Abstract
This study compared the relative efficacy of two contingency management (CM) interventions versus standard care. During a 12-week intervention, opioid dependent participants (N = 120) were maintained on thrice-a-week (M, W, F) buprenorphine plus therapist and computer-based counseling. They were randomized to receive: (a) medication contingencies (MC= thrice weekly dosing schedule vs. daily attendance and single-day 50% dose reduction imposed upon submission of an opioid and/or cocaine positive urine sample); (b) voucher contingency (VC=escalating schedule for opioid and/or cocaine negative samples with reset for drug-positive samples); or (c) standard care (SC), with no programmed consequences for urinalysis results. Voucher reinforcement resulted in better 12-week retention (85%) compared to contingent medication (58%; p=0.009), but neither differed from standard care (76% retained). The groups submitted a similar overall percentage of opioid and cocaine-free urines (MC = 79%, VC = 76%, SC = 69%). After adjusting for baseline differences in employment, the medication contingency group achieved 1.5 more continuous weeks of combined opioid/cocaine abstinence than standard care (p=0.030), while the voucher group had 2 more total weeks of abstinence than standard care (p=0.048). Drug use results suggest that the two interventions were both efficacious, with effects seen primarily in opioid rather than cocaine test results. Findings should be interpreted in light of the greater attrition associated with medication-based contingencies versus the greater monetary costs of voucher-based contingencies.
Keywords: buprenorphine, cocaine, community reinforcement approach, contingency management, opiate or opioid dependence
INTRODUCTION
Dependence or abuse of opioids is an increasing public health problem in the United States. Most recent data from the 2007 National Survey on Drug Use and Health (NSDUH) reveals that while there was a decline in the abuse of a number of illicit drugs, especially cocaine and methamphetamines, there also was a 12% increase compared to the previous year in the “nonmedical use of prescription pain relievers” especially in young adults aged 18 to 25 years (Substance Abuse and Mental Health Services Administration, 2008; news release at: http://oas.samhsa.gov/NSDUH/2k7NSDUH/press.htm). Earlier figures from 2006 suggested that more than 12.5 million people over age 12 years had used illicit pain relievers over the past year, and over 5 million had used them during the past month. Both represented statistically significant increases from the preceding year. Given this increase in the prescription-opioid abuse epidemic, the approval of buprenorphine (as Suboxone® and Subutex®) presents an advance in the treatment of opioid addiction, suitable for both heroin and prescription opioid abusers.
Concurrent cocaine abuse is often a problem in a substantial proportion of the opioid-abusing population. Indeed, comorbid cocaine abuse has been associated with worse outcomes, including persistent use of illicit opioids (Wasserman, Weinstein, Havassy, & Hall, 1998), poor retention in treatment (Magura, Nwakeze, & Demsky, 1998), and continued indulgence in drug-related behavior and criminal activity (Hunt et al., 1986; Kolar, Brown, Weddington, & Ball, 1990; Kosten, Rounsaville, & Kleber, 1988). Hence, treatment for opioid dependence should optimally address abstinence from cocaine as a concurrent treatment goal. Strategies using behavior therapy, derived from learning theory, and collectively referred to as Contingency Management (CM) techniques, have demonstrated success for the treatment of comorbid cocaine and opioid dependence (see reviews by Dutra et al., 2008; Prendergast et al., 2006; Stitzer & Petry, 2006).
However, one of the criticisms against the use of CM techniques has been that that they are expensive in terms of cost, and time-consuming to implement. Hence, an important objective for research on treatment options for addictive disorders is to identify innovations that are efficacious but that can also be readily adopted by treatment providers and clinics. One approach to cost reduction is the use of information technology to deliver CM or similar treatment options (Bickel, Marsch, Buchhalter, & Badger, 2008; Carrol et al., 2008). Another approach is the use of privileges intrinsic to the treatment setting as reinforcement. For example, providing take-home doses of opioid maintenance medications granted on condition of successful abstinence has the advantage of both low cost and high reinforcement value to the patients (Chutuape, Silverman, & Stitzer, 1999; Silverman, Robles, Mudric, Bigelow & Stitzer, 2004). A previous study (Gross, Marsch, Badger, & Bickel, 2006) showed that buprenorphine medication-contingencies, in which full dosing was contingent upon evidence of drug abstinence, produced nearly twice as many weeks of abstinence as a low-cost voucher condition with total possible earnings of US$269 over a 12-week period. In the same trial, the contingent voucher condition had a retention of 80%, which was not significantly different from the 65% retention with contingent medication. However, the efficacy of medication contingencies relative to the higher, more typical voucher-reinforcement magnitudes, (as originally described by Higgins et al., 1991) has remained unknown since vouchers of higher magnitude have consistently been shown to improve outcomes for substance-abuse disorders (see Lussier et al., 2006).
The present study was designed to compare the efficacy of buprenorphine medication contingencies to voucher contingencies with a reinforcer magnitude similar to that used in previous research (e.g. Higgins et al., 1991). The two experimental conditions were delivered using the Community Reinforcement Approach (CRA) format, which has been defined as a multifaceted biopsychosocial approach to change a lifestyle of substance abuse (Roozen et al., 2004). Our group has developed a computerized delivery of CRA that ensures consistency and lowers cost (Bickel et al., 2008). A standard-care control group was also included in the design to permit comparison of each intervention with a treatment condition in which no contingencies were placed on urinalysis test results. Findings of this study will be important for optimizing treatment interventions with opioid abusers being treated with buprenorphine maintenance pharmacotherapy.
MATERIALS AND METHODS
Participants
The study was initiated at the University of Vermont in 2003, and completed at the University of Arkansas for Medical Sciences (UAMS). The Institutional Review Boards of both institutions had approved the study. Participants were healthy volunteers between the ages of 18 and 55 years who met Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994) criteria for opioid dependence and Food and Drug Administration (FDA) qualification criteria for buprenorphine treatment (i.e., a history of opioid dependence and either objective evidence of significant current opioid use or signs of opioid withdrawal) and who signed written informed consent to participate in the study.
Participants were recruited through referrals, advertisements and public service announcements and all potential candidates received a standard medical evaluation, consisting of physical examination and laboratory tests (including blood-cell counts, chemistry and liver function tests, urinalysis and electrocardiogram) prior to enrollment. Individuals with evidence of any significant or unstable medical condition (e.g., cardiovascular disease) or an active psychiatric disorder that may interfere with participation in the research (e.g., psychosis or organic psychiatric disorders), or those who were pregnant were excluded from participation. So that the findings of this study were applicable to a general clinical setting, co-dependence or abuse of cocaine or alcohol were not among the exclusion criteria.
