis |
| Iridocyclitis |
| Secondary glaucoma |
| Cataract |
| Visceral |
| Gastrointestinal involvement (esophagitis, gastritis, colitis)* |
| Pneumonia* |
| Pericarditis |
| Cystitis |
| Hepatitis |
Most frequently seen in immunocompromised patients, particularly in those with marked reduction in CD4+ T cell counts
EPIDEMIOLOGY AND BURDEN OF DISEASE
Incidence
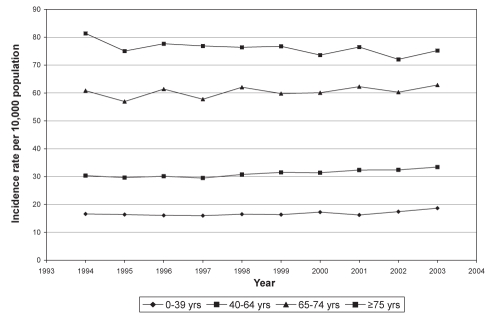
Most North Americans older than 40 years of age were infected with varicella in the past and are, therefore, susceptible to an episode of zoster (5). Zoster incidence ranges from 20 per 10,000 person-years to 34 per 10,000 person-years overall, but from 39 per 10,000 person-years to 118 per 10,000 person-years in elderly patients, who are thought to be increasingly susceptible due to senescence of cell-mediated immune responses (Figure 2) (6,7). This translates to a cumulative HZ lifetime risk of 25%; one-half of those who live to 85 years of age will experience an episode (6).
Figure 2).
Incidence rate of herpes zoster by age group per 10,000 population in British Columbia from 1994 to 2003 (adapted with permission from reference 8). yrs Years
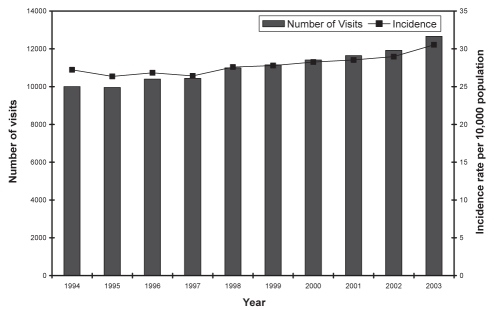
Most patients with zoster will visit a doctor; therefore, rates of physician visits are a reasonable estimate of incidence (6). Between 1994 and 2003, there was an average rate of 29 physician visits and one hospitalization per 10,000 population annually in British Columbia due to zoster (8) (Figure 3). Those older than 65 years of age had a higher incidence of both physician visits (70 per 10,000 population) and hospitalizations (five per 10,000 population). These observations indicate that burden of disease in a Canadian population is substantially similar in distribution to that observed in other developed countries. A recent study (9) has estimated that each year there are 130,000 new cases of HZ, 17,000 cases of PHN and 20 deaths in Canada (population in the 30 millions), which result in 252,000 physician consultations and 2000 hospitalizations.
Figure 3).
Number and rate of physician visits for herpes zoster in British Columbia, 1994 to 2003 (reproduced with permission from reference 8)
It is hypothesized that, with widespread varicella immunization and decreased varicella exposure, adults carrying wild-type VZV will be less likely to experience natural boosting of VZV-specific immunity and may experience increasing rates of zoster in the future (10–12). Some studies (6,8) may be showing early evidence of an increased rate although more observation is required. For those immunized against varicella, the Oka strain may also reactivate (13) but HZ is far less frequently observed and less severe in vaccinees than in those infected by wild-type VZV (14–16).
Morbidity
The risk of PHN, given an episode of zoster, increases with age (7,17). It occurs in more than 10% of patients with zoster (17,18), but in one-third of zoster patients older than 60 years of age (18,19) for an incidence of 14 cases per 10,000 person-years (17). In addition to advancing age, the severity of acute pain and rash, prodromal pain, ophthalmic location and possibly female sex are also risk factors for PHN (4,20–23).
