Abstract
BACKGROUND:
In February 2007, a general surgeon in Charlottetown, Prince Edward Island, tested positive for hepatitis C virus (HCV). The surgeon’s infection onset date could not be determined; however, episodic hepatic enzyme elevations were first detected in November 2004 and again in February 2007. HCV transmission during surgery, alhough rare, has been documented. A phased look-back HCV screening program was conducted to detect HCV transmission from this surgeon to patients who underwent the highest-risk procedures in the three years before his positive test.
METHODS:
Highest-risk procedures were defined as exposure-prone procedures (EPP) in which exposure to the surgeon’s blood was most likely. EPP patients from January 2004 to February 2007 were identified using hospital and administrative records. Linkages with the provincial notifiable disease for HCV was performed, and death records for deceased EPP patients were reviewed. Eligible patients were invited for screening.
RESULTS:
Of 6248 patients seen in phase 1, 272 (4.4%) were identified to be EPP. Of the 272 patients, 248 (91.1%) were invited for HCV testing and 24 (8.8%) were deceased. To date, 231 of 248 (93.1%) patients have presented for screening. Two patients (one alive, one deceased) were HCV positive before their EPP. Viral sequence of the surgeon’s isolate is unrelated to the first patient; the second individual has a resolved infection (polymerase chain reaction negative). No new transmission events were identified in the screened patients. The 95% CI of the transmission probability was estimated to be 0 to 0.016.
INTERPRETATION:
HCV transmission from the surgeon during a 38-month look back was unlikely. In the absence of protocols for investigating HCV transmission from infected health care workers, screening was initially prioritized to the highest-risk patients. The investigation has been satisfactorily terminated based on these results.
Keywords: Hepatitis C virus, Nosocomial infection, Provider-to-patient transmission, Phylogenetic analysis
Abstract
CONTEXTE :
En février 2007, un chirurgien général de Charlottetown, Île-du-Prince-Édouard, s’est révélé être séropositif à l’égard du virus de l’hépatite C (VHC). Il a été impossible de déterminer la date où le chirurgien avait contracté l’infection. Toutefois, des augmentations épisodiques des enzymes hépatiques ont d’abord été décelées en novembre 2004, puis en février 2007. Bien que rare, la transmission du VHC durant la chirurgie a déjà été documentée. Un programme de dépistage de la transmission du VHC a été appliqué afin de vérifier si ce chirurgien n’avait pas transmis le virus à ses patients lors d’interventions plus à risque au cours des trois années précédant ses résultats positifs.
MÉTHODE :
Les interventions à risque plus élevé comprenaient des interventions susceptibles de donner lieu à une exposition et au cours desquelles l’exposition au sang du chirurgien était plus probable. Les patients ayant subi ce genre d’intervention entre janvier 2004 et février 2007 ont été recensés à partir des dossiers hospitaliers et administratifs. Des liens avec le service provincial des maladies à déclaration obligatoire ont été établis pour le VHC et les dossiers de mortalité des patients décédés suite à une intervention à risque ont été passés en revue. Les patients admissibles ont ensuite été invités à subir un test de dépistage.
RÉSULTATS :
Parmi les 6 248 patients vus durant la phase 1, 272 (4,4 %) avaient subi une intervention à risque. Parmi ces 272 patients, 248 (91,1 %) ont été invités à subir un test de dépistage du VHC et 24 (8,8 %) étaient décédés. À ce jour, 231 patients sur 248 (93,1 %) se sont présentés au dépistage. Deux patients (un vivant et un décédé) étaient déjà VHC-positifs avant leur intervention. La séquence virale de l’isolat du chirurgien est sans lien avec le premier patient. Le second s’est débarrassé de son infection (RPC négative). Aucun nouveau cas de transmission n’a été identifié chez les patients soumis au dépistage. L’IC à 95 % pour la probabilité de transmission a été estimée à 0 – 0,016.