Wherever clinically warranted, those abusing benzodiazepines or other sedative-hypnotics were referred for detoxification from these co-dependencies prior to participation, as safety of concurrent administration of buprenorphine with sedative-hypnotics was a concern. The study also used a modified version of the Addiction Severity Index (ASI) scale, such that questions for the ‘Drug’ subscale were asked separately for both opioids and cocaine (McLellan et al., 1985). Thus the modified ASI used in this study had two additional items of ‘Opioids’ and ‘Cocaine’, in addition to the category of ‘Drugs’.
One hundred and seventy individuals consented to participate in the outpatient study and a total of 127 were randomly assigned to one of the treatment conditions. Forty-three participants who consented for the trial were unable to make it through the one-week induction phase prior to randomization for different reasons. Further, 14 of the participants were couples, and since it was likely that their outcomes were not independent of each other, information from one person in each couple was randomly selected for inclusion in the data analysis. The small number (7) of couples prevented any statistically meaningful inferences on potential inter-dependence in the results from a couple. After successfully being stabilized on one of the three maintenance doses of buprenorphine (6, 12 or 18 mg) during the one-week induction period, these 120 participants (75 from Vermont, 45 from Arkansas) were randomly assigned to one of three treatment groups (contingent medication, contingent voucher or standard counseling) using minimum-likelihood allocation (Aickin, 1982). This method of permutation has been shown to achieve balance between treatment groups on patient characteristics likely to influence treatment outcome.
Three characteristics were used to stratify patients to one of the three treatment groups: (1) stabilization dose of the buprenorphine/naloxone tablet (6, 12 or 18 mg of the sublingual tablet; see Buprenorphine section below); (2) cocaine use in the past month; and (3) distance from the clinic in minutes (i.e., near < 30 min, medium = 30 to 60 min, and far > 60 min). We have successfully used this allocation procedure in other trials to assign participants to the three intervention groups without any significant differences on any measure of baseline-intake characteristics (Bickel et al., 1997; Higgins et al., 1994). Thus, 69 male and 51 female Suboxone-maintained individuals participated in this out-patient, parallel-groups design study. The baseline characteristics of the three groups of participants are presented in Table 1.
Table 1. Demographic and Participant Characteristics of the Three Study Groups.
| Characteristic (Statistic) | Group |
p | ||
|---|---|---|---|---|
| Standard Treatment (n=37) |
Medication Contingency * (n=42) |
Voucher Incentive (n=41) |
||
| Demographics | ||||
| White (%) | 97.3 | 97.6 | 97.6 | 0.99 |
| Male (%) | 64.9 | 47.6 | 61.0 | 0.26 |
| Never married (%) | 48.6 | 52.4 | 58.5 | 0.67 |
| High school education (%) | 78.4 | 83.3 | 80.5 | 0.85 |
| Employed full-time (%) | 62.2 | 33.3 | 53.7 | 0.03 |
| Age (Yrs) M ± SD | 33.5 ± 11.1 | 31.6 ± 10.1 | 30.6 ± 9.1 | 0.44 |
| Monthly income ($) Md (Q1,Q3) | 1200 (700, 1933) | 1010 (600, 2100) | 1200 (490, 3200) | 0.92 |
| Opioid Use | ||||
| Prior treatment (%) | 67.6 | 71.4 | 80.5 | 0.41 |
| Regular use (Yrs) M ± SD | 7.0 ± 6.9 | 6.1 ± 5.7 | 5.6 ± 6.1 | 0.59 |
| Age of first use (Yrs) M ± SD | 22.5 ± 8.3 | 22.6 ± 7.7 | 21.5 ± 7.7 | 0.80 |
| Previous month’s spending on opioids ($) Md (Q1,Q3) |
1000 (300, 3000) | 1000 (400, 2250) | 1800 (500, 3000) | 0.61 |
| Preferred Route:** | ||||
| Intravenous (%) | 32.4 | 38.1 | 39.0 | |
| Intranasal (%) | 35.1 | 35.7 | 39.0 | |
| Oral (%) | 32.4 | 26.2 | 22.0 | 0.88 |
| Other Drug Dependence | ||||
| Alcohol (%) | 10.8 | 2.4 | 14.6 | 0.14 |
| Cocaine (%) | 18.9 | 11.9 | 24.4 | 0.34 |
| Sedative (%) | 8.1 | 7.1 | 14.6 | 0.47 |
| Cannabis (%) | 43.2 | 33.3 | 39.0 | 0.66 |
| Duration-cocaine use (Yrs) Md (Q1,Q3) |
1 (0, 5) | 1 (0, 3) | 1 (0, 5) | 0.50 |
| ASI Composite Scales | ||||
| Medical Md (Q1,Q3) | 0.08 (0, 0.49) | 0.08 (0, 0.51) | 0.00 (0, 0.34) | 0.42 |
| Employment Md (Q1,Q3) | 0.50 (0.31, 0.62) | 0.50 (0.29, 0.69) | 0.50 (0.18, 0.52) | 0.34 |
| Alcohol Md (Q1,Q3) | 0.00 (0, 0.06) | 0.00 (0, 0.04) | 0.00 (0, 0.08) | >0.99 |
| Drug Md (Q1,Q3) | 0.31 (0.18, 0.41) | 0.30 (0.20, 0.36) | 0.32 (0.20, 0.37) | 0.85 |
| Psychiatric Md (Q1,Q3) | 0.32 (0.05, 0.50) | 0.29 (0.09, 0.50) | 0.27 (0.09, 0.38) | 0.81 |
| Legal Md (Q1,Q3) | 0.19 (0, 0.35) | 0.13 (0, 0.40) | 0.20 (0, 0.31) | 0.92 |
| Family-Social Md (Q1,Q3) | 0.19 (0, 0.40) | 0.14 (0.02, 0.35) | 0.11 (0, 0.33) | 0.53 |
| Cocaine Md (Q1,Q3) | 0.00 (0, 0.01) | 0.00 (0, 0.01) | 0.00 (0, 0.03) | 0.97 |
| Opioids Md (Q1,Q3) | 0.70 (0.61, 0.73) | 0.65 (0.55, 0.72) | 0.70 (0.63, 0.74) | 0.02 |
| Buprenorphine dose Md (Q1,Q3) *** |
12 (12, 18) | 12 (12, 18) | 12 (12, 18) | >0.99 |
| 6 mg/day group (%) | 10.8 | 9.5 | 2.4 | |
| 12 mg/day group (%) | 54.1 | 50.0 | 58.5 | |
| 18 mg/day group (%) | 35.1 | 40.5 | 39.0 | 0.63 |
One subject in the medication contingency group provided no data; hence, the medication contingency group had n for denominator of 41.