Quality of life and cost issues
The effects of PHN on quality of life are comparable with those of diabetes, myocardial infarction, congestive heart failure and depression (24). Canadian hospitalizations for zoster complications cost approximately $82 million annually. Between 1979 and 1997, hospitalization in patients 65 years of age and older was nine per 10,000 individuals, with an average length of stay of 20 days (6,9,25). Hospitalizations and medications for PHN drive the overall cost of zoster (26). On the positive side, the rate of hospitalization is declining, and may be associated with increasing use of antiviral therapy (8).
ANTIVIRAL TREATMENT
The main objectives of antiviral treatment for HZ are to reduce viral replication, duration of rash and acute pain as well as to prevent complications seen mostly in immunocompromised patients. In addition, early antiviral therapy may also attenuate development of PHN. There are three antiviral agents approved for treatment of HZ in Canada: acyclovir with its prodrug valacyclovir and famciclovir. These agents are guanosine analogues that need to be first phosphorylated by the viral thymidine kinase and then by cellular kinases to their active triphosphate forms. The latter compounds inhibit viral DNA polymerase, which is essential for VZV replication. The newest agents, valacyclovir and famciclovir, are generally preferred to acyclovir due to increased bioavailability, which allows a reduction in daily doses. It is important to note that higher drug concentrations are required to inhibit VZV than HSV, which translates into higher doses for treatment of HZ compared with genital herpes (Table 2). Only acyclovir is available as an intravenous formulation that can be used to treat central nervous system complications (eg, encephalitis) or disseminated infections in immunocompromised patients. In general, these drugs are well tolerated and the most frequent side effects consist of nausea and headache. Dosage adjustment is required for all agents in the presence of renal insufficiency.
TABLE 2.
Antiviral agents for treatment of herpes zoster in immunocompetent subjects
| Antiviral | Dosage | Duration of treatment (days) | Reference |
|---|---|---|---|
| Acyclovir | 800 mg oral 5 times daily or 10 mg/kg IV every 8 h | 7 to 10 | (54–57) |
| Famciclovir | 500 mg oral 3 times daily | 7 | (30–32,58) |
| Valacyclovir | 1000 mg oral 3 times daily | 7 | (29,32,59) |
IV Intravenously
In immunocompetent patients, randomized trials have shown that orally administered acyclovir, valacyclovir and famciclovir can reduce the duration of viral shedding, accelerate rash healing and decrease the duration of acute pain (3). By inhibiting viral replication, antiviral agents could also decrease neuronal damage and, thus, alter the development of PHN if they are administered rapidly after rash onset. Indeed, the results of meta-analyses for acyclovir (27,28) and of randomized clinical trials for valacyclovir (29) and famciclovir (30,31) have confirmed the concept that early antiviral treatment (in less than 72 h of rash onset) can reduce the duration and, in some cases, the incidence of PHN. In one trial (30), famciclovir decreased the median time to cessation of pain from 119 (placebo) to 63 days. In another study (29), the median time to loss of pain was 38 days for valacyclovir compared with 51 days for acyclovir. A trial (32) that directly compared valacyclovir and famciclovir treatments found no difference in time to complete cessation of pain.
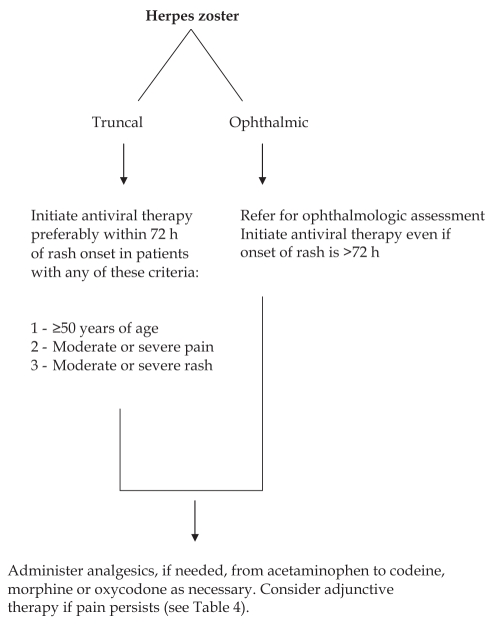
Oral therapy with one of the three antivirals is recommended as first-line treatment for all immunocompetent patients who consult rapidly (preferably within 72 h of rash onset) and who fulfill any of the following criteria: 50 years of age or older; moderate or severe acute pain; moderate or severe rash; or nontruncal involvement (3) (Figure 4). Because of its potential complications, ophthalmic zoster should be treated more aggressively, including referral for ophthalmological assessment and institution of antiviral therapy even beyond the 72 h recommended window period. Of note, the 72 h inclusion criterion has been arbitrarily chosen in randomized clinical trials and may not be optimal in clinical practice. The presence of new vesicles, which reflect active viral replication, may be an alternative way to select patients for antiviral treatment. Although showing some benefits in acute zoster pain, corticosteroids do not provide added value over acyclovir in reducing PHN, and are thus not recommended in the initial management of HZ (33,34).