INTERPRÉTATION :
La transmission du VHC par le chirurgien durant une période rétrospective de 38 mois est peu probable. En l’absence d’un protocole d’enquête sur la transmission du VHC par des professionnels de la santé infectés, le dépistage a initialement été offert aux patients les plus à risque. L’enquête s’est terminée de façon satisfaisante compte tenu des résultats obtenus.
In February 2007, one of four general surgeons working in the province of Prince Edward Island (PEI) (population 135,851) (1) tested positive for hepatitis C virus (HCV) infection genotype 1a (viral load 5.69 log IU HCV RNA/mL). He was asymptomatic at the time of diagnosis. The diagnosis had been made during a routine medical assessment follow-up, which included screening blood tests that showed elevation in his serum transaminases. The surgeon had never before been tested for HCV although transient low-level elevation in his transaminases was first documented in November 2004. No archived serum samples were available for HCV testing. Occupational exposure to HCV was deemed to be the surgeon’s likely source of infection. At the time of his positive test result, the surgeon voluntarily withdrew from performing further surgical procedures.
HCV transmission from a surgeon to patients during surgery has been documented (2–15). The purpose of the present paper is to describe the HCV look-back exercise conducted in the patients of this surgeon following his positive HCV test result.
BACKGROUND ON HCV AND TRANSMISSION DURING SURGERY
HCV is an enveloped single-stranded RNA virus that is a member of the family Flaviviridae. There are six major HCV genotypes and over 50 subtypes (16). In Canada, hepatitis C infection is the most common chronic blood-borne infection, and HCV cirrhosis is the leading cause of liver transplantation (16). It is estimated that approximately 250,000 Canadians are infected with HCV, and one-third are likely unaware of their infection status (16). Genotype 1a is the leading strain (27.8%) among genotyped HCV infections in PEI. Acute infection is asymptomatic in 60% to 75% of cases (16). Symptoms of infection may include malaise, fatigue, anorexia, abdominal pain, nausea and jaundice. Although some patients can spontaneously clear the virus, the majority of individuals (50% to 80%) become chronically infected (16).
Risk factors for HCV transmission include injection drug use, travel to or history of residence in an HCV-endemic country, history of transfusion before 1992 and, uncommonly, sexual or vertical transmission (16,17). In North America, unsafe injection practices have resulted in iatrogenic transmission of HCV in hematology and endoscopy units, and pain clinics (18,19).
HCV transmission during surgery has been documented to occur from infected anesthesiologists (20–22), an anesthesiology assistant (23), a surgical technician (24) and surgeons (2–15). Table 1 summarizes results of look-back exercises involving HCV-infected surgeons (2–15) with the transmission probability ranging from 0.0004 to 0.0225 per patient. The transmission risk is likely affected by the physician’s infection status (duration of viremia and viral load), as well as the type and duration of exposure-prone procedures (EPP) performed by the surgeon. EPP are any invasive procedures where the surgeon’s hands are fully or partly hidden in a body cavity in close proximity to a sharp instrument/needle tips or bone resulting in a potential for the surgeon to sustain a percutaneous injury and their blood to come into contact with the patient’s tissues (eg, via recontact of contaminated sharp objects with the patient) (25–28). A modelling exercise estimated the risk of a HCV viremic surgeon transmitting HCV to a patient at 0.006% to 0.057% or a mean 7% probability of infecting at least one patient in 500 invasive procedures (25).
TABLE 1.