No differences in the proportions of participants’ preferred routes of administration among the three experimental groups (χ2[df=4] =1.169, p=0.883).
No differences in the proportions of participants assigned to the three buprenorphine dose groups among the three experimental groups (χ2[df=4] =2.604, p=0.626).
M: mean; Md: median; SD: standard deviation; Q1,Q3: Inter-quartile range; Yrs: years; ASI: Addiction Severity Index.
Buprenorphine Induction and Stabilization
Buprenorphine maintenance doses were determined during the first week of participation. On Day 1, participants initially received a 2 mg dose of the buprenorphine-mono (Subutex) tablet sublingually. If they were able to tolerate this initial dose without any adverse events or worsening of withdrawal symptoms, they then received another 4 mg for a total dose of 6 mg on this day. Participants were evaluated daily during this induction week, and if they were experiencing withdrawal symptoms on Day 2, they received a total of 12 mg of buprenorphine (Subutex) on this day. If this dose had been insufficient to address withdrawal symptoms by Day 3, participants were given 18 mg of the buprenorphine/naloxone (Suboxone) combination tablets. Withdrawal symptoms were assessed using the Non-Narcan Challenge CINA Assessments and physiologic measures (like pulse rate, blood-pressure and diameter of pupils). Dose adjustments were made on the following days, if necessary, and participants reached one of the stable maintenance doses before the end of this week. Our prior experience with opioid treatment indicates that this method for determining a subject’s dose is safe and effective (Amass, Bickel, Higgins, & Badger, 1994; Bickel et al.,1997; Bickel, Amass, Crean, & Badger, 1999).
Following this one-week period of buprenorphine dose stabilization, participants began the study’s 12-week experimental phase, with participants receiving their maintenance dose on an alternate-day schedule. That is, participants attended the clinic three times a week, receiving a double of their daily buprenorphine/naloxone maintenance dose on Mondays and Wednesdays and a triple dose on Fridays, which has been shown to be effective because of the unique pharmacological profile of buprenorphine (e.g., Amass, Bickel, Crean, Blake, & Higgins, 1998; Amass et al., 1994; Bickel, Amass et al., 1999). The proportion of participants receiving each buprenorphine stabilization dose is also presented in Table 1. This dosing regimen remained in effect throughout the 12-week duration of the study. On completion of the study, participants had the option of being referred to other studies or appropriate treatment programs, or a buprenorphine taper with a 2 mg decrease in dose every week.
Buprenorphine-mono or buprenorphine/naloxone tablets were provided by the National Institute of Drug Abuse (NIDA). Buprenorphine and naloxone were available in the ratio of 4:1 in the combination tablets and medications were administered under single-blind conditions. Participants were instructed not to smoke any cigarettes for two hours prior to medication administration and were required to hold the tablets under their tongue for five minutes under observation. They were also required to drink a small glass of water both prior to and after medication administration.
Treatment Groups
Behavioral treatment (described below), including a computerized version of the CRA, was provided to the two contingency groups for the 12-week duration of the trial.
Medication Contingency Condition with CRA
The buprenorphine medication contingencies consisted of two parts. The first part involved alternate day dosing, wherein participants came to the clinic only three days a week, and received twice their daily maintenance dose on Mondays and Wednesdays and three times the daily dose on Fridays, if they provided opioid- and cocaine-free urine samples. If a urine sample was positive for one of the target drugs, the participant was required to report for dosing under a five-days-per-week schedule. This continued until they had provided three consecutive drug-free samples and attended daily for at least five consecutive weekdays. At this point, they could return to the three-day a week dosing schedule.
The second part of the medication contingency included the use of a dose alteration procedure, wherein the patients’ daily buprenorphine dose depended on both clinic attendance and recent abstinence. A cocaine- or opioid- positive urine sample resulted in the participant receiving only half their daily maintenance dose when they attended the clinic each weekday. If the participant submitted a drug positive sample on a Friday, they received double their daily maintenance dose for the weekend rather than a triple dose. Reduced dosing continued until the first opioid- and cocaine- free urine sample was submitted (while release from the daily dosing schedule required three consecutive drug-free urines) Thus, following a drug-positive urine, participants were under the daily dosing requirement for a longer time than the reduced dosing aspect of the contingency. The differential time course was implemented to limit any potential adverse impact of dose reduction on treatment retention. Collectively, this two-part medication contingency was designed to approximate the voucher-based contingency to the greatest extent possible.
Voucher Contingency Condition with CRA
The voucher system involved systematically reinforcing abstinence as indicated by urinalysis results. Staff informed patients of their urinalysis results immediately after testing. Specimens that were negative for opioids (opiates, propoxyphene, or methadone) and cocaine earned points that were recorded on vouchers and given to patients with each point worth $0.25. The first negative specimen was worth 10 points at $0.25 each or a total of $2.50. Each subsequent consecutive negative specimen increased the value of the voucher by 5 points (2nd =15 points, 3rd =20 points, etc.). As an additional incentive for continuous opioid and cocaine abstinence, a $10.00 bonus was provided to patients for each set of 3 consecutive negative samples. Continuous abstinence throughout the 12-week trial maintenance period during which these contingencies were imposed could potentially result in participants receiving vouchers equivalent to a total of $997.50.
Participants never received money directly. Instead, the cash equivalents of the points earned were used by staff members to buy material reinforcers requested by participants (e.g., fishing license, restaurant gift certificates, automobile parts, establish phone service). These material reinforcers could be obtained at any time during treatment and were selected by the participant, with the therapist retaining veto power over any item deemed to be inconsistent with a drug-free lifestyle. Submission of an opioid- and/or cocaine-positive urine sample, or failing to submit a scheduled specimen (which was counted as a positive sample) would reset the value of the vouchers to the initial $2.50 level. Submission of five consecutive opioid- and/or cocaine-negative specimens returned the value of the vouchers to the level obtained before the reset. Points, once earned, could not be lost.
Standard Treatment Condition with Counseling
Participants in this group did not receive any of the programmed consequences contingent on urinalysis results. Thus, these participants did not receive voucher points on submission of opioid- and/or cocaine-free urine samples, nor did they receive dose reductions and daily dosing on submission of opioid- and/or cocaine-positive urine samples. Participants in this condition continued to receive thrice-weekly dosing with buprenorphine/naloxone and standard methadone-style counseling sessions once a week throughout the period of the trial. Study therapists were informed about the urinalysis results and discussed them with the participants.