Figure 4).
Initial management of herpes zoster (adapted with permission from references 3 and 60)
Immunocompromised patients are at greater risk of developing disseminated and/or recurring HZ. Intravenous acyclovir at a dose of 10 mg/kg (or 500 mg/m2) every 8 h remains the therapy of choice for these patients. When the infection is under control (ie, when there are no new vesicles), therapy can be switched to an oral agent. In less severely immunocompromised patients, an option would be to initiate therapy with oral agents (especially valacyclovir or famciclovir) coupled with close monitoring. It is recommended to continue treatment until all lesions are crusted, which is often longer than the standard seven-day course. Clinical failure or recurrent HZ lesions in immunocompromised hosts such as in patients with AIDS may be caused by acyclovir-resistant VZV strains (35). In such cases, phenotypic and/or genotypic resistance tests may be ordered, and alternative therapy with foscarnet at a dosage of 40 mg/kg intravenously every 8 h should be considered.
THE MANAGEMENT OF ZOSTER-ASSOCIATED PAIN
Zoster-associated pain (ZAP) is a practical concept that includes both acute pain associated with rash and PHN. ZAP may be described as continuous or paroxysmal, evoked or spontaneous, burning or lancinating, and is often associated with other sensory abnormalities in the skin. This variability implies different pain mechanisms (36). The two primary pain mechanisms are believed to be increased excitability of damaged primary afferent neurons causing irritable nociceptors and central sensitization, resulting in pain and allodynia; and degeneration of nociceptive neurons in dorsal root ganglia or the spinal cord, leading to deafferentation with central hyperactivity, causing pain but typically without allodynia (37).
Pain assessment and documentation
In patients with prolonged ZAP, a simple office-based tool to assist the clinician to assess and document the pattern of pain, severity of pain and impact on function and quality of life is a modification of the Brief Pain Inventory, called the Zoster Brief Pain Inventory (Table 3) (38). It is especially useful in providing a baseline snapshot of pain and function before therapeutic trials. All patients should have a medical and psychosocial evaluation and targeted physical examination to confirm the diagnosis, document comorbid illness and provide a basis for treatment. Elderly patients may be socially isolated, may have cognitive impairment, depression or other life stressors that may impact treatment compliance and outcome. Anxiety or depression may also develop secondary to severe ZAP and can influence suffering.
TABLE 3.
Zoster Brief Pain Inventory
|
Adapted with permission from reference 38
Pain treatment
Patient education and general measures:
The disease and its time course should be explained, including the risk of viral transmission to individuals who have not had varicella. The rash should be kept clean and dry to reduce the risk of secondary bacterial infection. Acute skin discomfort may be reduced by sterile wet dressings. Topical antibiotic dressings with adhesives that can cause irritation and delay rash healing should be avoided.
Pharmacological agents:
ZAP should be assessed early and treatment should begin promptly. The principles of optimum pain management, such as the use of standardized pain measures, scheduled analgesia, and consistent and frequent follow-up to adjust dosing to the needs of the patient, should be applied to the management of pain in patients with HZ. It is important to recognize that ZAP changes over time and can become more severe as the acute infection progresses. The initial choice of treatment approaches depends on the patient’s pain severity, comorbid conditions and on any previous known response to specific medications. No randomized clinical trials of oral treatment for acute pain in patients with HZ have been published. However, the finding that more severe acute pain is a risk factor for PHN provides a clinical rationale that effective relief of acute pain may further lessen the risk of PHN beyond that achieved with antiviral therapy alone (39). No single treatment has been shown to be completely effective for all sufferers of PHN, and clinical experience suggests that combinations of analgesic drugs are usually required to achieve even partial pain relief. There are many published trials that compare various analgesics to placebo, but very few head-to-head trials or trials of combination therapies (40). For patients with refractory pain beyond four to six weeks after the onset of rash, additional pharmacological options are warranted (Table 4).