Probability of hepatitis C virus (HCV) transmission from infected surgeons to their patients: A summary of the literature
| Country (reference) | Time period of investigation | Surgical specialty | Patients tested, n | Patients with viral match, n | Transmission probability per patient, rate (95% CI) |
|---|---|---|---|---|---|
| Spain (2) | 1988–1994 | Cardiac surgeon* | 222 | 5 | 0.0225 (0.0074–0.0518) |
| UK, London (3,4) | 1993–1995 | Cardiac surgery resident (first assistant) | 278 | 1 | 0.0036 (0.0001–0.0199) |
| UK, Boston (5–9) | 1978–1999 | Gynecologist | 4500 | 8 | 0.0018 (0.0008–0.0035) |
| UK, West Midlands† (8,10) | NA | Surgeon | 723 notified | 1 | NA |
| UK, Southern Trusts† (11) | NA | Surgeon‡ | 228 notified | 1 | NA |
| Germany (12) | 1993–2000 | Gynecologist§ | 2286 | 1 | 0.0004 (0.0000–0.0024) |
| Germany (13) | 1999–2000 | Orthopedic surgeon | 207 | 1 | 0.0048 (0.0001–0.0266) |
| Germany (14) | 2002–2005 | General surgeon | 1194 | 1 | 0.0008 (0.0000–0.0047) |
| USA (15)¶ | NA | Cardiac surgeon | 937 | 14 | 0.0149 (0.0082–0.0249) |
This is limited investigation representing 35% of the surgeon’s surgical patients. No transmission events were identified in procedures in which the surgeon acted as an assistant and rarely performed sternotomy closure (n=138). In contrast, the five transmission events were identified in procedures in which the surgeon primarily performed the surgery including sternotomy closure with wires (n=84), giving a transmission rate of 5.95%;
Time period of these investigations is not available (NA): transmission probability is not estimated because final HCV screening results from these investigations have not been published;
Investigation was initiated as a look-back exercise following the identification of the one HCV-infected patient who underwent an exposure-prone procedure (EPP) by a health care worker; the type of surgery is not indicated. In total, 228 individuals were notified and final HCV screening results remain unpublished;
This extensive investigation categorized gynecological surgical procedures as low-risk (eg, dilatation and curettage, termination of pregnancy), medium-risk (eg, cone biopsies, perineal sutures) and high-risk (major gynecological surgery involving laparotomy, hysterectomies, major repairs, etc) EPPs. The surgeon performed 2339 procedures (1850 low and medium risk, 489 high risk) on 2286 women. No transmissions were identified in patients undergoing medium- and low-risk EPPs; only one transmission (the index patient) was identified in high-risk EPP (n=489) giving a transmission rate of 0.0020 (95% CI 0.0001–0.0113);
The details of this investigation remain unpublished; minimum details were obtained from the cited reference. UK United Kingdom; USA United States of America
METHODS
HCV notification and screening program
A public health investigation team consisting of members from the PEI Department of Health, Queen Elizabeth Hospital (QEH) and the Public Health Agency of Canada was assembled. Given that the physician had been working in PEI for approximately 30 years, and that his date of infection could not be definitively determined, a phased approach to screening was undertaken that prioritized individuals according to time period of greatest transmission risk and individuals at greatest risk of exposure.
Since the surgeon’s first hepatic laboratory abnormality was in November 2004, it was assumed that he may have become infected with HCV in early 2004 given an incubation period of six months. However, it is acknowledged that individuals infected with HCV may have normal or fluctuating levels of transaminases for several years. As an initial step, individuals who had undergone EPP performed by the surgeon from January 2004 (predating the assumed infection date) to February 2007 (last date of surgery) were screened. Two individuals who underwent non-EPP in which the surgeon sustained a percutaneous injury were also included. The period of phase 1 screening was based on two factors: the duration of documented elevation in the surgeon’s serum transaminases, and the knowledge that the HCV mutates over time. Thus, the probability of linking a transmission event preceeding this 38-month period based on finding homology in RNA sequence in the surgeon and infected patient’s virus would be difficult.