CRA Treatment
A computerized version of the Community Reinforcement Approach (CRA), along with contingency management, was provided to the two active treatment groups in three 30-minute sessions each week. Subjects in the contingency treatment conditions met with their assigned therapists once every other week (biweekly) for sessions of about 30-minute duration, which substituted for one computerized session that week. During these sessions, therapists formulated a treatment plan individualized for each participant, reviewed their cumulative progress and agreed on the order in which the participants could access their future computerized CRA topics.
The greatest emphasis in CRA is on behavioral skills training, along with behavioral rehearsal and role-playing being employed in a few treatment topics. These have been described in detail in the treatment manual for this intervention (Budney & Higgins, 1998). The content of the fluency-based skills training component of CRA was personalized to the situation of each participant and delivered via a computer-assisted instruction system. It included topics like drug-refusal skills training, relationship counseling and the like for the participant. The Behavioral Rehearsal involved practicing skills like assertiveness training or time management training, and involved practice homework in real life situations. Participants whose urinalysis was positive for one of the two target drugs completed a Functional Analysis of their situation. This computer-delivered CRA has been shown to be as efficacious and more cost-effective compared to therapist-delivered CRA when part of a multi-modal treatment package (Bickel et al., 2008).
Substance Use Monitoring
Urine specimens were collected under staff observation from all participants on Mondays, Wednesdays and Fridays and screened immediately on-site with the enzyme-multiplied immunoassay technique (Syva Corp., San Jose, CA). All specimens were screened for methadone, opiates, propoxyphene, and cocaine, with one randomly selected specimen per week also screened for benzodiazepines (positive results determined at >300 ng/mL). Breath samples were also analyzed at the time that urine specimens were collected and breath alcohol levels had to be less than 0.05 g/ml of air for participants to receive scheduled medications.
Statistical Analyses
Trial retention was examined using survival curves, and the groups were compared using log-rank chi-square tests for homogeneity of the Kaplan-Meier event-time functions. Proportions of participants in the three groups completing the trial were also compared with a chi-square test. Primary outcome measures of interest reflected abstinence from both opioids and cocaine, and from opioids alone. Measures reported are longest duration of continuous abstinence, total weeks of abstinence (3 consecutive negative urines provided within a calendar week) and percentage of negative urines provided per week. Mean percentages of weekly negative urine samples were calculated from the total number of urine samples provided within each of the intervention groups during the week, and hence were conditioned on the number of active participants for the given week.
Examination of the abstinence data revealed non-normal distributions; hence, we focused our inference on the medians rather than means of the number of continuous and total weeks abstinent. We tested the omnibus null hypothesis that neither of the experimental treatments was better than the standard with respect to median abstinence measures using permutation tests, a non-parametric method (Good, 2005). We analyzed the weekly percentages of negative urines within a repeated measures analysis of variance (ANOVA), having treatment, weeks, and their interaction as the factors, with weeks being a within-individual factor. The within-individual correlation was modeled with a compound symmetric structure. All p-values from these repeated measures ANOVAs were computed using permutation tests rather than F-tests due to violations of the normal assumption. When significant differences among the three treatment groups were found, planned pair-wise comparisons between the medication contingency group versus standard treatment and the voucher contingency group versus standard treatment were performed.
The three groups were compared on baseline measures using chi-square tests for differences among proportions, median tests for differences among ordinal or non-normal measures, and F-tests for normally distributed measures. At baseline, the three groups statistically differed on employment status and the opioid sub-scale of the ASI. A significant association between employment status and abstinence was found; no evidence existed relating ASI opioid subscale to abstinence. Permutation tests for evaluating group differences in abstinence were hence configured to include the (non-randomized) employment factor. Results from permutation tests controlling for employment are presented.
Cochran-Mantel-Haenszel Tests were used to examine for any differences in characteristics and outcome between the two sites while controlling for study group. Significant between-sites differences were noticed with regard the median participant income. Participants in Vermont (n=75) had a median (inter-quartile range [IQR]) income of $1800 (857, 3600), which was significantly greater (median test: p<0.001) than the median income of participants in Arkansas (n=41), whose median income was $620 (149, 1021.5). Site-related income differences were significant for the voucher contingency group (Vermont: $2000 [1000, 4000]; Arkansas $170 [40, 800]; median test: p<0.001) and standard treatment groups (Vermont: $1800 [1200, 3000]; Arkansas $706 [150, 1050.5]; median test: p=0.003), but not for those in the medication contingency group (Vermont: $1500 [600, 4500]; Arkansas $662.5 [150, 1600]; median test: p=0.100). No statistically significant correlation was observed between participant income and the outcome measures using Spearman’s rank order correlation. Further, using Cochran-Mantel-Haenszel tests, which controlled for treatment group effects, no differences between study sites (Vermont versus Arkansas) were found for any abstinence outcome (all p>0.16). However, when comparing abstinence measures between the sites within each treatment group with a median test, we did find the Arkansas participants in the contingent voucher group to have significantly better outcomes than their Vermont counterparts with regard to continuous weeks abstinent from both opiates and cocaine (median test: p=0.026).
To examine if the route of opioid abuse was associated with the outcomes, we median polished the abstinence data first, with respect to treatment group and then to employment status. That is, we first subtracted the respective group median from each individual’s outcome (thus effectively removing the group effect), and then from each individual’s residual, we subtracted the respective employment status median (thus effectively removing the employment-status effect). For each of continuous and total abstinence measures for both opioids and cocaine, or opioids alone, the medians of the polished data were compared among the different self-administration routes. No association between route of administration and outcome was found (median test: all four p>0.47).
RESULTS
Demographic and other baseline characteristics, including frequencies of opioid, cocaine and other substances abused by the three groups are presented in Table 1. The proportions of patients with fulltime employment differed among the groups (χ2[df=2] = 7.04, p= 0.030), as well as the medians of the opioid sub-scale of the ASI (median test: p= 0.019). The medication contingency group demonstrated the lowest percentage of fulltime employment and lowest median ASI opioid sub-scale score among the groups. Using abstinence residuals from group medians, those with full time employment produced lower continuous weeks of abstinence from both opioids and cocaine and from opioids alone compared to those without full time employment (median test: both p < 0.001), with similar results on total weeks of abstinence (median test: both p < 0.011). Correlation analyses failed to find any evidence of a relationship between the ASI opioid subscale and the median-polished abstinence data (all four p > 0.10).