TABLE 4.
First-, second- and third-line pharmacotherapy for neuropathic pain
| Agent | Starting dose and titration | Usual effective dose | Maximum recommended dose | Adverse effects | Comments | |
|---|---|---|---|---|---|---|
| First line | ||||||
| Tricyclics | ||||||
| Amitriptyline, nortriptyline, desipramine, imipramine | 10 to 25 mg hs. Increase by 10 mg every 3 to 7 days based on patient tolerance | 50 to 150 mg hs | 150 mg hs | Drowsiness, confusion, orthostatic hypotension, dry mouth, constipation, urinary retention, weight gain, arrhythmias | Use with caution in the elderly. Contraindicated in glaucoma, symptomatic prostatism and significant cardiovascular disease | |
| Anticonvulsants | ||||||
| Gabapentin | 100 to 300 mg hs. Increase by 100 to 300 mg tid every 1 to 4 weeks | 1200 to 2400 mg in 3 to 4 doses daily if using high doses | 3600 mg in 3–4 doses | Drowsiness, dizziness, peripheral edema, visual blurring | Adjust dose and interval in renal failure | |
| Pregabalin | 25 to 50 mg hs, bid or tid up to 75 mg bid | 300 to 600 mg a day in 2 or 3 divided doses | 600 mg in 2 or 3 doses | Drowsiness, dizziness, peripheral edema, visual blurring | Adjust dose and interval in renal failure | |
| Second line | ||||||
| Topical 5% lidocaine patch or 10% topical cream or gel | 5% patches applied to painful areas for 12 of 24 h. Apply cream tid to qid | 1 to 3 patches daily for 12 of 24 h | Maximum surface of 300 cm2 | Rare systemic adverse effects (tremor and increased heart rate) | Lidocaine patches not available in Canada | |
| Venlafaxine | 37.5 mg/day. Increase by 37.5–75 mg/day every 14 weeks | 75 to 225 mg/day | 300 mg | Nausea, dizziness, drowsiness, hypertension, constipation | Dose adjust in renal failure | |
| Duloxetine | 30 mg/day. Increase to 60 mg/day after 1 to 2 weeks | 60 mg/day | 120 mg/day | Sedation, nausea, constipation, ataxia, dry mouth, dizziness, somnolence, erectile dysfunction | Contraindicated in glaucoma | |
| Third line | ||||||
| Tramadol | 37.5 mg (with acetaminophen) 1–2 tabs qid prn | 150 to 300 mg daily | 8 tablets daily | Sedation, nausea, orthostatic hypotension, headaches, may decrease seizure threshold | Caution needed with other serotoninergic drugs | |
| CR/ER 100–150 mg once daily. Titrate every 2–7 days | 100 to 400 mg daily | 400 mg daily (300 mg max in elderly) | ||||
| Morphine, oxycodone, hydromorphone | IR morphine: 2.5 to 10 mg (or equivalent dose of other opioid) every 4 h prn. Switch to SR/CR formulation after 2 weeks or start lowest dose of SR/CR opioid every 12 h. Titrate every 3–7 days | Individual | See note* | Nausea, vomiting, sedation, dizziness, urinary retention, constipation | Constipation requires concurrent bowel regimen. Screen for addiction risk before starting long-term opioids | |
| Fentanyl patch | After 30 mg of morphine or equivalent daily switch to 12 μg/h patch every 3 days. Titrate every 6 days by 12–25 μg/h | Individual | Maximum dose may be limited by skin surface area required | Nausea, vomiting, sedation, dizziness, urinary retention, constipation, skin irritation | Constipation requires concurrent bowel regimen. Screen for addiction risk before starting long-term opioids | |
Note: There is no maximal dose if medication is titrated carefully, but attention should be paid to the possibility of causing excessive adverse effects at elevated doses of all opioids. Adapted from the Canadian Pain Society Guidelines, 2007 and the Quebec Pain Specialist Recommendations, 2008. bid Twice a day; CR Controlled release; ER Extended release; hs At bedtime; max Maximum; prn As needed; qid Four times a day; SR Sustained release; tid Three times a day
Antidepressants:
Tricyclic antidepressants have well-established efficacy in the treatment of PHN (41). The results of one placebo-controlled trial (42) of amitriptyline (25 mg once daily for three months beginning within 48 h of rash onset) and a reanalysis examining the subgroup of patients also treated with an antiviral suggested that amitriptyline reduced the incidence of PHN at six months by at least 50%. Two selective serotonin and nor-adrenaline reuptake inhibitors – venlafaxine and duloxetine – are better tolerated than tricyclic antidepressants and have recently shown efficacy in patients with diabetic polyneuropathy but not specifically PHN (43).
