Abstract
A method for testing changes in pain sensitivity of human subjects over the course of prolonged thermal stimulation is introduced. It uses a Peltier-device-based thermode to generate a thermal contact stimulus, an electronic visual analog scale to continuously record the pain intensity and a system that controls selected stimulus parameters (temperature or pulse timing) as a function of the pain intensity rating. The stimulus parameter that is modulated to clamp pain intensity near a desired setpoint serves as the response variable and is used to infer pain sensitivity. Advantages of the method are that it automatically finds the stimulus magnitude that elicits predetermined pain intensity, regardless of how sensitive or insensitive the subject is, and it allows prolonged stimulation, because it does not allow pain intensity to escalate to unacceptable levels due to progressive sensitization. The subject is blinded regarding experimental effects because average pain intensity remains constant regardless of sensitization or pharmacological interventions.
Keywords: Psychophysics, Pain, Stimulus response, Experimental pain, human
Introduction
Chronic clinical pain presents unique diagnostic challenges. Often there is no biologically detectable generator of the ongoing pain that is described by a patient. There may be a history suggesting that a generator exists, such as a prior injury, but critical features of the injury cannot be distinguished from similar injuries of others who are relatively pain free. Input-output relationships between receptor activation by some unknown process at the injury site and ratings by the patient of chronic ongoing pain cannot be determined. This suggests that the pain processing system is best probed with clearly defined experimental stimuli. Experimental pain models using brief phasic stimuli (electrical, mechanical or thermal) have been used extensively to characterize the human pain system, especially to determine the properties of afferents (Hardy, Wolff et al., 1952; Hardy, Goodell et al., 1952; Chen, Niddam et al., 2001; Gracely, Grant et al., 2003; Donaldson, Chapman et al., 2003). Likewise, the measurement of pain thresholds has been popular with investigators and led to many important insights (Giesecke, Williams et al., 2003). Unfortunately, these methods may not adequately model clinical pain conditions where the pain experience remains at suprathreshold levels for prolonged periods of time. Maintaining suprathreshold pain intensities for more than a few seconds is important for studies that are interested in how pain modulation is affected by the continued presence of a nociceptive input (e.g., sensitization and adaptation experiments). Paradigms using series of pulsed thermal stimuli (Price, Hu et al., 1977;Vierck, Jr., Cannon et al., 1997) or administration of chemical irritants (Sang, Gracely et al., 1996) have contributed to our understanding of central sensitization (e.g., wind-up). Most of these studies measured the pain intensity ratings elicited by a predetermined stimulus magnitude. The drawback of this approach is that choosing the stimulus magnitude may be difficult when the subject populations include individuals with vastly different pain sensitivities. Furthermore, the experiments may have to be terminated early when the pain intensity escalates to intolerable levels during the course of the stimulus exposure. This problem has been overcome by the introduction of a method where the administration of a pain-inducing substance was continuously adjusted depending on the pain intensity (Sternberg, Bailin et al., 1998;Byas-Smith, Bennett et al., 1999;Niddam, Graven-Nielsen et al., 2001). Unfortunately, chemical stimuli do not allow rapid and tight control over the nociceptive input they generate: the response to changes in the stimulus level is sluggish. The new rating-dependent thermal stimulation method that is the subject of this article largely overcomes this drawback and therefore represents a new addition to the repertoire of human experimental pain models. Furthermore, the new method helps to distinguish between exaggerated sensory rating due to abnormal pain processing and exaggerated ratings due to changes in rating bias. This abnormal pain processing vs. abnormal rating bias dilemma has led to ambiguity as to whether certain chronic pain conditions should be attributed to psychological or neurobiological causes or both (Mayer E, 2009).
Ideally, techniques of psychophysical evaluation should be able to reveal whether there are abnormalities of pain processing other than an exaggerated interpretation of pain intensity. Decisions concerning therapeutic approaches could differ for a patient with a genetic or psychological predisposition toward high sensitivity and a patient with other features of pain sensitivity suggestive of aberrant mechanisms of pain coding. In order to provide this analytical power, psychophysical procedures are needed which provide a great deal of parametric flexibility with automation. The subject should be blind as to which stimulus parameters are changed within a test, and automatic variation of stimulus features removes experimenter bias from the test. Traditionally, predetermined intensities and sequences of short duration stimuli are presented at long interstimulus intervals (ISIs) to avoid interaction, and they are rated individually (Arendt-Nielsen and Yarnitsky, 2009). This offers predictability for the subject, and such stimuli are not designed to reveal interactions between excitatory and inhibitory central processing which might be disrupted by the variety of neuronal injuries which can result in chronic pain.