Participant Retention
Participant retention in the three intervention groups is presented in Figure 1. Retention was found to not be homogenous among the treatment groups, log rank χ2[df=2] = 6.916, p= 0.032. Subsequent post hoc tests revealed that participants in the voucher contingency group adhered to treatment significantly better than those receiving medication contingencies (log rank χ2[df=1] = 6.503, p= 0.011). Differences between the standard treatment group and either the contingent voucher or the continent medication groups were not significant (log rank χ2[df=1] = 1.083, p= 0.298 and log rank χ2[df=1] = 2.138, p= 0.144, respectively). Thirty-five of 41 (85.4%) participants in the voucher contingency group and 28 of 37 (75.7%) participants in the standard treatment group completed the entire 12-week experimental phase of the study compared to 25 of 42 (59.5%) participants assigned to medication contingencies. The proportions of participants completing the trial among the three groups were significantly different (χ2[2] = 7.235, p = 0.027), with the voucher contingency group having a significantly better retention at 12 weeks than the medication contingency group (χ2[2] = 6.917, p=0.009). Of the 32 participants who failed to complete the experimental phase, three were receiving buprenorphine at 6 mg/day, while 14 were in the 12/mg/day and 15 were in the 18 mg/day dosing groups.
Figure 1.
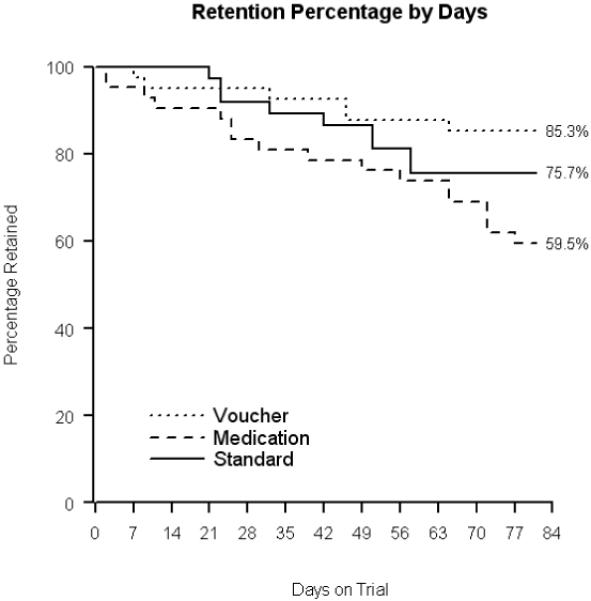
Participant retention in the three study groups.
The medians of missed visits were 0.5 (IQR: 0 - 3), 0 (IQR: 0 - 1) and 1 (IQR: 0 - 3) for participants in the contingent medication, contingent voucher and standard treatment groups respectively. Missed visits were not significantly different between the three treatment groups (median test: p= 0.300). However, 59% of those with fulltime employment had a number of missed clinic appointments that was greater than the grand median of missed appointments from all 120 participants. This compared with only 38% of those without fulltime employment who had a number of missed appointments greater than the grand median, and this difference was significant (median test: p = 0.028). The median (IQR) number of reduced doses for the participants assigned to the medication contingency group was 4 (1-8). Participants randomized to the voucher condition earned an average of $479.30 (± $382.33, IQR = $67.50 - $898.75) of the $997.50 possible.
Abstinence from Both Opioids and Cocaine
Median longest continuous weeks abstinent and total (including non-continuous) weeks abstinent from opioids and cocaine for the three intervention groups at each employment status (fulltime or not) are presented in Table 2. There was evidence that at least one of the experimental groups had longer continuous weeks in abstinence than standard treatment (permutation test: p= 0.009). Planned a priori pair-wise comparisons revealed that the medication contingency group achieved 1.5 more continuous weeks of combined target drug abstinence compared to standard treatment (permutation test: p= 0.029) while the voucher contingency group was not found to be statistically better than the standard treatment group (permutation test: p = 0.086).
Table 2. Weeks of Abstinence from Opioids and Cocaine, and Opioids Alone During the Trial.
| WEEKS ABSTINENT FROM * |
|||||
|---|---|---|---|---|---|
| Opioids And Cocaine | Opioids Alone | ||||
| Continuous | Total | Continuous | Total | ||
|
Across
Employment Status |
Standard | 4 (1, 10) | 5 (1, 11) | 4 (2, 11) | 6 (2, 11) |
| Medication | 6 (2, 9) | 8 (3, 10) | 6 (2, 10) | 8 (3, 11) | |
| Voucher | 4 (1, 11) | 9 (2, 11) | 6 (3, 12) | 10 (5, 12) | |
|
Employed
Fulltime |
Standard | 3 (1, 6) | 5 (1, 8) | 3 (1, 6) | 6 (2, 9) |
| Medication | 5.5 (2, 6) | 5.5 (3, 8) | 5.5 (2, 6) | 5.5 (3, 10) | |
| Voucher | 4 (1, 8) | 6 (1, 10) | 4 (2, 12) | 6 (2, 12) | |
|
Not
Employed Fulltime |
Standard | 6 (2, 12) | 7.5 (2, 12) | 6 (2, 12) | 7.5 (2, 12) |
| Medication | 6 (2.5, 10.5) | 8.5 (3.5, 11) | 8 (2.5, 11) | 8.5 (4.5, 11) | |
| Voucher | 8 (3, 12) | 10 (6, 12) | 10 (4, 12) | 11 (10, 12) | |
| p-values | Omnibus | 0.003 | 0.028 | 0.003 | 0.012 |
| Medication vs. Standard | 0.029 | 0.180 | 0.023 | 0.235 | |
| Voucher vs. Standard | 0.086 | 0.043 | 0.040 | 0.025 | |
| Medication vs. Voucher | 0.511 | 0.382 | 0.827 | 0.275 | |
Presented as Median (Inter-Quartile Range)
Comparison of total weeks abstinent from both drugs during the trial revealed at least one experimental group was better than the standard group (permutation test: p= 0.028). Planned pair-wise comparisons revealed that the voucher incentive group had 2 more total weeks of abstinence than standard treatment, and this difference was significant (permutation test: p= 0.043), but the buprenorphine contingency group failed to be statistically better than standard treatment (permutation test: p= 0.180).
The percentages of weekly urine samples that were free from both opioids and cocaine are shown in Figure 2. There was no evidence of a treatment by time interaction nor of a time effect (permutation test: p= 0.803 and p= 0.513, respectively). The medication contingency group averaged 79% drug-free urines (from opioids and cocaine) over the 12 week trial, which, though 10 percentage points greater than the average percent (69%) of drug-free urines provided by the standard treatment group, was not statistically different from the standard (permutation test: p= 0.067). The voucher contingency group provided an average of 76% drug-free urine samples (from opioids and cocaine); this increase of seven percentage points from the standard treatment groups’ mean also failed to reach statistical significance (permutation test: p= 0.144).
Figure 2.