Anticonvulsants:
Multiple studies have demonstrated the efficacy of gabapentin or pregabalin in patients with PHN (44). The combination of satisfactory tolerability, safety and lack of drug interactions distinguish them from other oral medications used in the treatment of neuropathic pain. One open-label study (3) reported that treatment with the combination of gabapentin and valacyclovir reduced the incidence of PHN at three and six months compared with traditional antiviral monotherapy. Other anticonvulsant medications are not currently recommended for patients with ZAP because of their lack of proven efficacy in PHN, poor tolerability or risks of clinically significant adverse events.
Opioids:
The combination of opioids with acetaminophen or nonsteroidal anti-inflammatory drugs has not been systematically studied in patients with HZ. Tramadol, a weak muopioid agonist that also inhibits the reuptake of noradrenaline and serotonin, has not been studied as a treatment for acute HZ but has shown evidence of activity in PHN (45). There is evidence from randomized controlled trials to support the use of potent opioids such as morphine and oxycodone in PHN (40).
Topical treatments:
Topical lidocaine patch 5%, applied twice a day on painful but intact skin, may serve as a well-tolerated and effective modality to relieve moderate to severe pain associated with acute HZ (46). For PHN, a recent Cochrane review (47) of topical therapy concluded that “there is insufficient evidence to recommend topical lidocaine as a first-line agent in the treatment of postherpetic neuralgia with allodynia”. Nonetheless, topical treatments have a lower potential for systemic adverse effects; therefore, some clinicians consider the early use of topical lidocaine or capsaicin, especially where quantitative sensory testing has indicated that the patient has ‘irritable nociceptors’ as opposed to ‘deafferentation’ pain.
Interventional treatments:
Sympathetic and epidural nerve blocks have been used for the treatment of severe pain in patients with ZAP for many years, but few controlled studies have examined their effects on acute pain or PHN (48). For patients with pain that is inadequately controlled by other combination pharmacotherapies, referral for a trial of neural blockade may be reasonable. Patients with the most severe pain may benefit from hospitalization and administration of epidural analgesics. The results of a randomized trial (49) involving patients with HZ treated with oral antiviral therapy demonstrated that a single epidural injection of steroids and local anesthetics relieved acute pain within the first month after rash onset significantly better than standard care, but did not reduce the risk of developing PHN. Continuous epidural infusion of local anesthetic with intermittent additional epidural anesthetic boluses was superior to continuous infusion of saline and intermittent anesthetic boluses in reducing the time to complete cessation of pain in patients with HZ treated with acyclovir (50). However, treatment of HZ patients with multiple epidural injections or continuous epidural infusions is unlikely to be feasible in most settings.
ZOSTER VACCINE
Zoster vaccine (Zostavax, Merck & Co Inc, USA) has been licensed for individuals 60 years of age and older in the United States since May 2006 and in Canada since August 2008. It is not licensed for immunocompromised individuals, pregnant women or children. The vaccine is a lyophilized preparation of the live, attenuated Oka VZV strain. This is the same preparation used in the varicella vaccine but at a higher dose (14-fold greater) (17). Zoster vaccine has been demonstrated to boost cellular immunity in older adults through a range of subcutaneous doses with a good safety profile (2,51).