A comprehensive list of the surgeon’s surgical procedures and consultations were obtained from his Medicare billing records. These procedures were categorized as EPP and non-EPP based on national and international guidance documents (26–28). Clarification was also sought from the surgeon regarding a few billing codes and his practice patterns for certain procedures (eg, use of instrument or fingers to facilitate suturing of abdominal incisions following a laparoscopic cholecystectomy). Electronic files of all patients seen by the surgeon were obtained from Medicare and QEH medical records. These were merged by SAS v9.1 (SAS Inc, USA) using a personal health number as a unique identifier. EPP patients were identified using Medicare fee codes. In addition, International Classification of Disease, 10 edition codes or chart review were used to identify EPP patients among the records that were missing Medicare fee codes. All identified individuals were notified by registered mail and invited to participate in HCV screening clinics organized for this investigation. Patients who had EPP on October 1, 2006, or later were invited for a baseline HCV test and a follow-up test six months from their EPP date. In addition, HCV testing was also offered to other patients of the surgeon who were not eligible for phase 1 screening but were interested in being screened.
The list of phase 1 EPP patients was compared with the provincial notifiable disease registry to identify individuals who were known to be HCV positive. Given that previous HCV infection does not protect against reinfection, these individuals were invited to undergo repeat HCV screening.
Death records for deceased phase 1 EPP patients were reviewed to identify whether HCV infection or its sequelae were identified on the records.
HCV screening tests were conducted in the QEH microbiology laboratory using a third-generation anti-HCV enzyme-linked immunoabsorbent assay test (AxSYM, Abbott Laboratories, USA). Confirmatory testing was performed in Georges L. Dumont Regional Hospital in Moncton, New Brunswick, or at Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia. Both centres used a qualitative HCV polymerase chain reaction (PCR) (COBAS Amplicor HCV Test, version 2.0, Roche Diagnostics, Canada) as the primary confirmatory test. PCR-negative tests were reassessed for specific HCV antibodies using line immunoassay (Inno-LIA HCV Ab III, Innogenetics, Belgium) in Moncton and recombinant immunoblot assay (Chiron RIBA HCV 3.0 SIA, Chiron Corporation, USA) in Halifax. HCV genotyping and viral load assays were also performed at the Queen Elizabeth II Health Sciences Centre. Molecular sequencing of HCV 5’-non-coding region and C, E1 and Ns5b genes with phylogenetic analysis were conducted at the National Microbiology Laboratory in Winnipeg, Manitoba. HCV genotypes and subgenotypes were determined on the basis of sequence relationships and the degree of sequence divergence projected into phylogenetic tree structure. Phylogenetic trees and bootstrap analysis were constructed by neighbour-joining methods using the MEGA3 program (29). Reference nucleotide sequences of different HCV subgenotypes were obtained from GenBank. Additional nucleotide sequences originating from Canadian clinical isolates collected in 2005 and 2006, but not associated with the current outbreak, were also included in the phylogenetic tree analysis. Transmission probability and 95% CI were calculated using the binomial distribution (30).
The chief health officer and the director of hospital services, QEH, jointly implemented a proactive communication strategy. This included two media announcements, an advertisement about the screening program in all PEI newspapers, media interviews and the creation of a toll-free Hepatitis C Information Line accessible in the provinces of PEI, Nova Scotia and New Brunswick.
RESULTS
The surgeon saw 6248 patients in the 38-month time period of this investigation. His services to Medicare were billed using 166 fee codes; of these, 28 (16.9%) coded for various combinations of 11 EPP (Table 2). The EPP included procedures such as open cholecystectomies, bowel resection, repair of epigastric hernias, appendectomies, drainage of abscesses and salpingectomy. Non-EPP procedures included superficial procedures such as placement of central intravenous lines and Hickman’s catheters, as well as mastectomies, partial or complete thyroidectomies, and laparoscopic cholecystectomies.
TABLE 2.