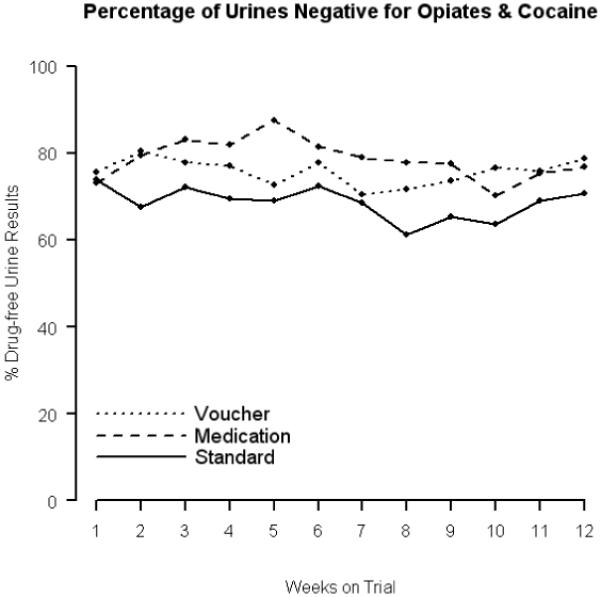
Percentage of weekly urinalysis samples negative for both opioids and cocaine in each of the three study groups.
Abstinence from Opioids Alone
Median continuous weeks abstinent and total weeks abstinent from opioids alone for the groups at each employment status (fulltime or not) are also presented in Table 2. Analyses revealed at least one experimental group was better than the standard treatment with regards to both continuous (permutation test: p= 0.003) and total weeks (permutation test: p= 0.012) opioid free. Planned pair-wise comparisons for continuous weeks of abstinence revealed that both the medication and voucher contingency groups were each significantly better than standard treatment (permutation tests: p= 0.023 and p= 0.040, respectively). Similar pair-wise comparisons for total weeks free from opioids alone revealed evidence that the contingent voucher was better than the standard treatment group (permutation test: p= 0.025), but failed to find the medication contingency to be so (permutation test: p= 0.235).
The percentage of weekly urine samples that were opioid-free is shown in Figure 3. There was no evidence of a treatment by number of weeks interaction, nor an effect of the number of weeks (permutation test: p= 0.939 and p= 0.468, respectively). The voucher contingency group had an average of 84% opioid-free urine samples over the 12 weeks of the trial, which was 12 percentage points greater than the average number (72%) of opioid-free urine samples provided by the standard treatment group (permutation test: p=0.010). The medication contingency group provided an average of 81% opioid-free urine samples, which was 9 percentage points higher than that of the standard treatment group, but this difference failed to reach statistical significance (permutation test: p=0.055).
Figure 3.
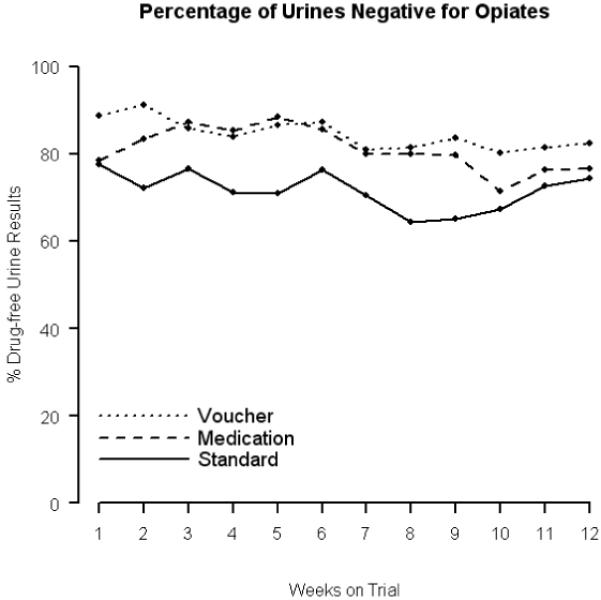
Percentage of weekly urinalysis samples negative for opioids alone in each of the three study groups.
Abstinence from Cocaine
Analyzing the proportions of urinalysis results negative for cocaine (similar to the proportions negative for both opioids and cocaine, or opioid alone) revealed no differences between the groups (permutation test: p = 0.340), no effect of time (permutation test: p = 0.720), and no interaction between groups and time (permutation test: p = 0.174). The medication contingency group had 87.9% cocaine-free urinalysis results, which was 3.5 percentage points higher than the average number (84.4%) of cocaine-free urine samples provided by the standard treatment group (permutation test: p=0.730). The voucher contingency group had provided an average of 83.3% urinalysis results negative for cocaine, which was 1.1 percentage points lower than the standard-care group, and these differences were not significant either (permutation test: p = 0.435). The percentage off weekly urinalysis results negative for cocaine alone in each of the three intervention groups is presented in Figure 4.
Figure 4.
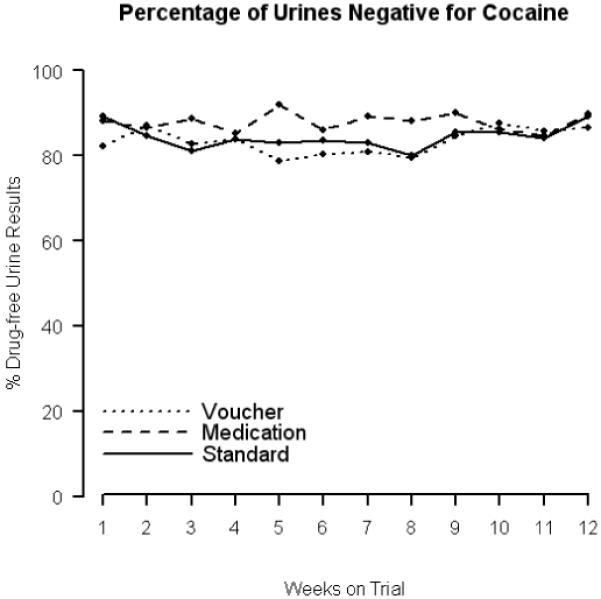
Percentage of weekly urinalysis samples negative for cocaine alone in each of the three study groups.
ASI Composite Scores
Significant improvements from intake (baseline) scores at 6 and 12 weeks were found on the employment, legal issues, psychological issues, opioid and drug abuse subscales (6 weeks: all p < 0.042; 12 weeks: all p < .012), but no changes from baseline were observed on either weeks 6 or 12 of the trial for the cocaine, alcohol or medication subscales (6 weeks: all p > 0.09; 12 weeks: all p > 0.30). An examination of the differential change in ASI subscale scores from baseline to the end of the experimental phase revealed no significant difference among the treatment groups for any of the nine ASI subscales (permutation test: all p>0.10). Scores on the family-social subscale of the ASI showed a complex group-by-assessment point interaction (p = 0.044), such that the medication contingency group showed marginal evidence of improvement at both 6 and 12 weeks (p=0.050 and p=0.056, respectively), the voucher incentive group did not show any significant change at either Week 6 or Week 12 (p=0.768 and p=0.196, respectively), and the standard treatment showed improvement at 6 weeks (p=0.004), but the improvement was not sustained at Week 12 of the trial (p=0.474).