The Shingles Prevention Study (SPS) was a large controlled trial that randomly assigned more than 38,000 people to zoster vaccine or placebo (17). The main outcome was a burden of illness score based on severity and duration of symptoms. The vaccine reduced the burden of illness by 61%, the incidence of zoster by 51% (11.1 to 5.2 cases per 1000 person-years) and the incidence of PHN by 67% (1.4 to 0.5 per 1000 person-years). The vaccine was more efficacious in reducing the incidence of HZ in those 60 to 69 years of age than those older than 70 years of age (vaccine efficacy 63.9% versus 37.6%, respectively). However, the reduction in incidence of PHN was similar in these two age groups. The SPS followed patients for more than three years and noted no decline in efficacy. Results of longer follow-up will help resolve the question of duration of protection.
An adverse events subgroup of the SPS, with more than 6500 subjects, found that vaccination was associated with injection-site events: erythema (36%), pain or tenderness (35%), swelling (26%) and pruritis (7%) (P<0.05 for all cited events) (17). A review of serious adverse events conducted by the writing committee revealed no clinically meaningful differences between these groups. In the entire SPS population, vaccine-related serious adverse events during the 42 days after vaccination occurred in less than 0.01% of patients in each of the trial arms. Postlicensure passive surveillance through vaccine adverse events reporting systems in the United States has demonstrated a good safety profile. The most common adverse event remained injection-site reaction. There were no causally linked severe adverse events.
Program issues and cost-effectiveness
Age at vaccination is an important consideration for a zoster vaccine program. Although cost-effectiveness appears to be greatest for vaccination at 60 years of age (26), adding zoster vaccine to an existing vaccination program at 65 years of age may substantially drive down program costs. Concomitant administration of zoster and influenza vaccines appears safe and without evidence of immunological interference (26). Evaluations of administration with pneumococcal and tetanus vaccines are underway but not yet published.
Two studies (9,26) have addressed cost-effectiveness in the Canadian context, both taking the perspective of the health system. A model from British Columbia (26) estimated the incremental cost-effectiveness ratio for vaccination of all adults older than 60 years of age to be $40,000 per quality-adjusted life year (QALY). Brisson et al (9) concluded a similar cost per QALY of $33,000 if immunizing adults 65 years of age. Both studies concluded that it would be less cost-effective to immunize older seniors (because of less time to accumulate benefit as well as lower efficacy) and that vaccination between 65 and 75 years of age is optimal. Studies from elsewhere used various assumptions but also concluded on the importance of age at immunization to cost-effectiveness. Cost-effectiveness of a zoster vaccine ranged from $16,229/QALY for a 60-year-old American to $191,000/QALY for an 81-year-old American in these studies (52,53). An increasing incidence of zoster or a decreased cost of vaccine would further increase the cost-effectiveness of zoster immunization. Societal costs (eg, lost income, caregivers not reimbursed by the health care system, etc) were not measured by these studies and may also be reduced by immunization. In summary, zoster vaccination has comparable cost-effectiveness with recently funded vaccination programs.
CONCLUSIONS
Zoster causes substantial morbidity, with approximately 130,000 new cases occurring annually in Canada. Many of these cases cause debilitating pain, which may last for months or even years. A significant number of patients may not obtain timely antiviral treatment and, even when they do, the treatment is only partially effective at alleviating the symptoms and shortening the duration of PHN. The effect of immunization against VZV shows that naturally acquired CMI can be enhanced and such an effect can attenuate the clinical consequences associated with this persistent (latent) viral pathogen. The recently approved zoster vaccine appears safe and effective in reducing HZ prevalence and more importantly PHN, which is a devastating condition in the elderly population. The next unresolved question is how long such a boost in VZV-specific memory T cells will last. Furthermore, the influence of the chickenpox vaccine on adulthood VZV immunity and the need for different boosting regimens in the future should be carefully monitored.
Acknowledgments
GB received an unrestricted grant-in-aid from Merck Frost Canada to coordinate the review.