List of exposure-prone surgical procedures in the investigation
| Procedures |
|---|
| Appendectomy, with or without gross perforation |
| Bowel resection – any of the following: abdomino-perineal resection, enterectomy, hemicolectomy, total colectomy, proctectomy, terminal ileum, caecum or ascending colon resection |
| Cholecystectomy – laporotomy |
| Closure of perforated ulcer |
| Closure of other bowel perforation |
| Drainage of subphrenic abscess |
| Epigastric hernia repair |
| Intestinal obstruction with or without resection |
| Laporotomy for deep exploration/biopsy |
| Ostomies: colostomy, entero-enterostomy, gastroduodenostomy, ileostomy |
| Salpingectomy and salpingo-oophorectomy |
Among the 6248 patients, 270 (4.3%) individuals had undergone EPP, 3454 (55.3%) had undergone other procedures, and 2524 (40.4%) individuals had received office consultations only. A total of 272 (4.4%) individuals were eligible for HCV screening; 270 who had undergone EPP procedures and two non-EPP patients for whom an intraoperative needle-stick injury to the physician had been identified. Of these, 248 (91.1%) were alive at the time of the look-back investigation, 231 (93.1%) presented for screening and 228 (98.7%) completed HCV screening. One individual (0.4%), patient A, tested HCV antibody positive (Table 3). From the linkage with the provincial HCV database, this individual was known to have been HCV positive, but PCR negative five years before an open cholecystectomy performed by the surgeon, and the individual remained HCV PCR negative at the time of the look-back investigation. There was thus no viral RNA available for phylogenetic analysis.
TABLE 3.
Hepatitis C virus (HCV) antibody-positive patients identified in the investigation
| Patient | Vital status | Age, years | Surgical procedures | Date of surgery | PCR +/− | HCV genotype | Notes |
|---|---|---|---|---|---|---|---|
| A | Alive | 51 | Open cholecystectomy | 2004 | − | −* | PCR negative in 1999 |
| B | Deceased | 62 | Ileostomy | 2006 | + | Ia† | Cause of death unrelated to HCV infection |
| C | Alive | 54 | Sigmoidoscopy | 2003 | − | −* | Elevated transaminases precholecystectomy |
| Endoscopy | 2004 | ||||||
| Laparoscopic cholecystectomy | 2006 | ||||||
| D | Alive | 51 | Office consultation only | 2006 | + | Ia† | HCV antibody positive in 1998, no PCR test at that time |
Unable to perform HCV genotyping in the absence of viremic blood samples;
Phylogenetic analysis indicates that neither of these two viruses are related to the surgeon’s virus. − Negative; + Positive; PCR Polymerase chain reaction
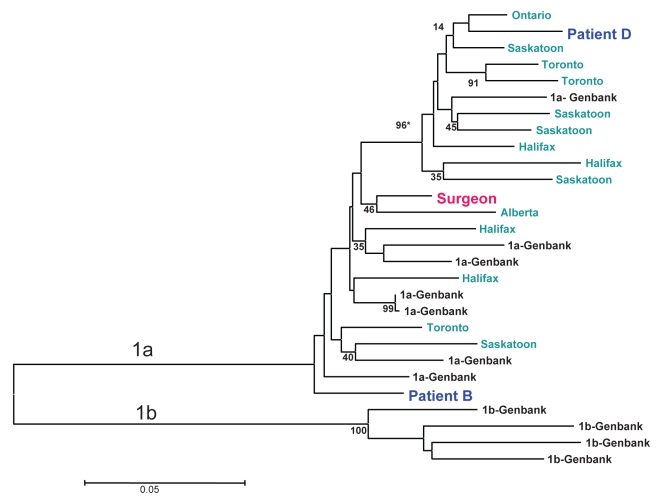
Death certificates were reviewed for 22 of 24 (91.7%) deceased EPP patients; liver failure was not identified as a cause of death for any of these patients. Linkage with the provincial HCV database revealed one HCV-positive individual, patient B, among the deceased EPP patients. Stored sera on this individual tested positive for HCV genotype 1a, but the RNA sequences did not match with those from the surgeon’s viral strain Figure 1 and Table 3). No HCV transmission events linked to the surgeon were identified; 95% CI of the transmission probability was estimated to be 0 to 0.016.