Psychophysical testing sessions for patients who experience ongoing pain must avoid presentation of intolerable intensities of stimulation. This is difficult with paradigms that predetermine the stimulus parameters. A fixed set of stimulus parameters is not likely to evoke a range of minimal to moderate or strong intensities of pain (an appropriate stimulus-response function) for normal subjects and patients with altered pain processing. A range of intensities that is appropriate for some subjects can be intolerable or not painful for others. Also, sensitivity can change with repeated presentation of painful stimulation (Vierck, Jr., Cannon et al., 1997;Wong F, 2010). This is an instructive source of variability that is typically unrecognized by fixed series of stimuli.
The psychophysical procedures available with the equipment described here incorporate features that overcome limitations inherent to most previous methods of pain sensitivity testing. The equipment can present fixed series of single stimuli (the independent variable), to describe pain thresholds and stimulus-response functions generated by traditional methods in which pain ratings are the dependent variable. For the paradigms described here, these relationships are reversed, and some stimulus parameter is utilized as the dependent variable. For example, stimulus intensity can be varied to maintain a desired setpoint of pain ratings that are generated continuously by electronic visual analog scaling (eVAS). Maintenance of a tolerable setpoint by variation of intensity or another stimulus parameter permits long trials of continuous or intermittent stimulation that can reveal the normality of central inhibitory and excitatory influences. Different setpoints can be utilized to establish a stimulus-response function for long duration stimuli that more closely mimic clinical pain than individual short stimuli. The subject is not aware of the setpoint value or parametric variations to maintain it, which provides important checks on the reliability of the subject’s ratings. Simple exaggeration of pain intensity across the board will not interfere with detection of central excitatory and inhibitory influences that are manipulated by parametric variation.
System Components
Background
The stimulator system described here (Figure 1) was designed and built as a prototype by one of the authors (APM). A key feature of the design is that the hardware components (stimulator and electronic rating scale) are integrated and linked by software. This allows addition of capabilities over time without modification of the hardware. Initially, the equipment was designed for traditional thermal pain sensitivity testing where the pain intensity rating is the dependent variable. Over time, the control software was expanded to enable the novel test protocols described below.
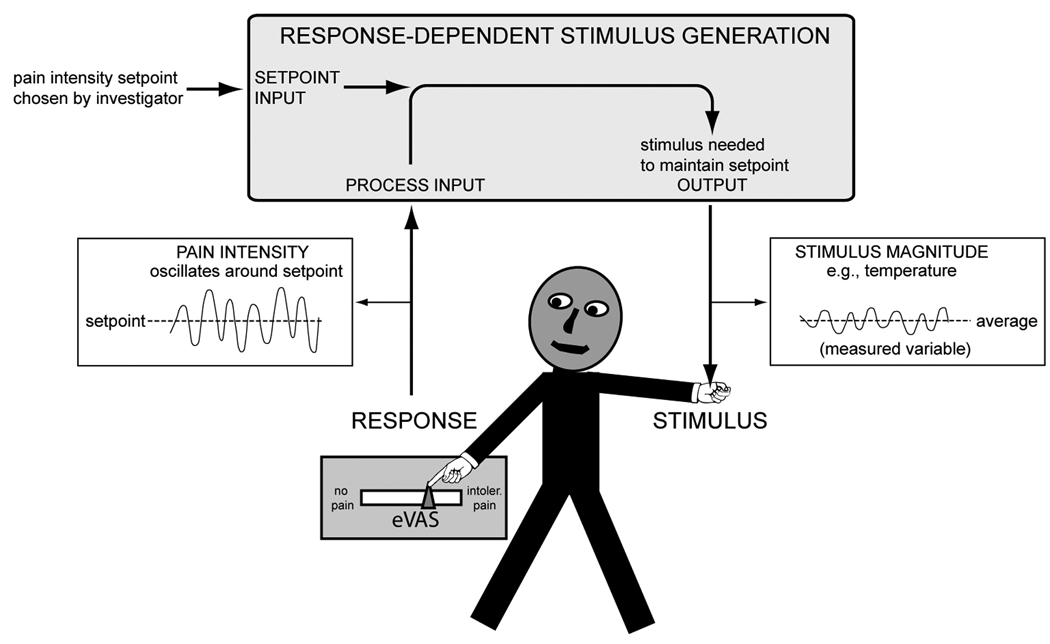
Figure 1.
A. A functional prototype of the thermal stimulation and response processing system is shown (TSAR). B. The system components and the subject are linked to form a proportional control loop. The computer software adjusts a stimulus parameter (temperature, ISI, or PD) so the pain intensity rating remains near the setpoint).