REFERENCES
- 1.Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005;352:2266–7. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 2.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44:S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 4.Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545–51. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- 5.Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: Implications for control through routine immunization. J Med Virol. 2003;70:S111–8. doi: 10.1002/jmv.10364. [DOI] [PubMed] [Google Scholar]
- 6.Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi: 10.1017/s0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope-Simpson RE. The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar BL, Galanis E, Kay C, Skowronski D, Naus M, Patrick D. The burden of varicella and zoster in British Columbia 1994–2003: Baseline assessment prior to universal vaccination. Can Commun Dis Rep. 2007;33:1–15. [PubMed] [Google Scholar]
- 9.Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin. 2008;4 doi: 10.4161/hv.4.3.5686. [DOI] [PubMed] [Google Scholar]
- 10.Brisson M, Edmunds WJ, Gay NJ. Varicella vaccination: Impact of vaccine efficacy on the epidemiology of VZV. J Med Virol. 2003;70:S31–7. doi: 10.1002/jmv.10317. [DOI] [PubMed] [Google Scholar]
- 11.Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect. 2002;44:211–9. doi: 10.1053/jinf.2002.0988. [DOI] [PubMed] [Google Scholar]
- 12.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 13.Takayama N, Takayama M, Takita J. Herpes zoster in healthy children immunized with varicella vaccine. Pediatr Infect Dis J. 2000;19:169–70. doi: 10.1097/00006454-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Brunell PA, Taylor-Wiedeman J, Geiser CF, Frierson L, Lydick E. Risk of herpes zoster in children with leukemia: Varicella vaccine compared with history of chickenpox. Pediatrics. 1986;77:53–6. [PubMed] [Google Scholar]
- 15.Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med. 1991;325:1545–50. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- 16.Broyer M, Tete MJ, Guest G, Gagnadoux MF, Rouzioux C. Varicella and zoster in children after kidney transplantation: Long-term results of vaccination. Pediatrics. 1997;99:35–9. doi: 10.1542/peds.99.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 18.Kost RG, Straus SE. Postherpetic neuralgia – pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 19.Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Higa K, Mori M, Hirata K, Hori K, Manabe H, Dan K. Severity of skin lesions of herpes zoster at the worst phase rather than age and involved region most influences the duration of acute herpetic pain. Pain. 1997;69:245–53. doi: 10.1016/S0304-3959(96)03229-0. [DOI] [PubMed] [Google Scholar]
- 21.Whitley RJ, Weiss HL, Soong SJ, Gnann JW. Herpes zoster: Risk categories for persistent pain. J Infect Dis. 1999;179:9–15. doi: 10.1086/314562. [DOI] [PubMed] [Google Scholar]
- 22.Scott FT, Leedham-Green ME, Barrett-Muir WY, et al. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol. 2003;70:S24–30. doi: 10.1002/jmv.10316. [DOI] [PubMed] [Google Scholar]
- 23.Zaal MJ, Volker-Dieben HJ, D’Amaro J. Risk and prognostic factors of postherpetic neuralgia and focal sensory denervation: A prospective evaluation in acute herpes zoster ophthalmicus. Clin J Pain. 2000;16:345–51. doi: 10.1097/00002508-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: A self-limited disease with severe impact. Neurology. 1995;45:S52–3. doi: 10.1212/wnl.45.12_suppl_8.s52. [DOI] [PubMed] [Google Scholar]
- 25.Nowgesic E, Skowronski D, King A, Hockin J. Direct costs attributed to chickenpox and herpes zoster in British Columbia – 1992 to 1996. Can Commun Dis Rep. 1999;25:100–4. [PubMed] [Google Scholar]
- 26.Najafzadeh M, Sadatsafavi M, Marra CA. Interpretation of results of the cost-effectiveness analysis reported by Pellissier et al. on October 2007. Vaccine. 2008;26:5244. doi: 10.1016/j.vaccine.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 27.Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: A meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341–7. doi: 10.1093/clinids/22.2.341. [DOI] [PubMed] [Google Scholar]
- 28.Jackson JL, Gibbons R, Meyer G, Inouye L. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909–12. [PubMed] [Google Scholar]
- 29.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: Effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, Boon RJ, Griffin DR, Phung D. Postherpetic neuralgia: Impact of famciclovir, age, rash severity, and acute pain in herpes zoster patients. J Infect Dis. 1998;178:S76–80. doi: 10.1086/514260. [DOI] [PubMed] [Google Scholar]