Figure 1).
Neighbour-joining phylogenetic tree analysis comparing hepatitis C virus (HCV) E1 gene sequences from the surgeon (red colour) and two of his patients (blue colour) with unrelated HCV strains from different provinces in Canada (green colour) and HCV strains from GenBank (black colour). *Phylogenetic relatedness is described as a bootstrapping value >70
An additional 184 non-EPP patients of this surgeon voluntarily sought testing; of these, two (1.1%) are HCV positive. Patient C had a laparoscopic cholecystectomy performed in November 2006. A review of this patient’s history suggested other risk factors for HCV acquisition. This individual has subsequently been tested twice, continues to remain PCR negative, and thus appears to have a resolved infection. In the absence of HCV viremia, it was not possible to perform phylogentic analysis. The second individual, patient D, met the surgeon for an office consultation only, not involving any surgical procedures; this patient was known to be previously HCV positive and had other risk factors for HCV acquisition. Although this individual is infected with HCV genotype Ia, phylogenetic analysis showed a different viral strain from the surgeon’s (Figure 1).
INTERPRETATION
Public health jurisdictions across the country have undertaken extensive HCV screening exercises in response to the transfusion of potentially infected blood products; however, this is the first Canadian look-back exercise following identification of a HCV infection in a health care worker. Canadian guidelines recommend that patient notification exercises be undertaken in the presence of known transmission of HCV from an infected health care worker to a patient (26). However, in the absence of known transmission to a patient, the decision to embark on a notification exercise is determined following a situational analysis (12,26,28). Recognizing the small but possible risk of spread of HCV in this situation, the PEI Department of Health decided that a look-back exercise was necessary. It was decided to prioritize public health resources by employing a staged approach. For the first phase, a lengthy time period was chosen for investigation (38 months), and a comprehensive list of patients were carefully identified to be at potential risk of HCV acquisition.
The initial investigation concluded that HCV transmission events linked to the surgeon during the 38-month period were unlikely. Reasons for this conclusion are as follows:
There was a high response rate to HCV screening among the eligible high-risk EPP patients.
The only two EPP individuals identified as HCV positive (patients A and B) were known to be positive before their surgery (based on phylogenetic analysis, patient B also has a different HCV virus lineage from the surgeon).
A nonprobabilistic sample of the surgeon’s low-risk or non-EPP patients were also screened for HCV infection; among these, two patients tested HCV positive. One of these, patient D, who only had consultation contact with the surgeon, was previously known to be HCV positive and has a different HCV virus lineage from the surgeon.
The only potentially significant finding was the newly diagnosed infection in a non-EPP patient, patient C. This patient has other risk factors for HCV infection, and in the absence of viral RNA a transmission link with the surgeon could not be identified.
The proactive communication strategy proved successful. The surgeon independently and voluntarily released his name to decrease alarm among the patients of the other three general surgeons in the same city. Media coverage of this event was comprehensive, nonalarming and proved to be a good venue for dissemination of public health information. The Hepatitis C Information Line was a successful mechanism for scheduling screening clinic visits and for connecting with out-of-province patients.