Electronic visual analog scale (eVAS)
The subject rates pain intensity by adjusting a linear low friction potentiometer of 100mm travel. The voltage generated by the potentiometer is linearly related to the position of the slider. The left end point of the slider is defined as “no pain”, the right end point as “intolerably intense pain”. The scale has no intermediate anchor points. This electronic version of the standard method for magnitude estimation of pain intensity (Price, Bush et al., 1994) permits continuous rating, in contrast to mechanical devices or verbal reporting.
Contact Thermode
The thermode consists of a flat copper surface of 23×23 mm. It is heated or cooled by a Thermo Electric Cooler (Peltier Device). A thermistor, located in the center of the thermode, 0.2mm below the surface, transforms the thermode temperature into an electrical signal with a time constant of < 1 sec and an accuracy of +/− 0.1 deg C. The processed temperature signal of the thermistor is routed to the data-acquisition system of the computer that controls the stimulator.
Thermode Motion System
At rest, the thermode is recessed behind a cutout in a thermally neutral plastic surface, out of contact with the skin, which rests on the plastic surface. The thermode is brought into skin contact by a solenoid-powered mechanism. The endpoint of the protrusion of the thermode, and therefore the contact pressure between thermode and skin, is adjustable.
Adjustable Support Arm
The thermode assembly is mounted on a stable support arm which—similar to the arm of a dental x-ray machine—can be adjusted and locked into position, allowing the stimulus to be directed at a desired anatomical location -- e.g., the cheek, arm, hand or leg.
Stimulus Timing
Stimuli can be timed by activating the solenoid mechanism, bringing the preheated (or cooled) thermode in and out of skin contact, or by ramping the thermode temperature up and down while maintaining uninterrupted skin contact.
Parameters Determining the Magnitude of Pain
Thermode temperature, contact duration and the interval between thermode contacts all have an effect on pain intensity. Any of these parameters can be selected as the dependent variable during a testing session.
Methods of Parametric Variation
The device can be programmed to generate pulsed thermal stimuli by bringing the thermode into and out of skin contact with the solenoid mechanism. Alternatively, the thermode can maintain skin contact throughout a session, and thermal pulses can be generated by ramping up and down the thermode temperature. In both cases, pulse duration, ISI and thermode temperature are the parameters that can be varied, either according to a predetermined schedule or automatically as a function of pain intensity ratings. Both pulse duration and ISI have an effect on temporal integration of the nociceptive signal. Nociception-induced sensitization occurs while the stimulus is present -- i.e., it depends on the duration of the pulses. Decay of sensitization progresses unopposed as long as no stimulus is present -- i.e., during an ISI. Overall temporal integration is the net effect of sensitization and decay. It is conceivable that certain interventions (e.g., drugs) affect sensitization and decay differently. The relative effect of manipulating pulse duration and ISI may depend on the type of intervention. While ISI can be varied over a wide range, this is not the case for pulse duration: Pain intensity ratings become progressively more difficult and unreliable as pulse duration shortens. In our experience, when compared with healthy subjects, chronic pain patients (eg, fibromyalgia patients) appear to require longer stimulus pulses for producing reliable ratings. The use of prolonged stimulation may level the playing field and reduce the variability of pain sensitivity data.
The Principle of Pain Intensity Clamping
The eVAS and contact thermode serve as man-machine interfaces, linking subject and stimulator in a closed proportional control loop. The stimulus parameter that changes during a session is the dependent variable that is used to make inferences regarding the functional state of pain transmission and modulation systems. The stimulator software uses deviations of the subject’s pain intensity rating from a predetermined setpoint and the derivative of these deviations as the basis for automatic adjustments of a stimulus parameter. The mechanism of parameter control during a clamping session depends on the mode of stimulation. For pulsed thermode contacts, the investigator chooses whether pulse duration, ISI or temperature is automatically adjusted to maintain (clamp) pain intensity at a predetermined setpoint. For continuous thermode contact, the system automatically adjusts the thermode temperature as necessary to keep the rating on the eVAS near the predetermined setpoint.
The system consists of 3 components: (a) a transducer that records the subject’s pain intensity rating (eVAS); (b) a thermal contact stimulator; and (c) a computer with software that controls the timing and magnitude of stimulation and assures that it remains within safe limits (Figure 1).
Pain Intensity Clamping Sequence
Predetermined pain intensity levels have been maintained by titrating the infusion of chemical irritants according to the subjects’ pain intensity rating (Zubieta and Stohler, 2009). However, pain intensity clamping by real-time proportional control of a thermal stimulus by electronically sampled pain intensity ratings is a new addition to the repertoire of pain sensitivity measurement methods.
A pain intensity clamping sequence begins with an induction phase and continues with a maintenance phase (see Figure 2).