DISCUSSION
This study examined two contingency-based interventions for their ability to improve treatment outcomes of buprenorphine-maintained opioid abusers (some of whom also abused cocaine) over those abusers treated with standard care. To our knowledge, this study is the first to simultaneously compare buprenorphine medication and voucher-based contingencies with standard care using incentive magnitudes with previously demonstrated efficacy. The primary finding was that both voucher reinforcement and the medication-based intervention improved outcomes compared to standard-care. This demonstration of efficacy for the voucher intervention replicates other findings in the research literature (Dutra et al., 2008; Prendergast et al., 2006; Stitzer & Petry, 2006) and is not surprising given the magnitude of the vouchers employed in this study. The intervention provides a positive control demonstrating that the study participants were sensitive to a contingent reinforcement intervention.
Perhaps more important is the new data that this study provides about the efficacy and limitations of a medication-based contingency in which treatment patients were required to increase their clinic attendance (from 3- to 5-days per week) and receive only a partial dose of buprenorphine for a brief period of time when there was evidence of recent opioid and/or cocaine abuse. Consistent with previous observations (Gross et al., 2006; Stitzer, Bickel, Bigelow, & Liebson, 1986), this intervention showed efficacy, particularly on measures of opioid abstinence (Fig. 3). Unfortunately, the medication-based intervention was also associated with higher rates of study attrition compared to the other interventions, especially voucher incentives (Fig. 1). This greater than usual attrition rate is consistent with findings from other trials using punishment procedures, like decreasing medication doses or withholding medications (Leal & Galanter, 1995; Stitzer et al., 1986). It represents a clinical limitation of the procedure as implemented since one consistent finding of substance-abuse research is that those retained in treatment, as a group, fare better than those who drop out of treatment.
One possible reason for increased attrition is a possible adverse impact of the dose-reduction contingency on participants’ ability to resume opiate abstinence in order to have the contingency withdrawn. To explore this possibility, we examined urinalysis data from the 17 medication contingency participants who did not complete the trial. Four of the 17 participants terminated the trial without ever experiencing a dose-reduction. Of the remaining 13 participants, 4 had poor treatment performance; they never provided even one drug-negative urinalysis specimen and tended to drop out early. The majority of remaining participants who experienced a dose reduction (6 of 9) returned to abstinence and to their full buprenorphine maintenance dose after experiencing the dose-reduction contingency, whereas three dropped out of the trial before returning to their full maintenance dose. These data, while they may call into question the acceptability to participants of the dose reduction contingency, suggest that the procedure is not necessarily detrimental to resumption of abstinence since several participants could return to abstinence after experiencing the contingent dose reduction.
The proportions retained in both the current and previous buprenorphine-contingency studies are very similar to the retention rates observed in the studies by Iguchi, Stitzer, Bigelow, & Liebson, (1988) and Nolimal and Crowley, (1990), where methadone contingencies were used. Greater attrition is a limitation of potentially effective medication-based interventions that needs to be addressed through further research and development. An innovative example has been described by Brooner and colleagues, (2007) who examined a Motivated-Stepped Care approach, which used a strategy of adaptive treatment, using principles of both negative reinforcement and avoidance, to motivate participants’ adherence to both abstinence and attendance to varying levels of counseling services upon demonstration of a slip in treatment.
At this time, it is not possible to ascertain which part of the two-part medication contingency intervention, i.e. whether receiving half the scheduled buprenorphine dose or the temporary increase in the frequency of clinic attendance, might be associated with a higher attrition rate and which sub-part possibly resulted in improved abstinence. In contrast, it is very likely that the relatively high magnitude vouchers program resulted in both better retention and less drug use compared to the standard treatment program, as has been shown in other studies of high magnitude voucher incentives for substance-abuse disorders (Lussier et al., 2006). It is encouraging to observe the beneficial effects of both medication and voucher contingencies when combined opioid and cocaine use were targeted. Some previous studies targeting concurrent abstinence from multiple drugs have shown very poor results with participants often unable to provide even a single drug-free urine specimen (Downey, Helmus, & Schuster, 2000; Stitzer et al., 2007). However, other studies have been successful using a combined opioid and cocaine abstinence target.
One hesitation in the wide-spread adoption of CM techniques is the perception that voucher incentives are costly (Kirby, Benishek, Dugosh, & Kerwin, 2006; McGovern, Fox, Xie, & Drake, 2004). Our findings of medication and voucher contingency groups performing similarly are important as they might facilitate the general adoption of contingency management practices depending on the technique that the clinic practitioners are comfortable with. It is also important to acknowledge that cocaine use at baseline in study subjects was low, which reduced the sensitivity of the study to show effects of either CM intervention in promoting abstinence from cocaine. Hence, no significant differences were observed for cocaine use.
The study was designed to detect a mean difference of three weeks of continuous abstinence among any pair of the three intervention groups (each with 85 participants) with a power of at least 0.80 and a Type-I error rate of 0.05 using parametric tests. However, this sample size was not attained. Further, the non-normal nature of the data called for nonparametric methods. Thus, inadequate power may be one reason for inconsistent findings across measures of drug abstinence when intervention groups were compared to the standard care group (Table 2). A surprising observation in our data was that those employed fulltime experienced poorer outcomes than those without fulltime employment. Employment has usually been associated with better outcomes, as validated by a recent study showing improvements in post-treatment employment was an independent predictor of improved ‘opiate free success’ following naltrexone implantation for opiate dependence (Reece, 2007). Additional analyses revealed that those with fulltime employment had a greater number of missed appointments compared to those who were not. Since missed clinic appointments were considered equivalent to providing a drug-positive urine specimen, it is likely that a greater proportion of missed appointments is the likely explanation for an association between fulltime employment and poorer outcomes.
There are several limitations to this study. First, the relatively small sample size may have precluded detection of significant between group differences. Similarly, relatively low overall rates of drug use may have reduced sensitivity for detecting effects of contingencies. Second, the standard treatment condition received only once-a-week methadone-style counseling and did not receive any computer-delivered CRA. Effects of the two contingencies could have been more clearly differentiated if all groups had received the CRA component of therapy. Third, successful implementation of medication contingencies depends upon frequent urinalysis, performed up to three-times-a-week. This might be a higher frequency than that which community treatment programs are willing or able to employ. Next, the study did not collect any data regarding the study participants’ or staffs’ perceptions of the two contingency interventions. The information might have provided additional insights into reasons for the higher drop-out rate experienced by the medication contingency intervention. Finally, the trial was carried out at two centers, and it is unclear whether certain unexplored differences in patient characteristics between the two sites influenced outcome in some unknown way.