There are a few limitations to this investigation that may affect our ability to identify and thus screen all at-risk patients. First, the date of the surgeon’s HCV infection onset could not be determined. While individuals who had EPP over a three-year period were screened, the possibility that the surgeon might have been infected before 2004 cannot be ruled out. Second, in the absence of scientific evidence, the EPP list was based on expert opinion in the literature and consultation with the surgeon. Additional consultation from noninfected surgeons or operating room personnel to assess the frequency of percutaneous injuries and rates of glove performations in classifying EPP was not sought (12). A misclassification of an EPP as a non-EPP would decrease the sensitivity of the case definition. Third, for the time period of risk, patients of this surgeon were identified by matching electronic administrative databases. Chart review of a few records identified errors in the respective electronic records. Thus, an error in the coding of the surgeon’s name or in the procedure fee code would have resulted in an eligible patient not being offered screening. Fourth, while percutaneous injuries to surgeons during non-EPPs are a risk for HCV transmission, without sufficient documentation of these incidents, it is not possible to identify all the patients involved in these episodes. Finally, one cannot exclude the remote possibility of transmission even though no transmission events linked to the surgeon were identified by our look-back exercise. A relatively small number of patients limited to a 38-month period were examined. If no evidence of transmission from the surgeons occurred in patients who underwent highest transmission risk procedures, it is unlikely that transmission to other patients occurred in other settings.
To efficiently utilize public health resources, a phased approach was undertaken that focused on screening the ‘highest-risk’ patients of the newly identified HCV-positive general surgeon. The lack of HCV transmission within this ‘highest-risk’ group is encouraging and resulted in the investigation committee deciding not to further expand the screening program to ‘low-risk’ patients for the same time period or to EPP patients preceding the 38 months look-back period.
Acknowledgments
The authors would like to acknowledge the following individuals who participated in this investigation: public health nurses, Prince Edward Island; QEH Hepatitis C Investigation Committee members – Ms Connie Cheverie, Epidemiology, Department of Health, Prince Edward Island; Dr Shirley Paton, and Dr Jun Wu, Blood Safety Surveillance and Health Care Acquired Infections Division.
Footnotes
This investigation was presented at the 2007 European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), Stockholm, October 19, 2007.
REFERENCES
- 1.Statistics Canada Population and dwelling counts, for Canada, provinces and territories, 2006 and 2001 censuses<www12.statcan.ca/english/census06/data/popdwell/Table.cfm?T=101> (Version current at March 14, 2008). [Google Scholar]
- 2.Esteban Jl, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555–60. doi: 10.1056/NEJM199602293340902. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth GJ, Heptonstall J, Aitken C. Transmission of hepatitis C virus from a surgeon to a patient. The Incident Control Team. Commun Dis Public Health. 1999;2:188–92. [PubMed] [Google Scholar]
- 4.Hepatitis C virus transmission from health care worker to patient. Commun Dis Rep CDR Wkly. 1995;5:121. [PubMed] [Google Scholar]
- 5.Brown P. Surgeon infects patient with hepatitis C. BMJ. 1999;319:1219. doi: 10.1136/bmj.319.7219.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Transmission of hepatitis C virus from surgeon to patient prompts lookback. Commun Dis Rep CDR Wkly. 1999;9:387. [PubMed] [Google Scholar]
- 7.Two hepatitis C lookback exercises – national and in London. Commun Dis Rep CDR Wkly. 2000;10:125, 128. [PubMed] [Google Scholar]
- 8.Hepatitis C lookback exercise. Commun Dis Rep CDR Wkly. 2000;10:203–206. [PubMed] [Google Scholar]
- 9.Health care worker-to-patient transmission of HCV in the United Kingdom. Infect Control Hosp Epidemiol. 2000;21:619. doi: 10.1017/s019594170004368x. [DOI] [PubMed] [Google Scholar]
- 10.Gunson RN, Shouval D, Roggendorf M, et al. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in health care workers (HCWs): Guidelines for prevention of transmission of HBV and HCV from HCW to patients. J Clin Virol. 2003;27:213–30. doi: 10.1016/s1386-6532(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 11.Hepatitis C lookback in two Trusts in the south of England. Commun Dis Rep CDR Wkly. 2001;11:21. [Google Scholar]
- 12.Ross RS, Viazov S, Thormahlen M, et al. Risk of hepatitis C virus transmission from an infected gynaecologist to patients. Arch Intern Med. 2002;162:805–10. doi: 10.1001/archinte.162.7.805. [DOI] [PubMed] [Google Scholar]
- 13.Ross RS, Viazov S, Roggendorf M. Phylogenetic analysis indicates transmission of hepatitis C virus from an infected orthopaedic surgeon to a patient. J Med Virol. 2002;66:461–7. [PubMed] [Google Scholar]
- 14.Ross RS, Steinbruckner B, Bohm S, Viazov S, Jilg W, Roggendorf M. Outcome of an exercise to notify patients treated by a general surgeon infected with the hepatitis C virus. J Clin Virol. 2008;41:314–7. doi: 10.1016/j.jcv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis. 2004;38:1592–8. doi: 10.1086/420935. [DOI] [PubMed] [Google Scholar]
- 16.Wong T, Lee SS. Hepatitis C: A review for primary care physicians. CMAJ. 2006;174:649–59. doi: 10.1503/cmaj.1030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ontario Assessment Guide for Hepatitis C Risk Factors <www.health.gov.on.ca/english/providers/program/hepc/hepc_pdf/hepc_assess_e.pdf> (Version current March 18, 2008).