- 32.Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: Randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9:863–9. doi: 10.1001/archfami.9.9.863. [DOI] [PubMed] [Google Scholar]
- 33.Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–83. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896–900. doi: 10.1056/NEJM199403313301304. [DOI] [PubMed] [Google Scholar]
- 35.Boivin G, Edelman CK, Pedneault L, Talarico CL, Biron KK, Balfour HH., Jr Phenotypic and genotypic characterization of acyclovir-resistant varicella-zoster viruses isolated from persons with AIDS. J Infect Dis. 1994;170:68–75. doi: 10.1093/infdis/170.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Dworkin RH, Gnann JW, Jr, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9:S37–44. doi: 10.1016/j.jpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: Irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–27. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- 38.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: Adaptation of the brief pain inventory. J Pain. 2004;5:344–56. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003;36:877–82. doi: 10.1086/368196. [DOI] [PubMed] [Google Scholar]
- 40.Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: A quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217–27. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 42.Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: A randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–31. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: Results of a randomised, placebo-controlled clinical trial. Pain. 2004;109:26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Boureau F, Legallicier P, Kabir-Ahmadi M. Tramadol in post-herpetic neuralgia: A randomized, double-blind, placebo-controlled trial. Pain. 2003;104:323–31. doi: 10.1016/s0304-3959(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 46.Lin PL, Fan SZ, Huang CH, et al. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: A double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008;33:320–5. doi: 10.1016/j.rapm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Khaliq W, Alam S, Puri N. Topical lidocaine for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev. 2007:CD004846. doi: 10.1002/14651858.CD004846.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Kumar V, Krone K, Mathieu A. Neuraxial and sympathetic blocks in herpes zoster and postherpetic neuralgia: An appraisal of current evidence. Reg Anesth Pain Med. 2004;29:454–61. doi: 10.1016/j.rapm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 49.van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: A randomised controlled trial. Lancet. 2006;367:219–24. doi: 10.1016/S0140-6736(06)68032-X. [DOI] [PubMed] [Google Scholar]
- 50.Manabe H, Dan K, Hirata K, et al. Optimum pain relief with continuous epidural infusion of local anesthetics shortens the duration of zoster-associated pain. Clin J Pain. 2004;20:302–8. doi: 10.1097/00002508-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Trannoy E, Berger R, Hollander G, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: A randomized, controlled, dose-response trial. Vaccine. 2000;18:1700–6. doi: 10.1016/s0264-410x(99)00510-1. [DOI] [PubMed] [Google Scholar]
- 52.Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25:8326–37. doi: 10.1016/j.vaccine.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 53.Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis. 2007;44:1280–8. doi: 10.1086/514342. [DOI] [PubMed] [Google Scholar]
- 54.McKendrick MW, McGill JI, Wood MJ. Lack of effect of acyclovir on postherpetic neuralgia. BMJ. 1989;298:431. doi: 10.1136/bmj.298.6671.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huff JC, Bean B, Balfour HH, Jr, et al. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988;85:84–9. [PubMed] [Google Scholar]
- 56.Wood MJ, Ogan PH, McKendrick MW, Care CD, McGill JI, Webb EM. Efficacy of oral acyclovir treatment of acute herpes zoster. Am J Med. 1988;85:79–83. [PubMed] [Google Scholar]
- 57.Harding SP, Porter SM. Oral acyclovir in herpes zoster ophthalmicus. Curr Eye Res. 1991;10:177–82. doi: 10.3109/02713689109020376. [DOI] [PubMed] [Google Scholar]
- 58.Shafran SD, Tyring SK, Ashton R, et al. Once, twice, or three times daily famciclovir compared with aciclovir for the oral treatment of herpes zoster in immunocompetent adults: A randomized, multicenter, double-blind clinical trial. J Clin Virol. 2004;29:248–53. doi: 10.1016/S1386-6532(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 59.Lin WR, Lin HH, Lee SS, et al. Comparative study of the efficacy and safety of valaciclovir versus acyclovir in the treatment of herpes zoster. J Microbiol Immunol Infect. 2001;34:138–42. [PubMed] [Google Scholar]
- 60.Dworkin RH. Post-herpetic neuralgia. Herpes. 2006;13:21A–7A. [Google Scholar]