In conclusion, while contingency management techniques have been shown to promote abstinence in substance abuse treatment paradigms, implementation of these techniques can be facilitated if they are readily available and easy to implement. The current study demonstrates that both medication and voucher reinforcement contingencies may be useful for improving during-treatment outcomes in buprenorphine maintained opioid abusers, with the caveat being that medication contingencies, as used in this study, resulted in a higher drop-out from treatment than that observed from voucher-based reinforcement. Additional research may lead to methods that could reduce this unwanted attrition while still retaining the benefits of contingent medication–based contingencies.
Acknowledgements
The study was sponsored by grant 7R01 DA012997-06 from the National Institute on Drug Abuse (NIDA), Bethesda, MD. The results from this study were presented, in part, at the 70th Annual meeting of the College on Problems of Drug Dependence (CPDD), in June 2007, San Juan, Puerto Rico. Mohit P Chopra, MD was employed by the UAMS at the time of the study. Drs. Bickel and Marsch, as well as Dr. Marsch’s husband, have an affiliation with the company that developed and owns the computerized Computerized Reinforcement Approach (CRA) intervention that was used in this trial.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
Contributor Information
Mohit P. Chopra, VA Boston Healthcare System. He was employed by the University of Arkansas for Medical Sciences at the time of the study
August R Buchhalter, Pinney Associates.
Maxine L. Stitzer, Johns Hopkins University School of Medicine
Lisa A. Marsch, National Development and Research Institutes
Warren K. Bickel, University of Arkansas for Medical Sciences
References
- Aickin M. A program for balancing the allocation of subjects to treatment in a clinical trial. Computers and Biomedical Research. 1982;15:519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Amass L, Bickel WK, Crean JP, Blake J, Higgins ST. Alternate-day buprenorphine dosing is preferred to daily dosing by opioid-dependent humans. Psychopharmacology. 1998;136:217–225. doi: 10.1007/s002130050559. [DOI] [PubMed] [Google Scholar]
- Amass L, Bickel WK, Higgins ST, Badger GJ. Alternate-day dosing during buprenorphine treatment of opioid dependence. Life Sciences. 1994;54:1215–1228. doi: 10.1016/0024-3205(94)00848-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical manual of Mental Disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch R. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting and Clinical Psychology. 1997;65:803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Experimental and Clinical Psychopharmacology. 2008;16:132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS, King VL, Stoller KB, Neufeld KJ, Kolodner K. Comparing adaptive stepped care and monetary-based voucher interventions for opioid dependence. Drug and Alcohol Dependence. 2007;88(Suppl 2):S14–23. doi: 10.1016/j.drugalcdep.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. U.S. Department of Health and Human Services, National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. American Journal of Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Silverman K, Stitzer ML. Use of methadone take-home contingencies with persistent opiate and cocaine abusers. Journal of Substance Abuse Treatment. 1999;16:23–30. doi: 10.1016/s0740-5472(97)00318-8. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Experimental and Clinical Psychopharmacology. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Good P. Permutation, Parametric and Bootstrap Tests of Hypotheses. 3rd ed. Springer Science+Business Media, Inc.; New York: 2005. [Google Scholar]
- Gross A, Marsch LA, Badger GJ, Bickel WK. A comparison between low-magnitude voucher and buprenorphine medication contingencies in promoting abstinence from opioids and cocaine. Experimental and Clinical Psychopharmacology. 2006;14:148–156. doi: 10.1037/1064-1297.14.2.148. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney D, Budney AJ, Bickel WK, Hughes JR, Feorg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hunt D, Spunt B, Lipton D, Goldsmith D, Strug D. The costly bonus: cocaine related crime among methadone treatment clients. Advances in Alcohol and Substance Abuse. 1986;6:107–122. doi: 10.1300/J251v06n02_08. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Stitzer ML, Bigelow GE, Liebson IA. Contingent methadone delivery: Effects on illicit-opiate use. Drug and Alcohol Dependence. 1988;17:311–322. doi: 10.1016/0376-8716(86)90080-3. [DOI] [PubMed] [Google Scholar]
- Leal J, Galanter M. The use of contingency contracting to improve outcome in methadone maintenance. Substance Abuse. 1995;16:155–165. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug and Alcohol Dependence. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kolar AF, Brown BS, Weddington WW, Ball JC. A treatment crisis: cocaine use by clients in methadone maintenance programs. Journal of Substance Abuse Treatment. 1990;7:101–107. doi: 10.1016/0740-5472(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Antecedents and consequences of cocaine abuse among opioid addicts: a 2.5-year follow-up. Journal of Nervous and Mental Disease. 1988;176:176–181. doi: 10.1097/00005053-198803000-00006. [DOI] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93:51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: dissemination research in an addiction treatment system. Journal of Substance Abuse Treatment. 2004;26:305–312. doi: 10.1016/j.jsat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr H, O’Brien C. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Diseases. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Nolimal D, Crowley TJ. Difficulties in a clinical application of methadone-dose contingency contracting. Journal of Substance Abuse Treatment. 1990;7:219–224. doi: 10.1016/0740-5472(90)90044-q. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for the treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Reece AS. Psychosocial and treatment correlates of opiate free success in a clinical review of a naltrexone implant program. Subst Abuse Treat Prev Policy. 2007;2:35–50. doi: 10.1186/1747-597X-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen HG, Boulogne JJ, van Tulder MW, van den Brink W, De Jong CA, Kerkhof AJ. A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug and Alcohol Dependence. 2004;74:1–13. doi: 10.1016/j.drugalcdep.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, Department of Health and Human Services; Rockville, MD: 2008. Also online at: http://oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.pdf. [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. Journal of Consulting and Clinical Psychology. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bickel WK, Bigelow GE, Liebson IA. Effects of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug and Alcohol Dependence. 1986;18:341–348. doi: 10.1016/0376-8716(86)90097-9. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Cohen A, Blaine J, Vandrey R, Kolodner K, Li R. Abstinence-based incentives in methadone maintenance: interaction with intake stimulant test results. Experimental and Clinical Psychopharmacology. 2007;15:344–350. doi: 10.1037/1064-1297.15.4.344. [DOI] [PubMed] [Google Scholar]
- Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug and Alcohol Dependence. 1998;52:183–19. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]