- 18.Balter S, Layton M, Bornschlegel K, et al. Transmission of hepatitis B and C viruses in outpatient settings – New York, Oklahoma, and Nebraska, 2000–2002. MMWR. 2003;52:901–6. [PubMed] [Google Scholar]
- 19.Hepatitis C Investigation Southern Nevada Health District <www.southernnevadahealthdistrict.org/outbreaks/index.htm> (Version current March 14, 2008).
- 20.Bosch X. Newspaper apportions blame in Spanish hepatitis C scandal. Lancet. 2000;355:818. doi: 10.1016/s0140-6736(05)72446-6. [DOI] [PubMed] [Google Scholar]
- 21.Siegel-Itzkovich J. Doctor allegedly infected patients with hepatitis C. BMJ. 2003;327:414. doi: 10.1136/bmj.327.7412.414-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cody SH, Nainan OV, Garfein RS, et al. Hepatitis C virus transmission from an infected anaesthesiologist to a patient. Arch Intern Med. 2002;162:345–50. doi: 10.1001/archinte.162.3.345. [DOI] [PubMed] [Google Scholar]
- 23.Ross RS, Viazov S, Gross T, Hofmann F, Seipp H-M, Roggendorf M. Transmission of hepatitis C virus from a patient to an anaesthesiology assistant to five patients. N Engl J Med. 2000;343:1851–4. doi: 10.1056/NEJM200012213432505. [DOI] [PubMed] [Google Scholar]
- 24.Sehulster L, Taylor J, Hendricks K, VanEgdom M, Whiteley S, Manning S. Hepatitis C outbreak linked to narcotic tampering in an ambulatory surgical centre. Abstracts of the 1997 Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: American Society of Microbiology Press; 1997. p. 293. [Google Scholar]
- 25.Ross RS, Viazov S, Roggendorf M. Risk of hepatitis C transmission from infected medical staff to patients. Arch Intern Med. 2000;160:2313–6. doi: 10.1001/archinte.160.15.2313. [DOI] [PubMed] [Google Scholar]
- 26.Proceedings of the Consensus Conference on Infected Health Care Workers: Risk for transmission of bloodborne pathogens Canada Communicable Disease Report 199824S4 <www.phac-aspc.gc.ca/publicat/ccdr-rmtc/98vol24/24s4/index.html> (Version current March 1, 2007). [PubMed] [Google Scholar]
- 27.College of Physicians and Surgeons of BC Guidelines of the advisory committee on blood-borne communicable diseases <www.cpsbc.ca/cps/college_programs/bbcd_panel> (Version current March 1, 2007).
- 28.Department of Health Hepatitis C infected health care workers <www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4010554> (Version current March 1, 2007).
- 29.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–63. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 30.Exact Binomial and Poisson Confidence Intervals <http://statpages.org/confint.html> (Version current at January 21, 2009).