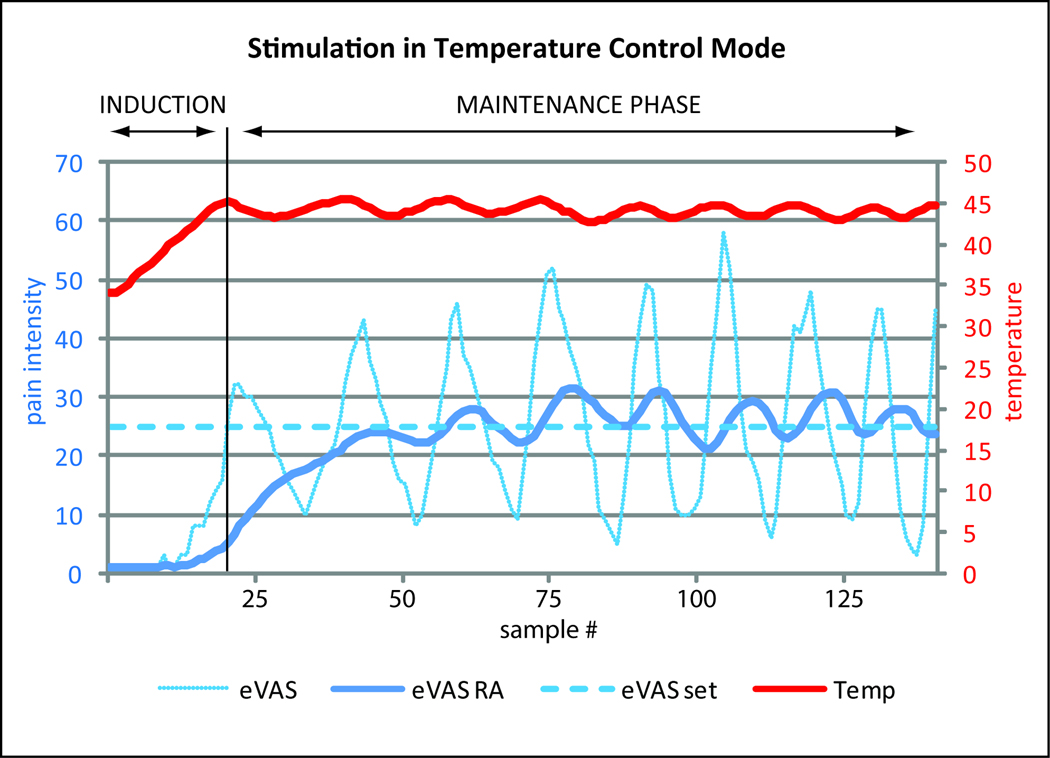
Figure 2.
Example of a pain intensity clamping experiment with continuous stimulation and temperature variations during a test session with a healthy male subject. The position of the eVAS slider was sampled at 1.5 sec intervals. The thermode temperature was adjusted after each sample as a function of eVAS deviation from the pain intensity setpoint. During the induction phase, the thermode temperature gradually increased (in steps of 0.1–0.3 °C) until the eVAS rating reached the setpoint (dotted line). Subsequently it oscillated around the setpoint (eVASset). The thick line in the lower half of the graph (eVAS RA) represents the running average of the last 20 samples of the pain rating. The stimulus temperature (Temp) oscillated around the mean in a corresponding fashion.
Induction Phase
The temperature needed to keep pain intensity near the setpoint under given circumstances varies from individual to individual and between disease- and control groups. The thermode temperature is at a non-nociceptive level at the beginning of a session. Then the temperature is increased in investigator-defined steps. The temperature steps decrease in size as pain ratings approach the setpoint. A key characteristic of the induction phase is that the magnitude of the stimulus progressively increases or –when pain intensity increases rapidly—remains the same, but it never decreases. Once pain intensity reaches the setpoint the maintenance phase begins.
Maintenance Phase
A stimulus parameter, e.g. thermode temperature, is adjusted periodically as a function of the pain intensity rating. In continuous stimulation mode the pain intensity signal from the eVAS is sampled and the thermode temperature adjusted at time intervals chosen by the investigator. In pulsed stimulation mode pain intensity is sampled and thermode temperature is adjusted after each pulse. When pain intensity is below setpoint the stimulus temperature or pulse duration is stepped up, or the ISI is stepped down. Conversely, when the pain exceeds the setpoint the temperature or pulse duration (PD) is stepped down or the ISI is stepped up. The size of the step increments is: (a) proportional to the deviation from setpoint, (b) a function of the rate of change of pain intensity and (c) dependent on whether the ratings are increasing, decreasing or not changing. A temperature limit can be set to prevent thermal injury of subjects that are pain insensitive.
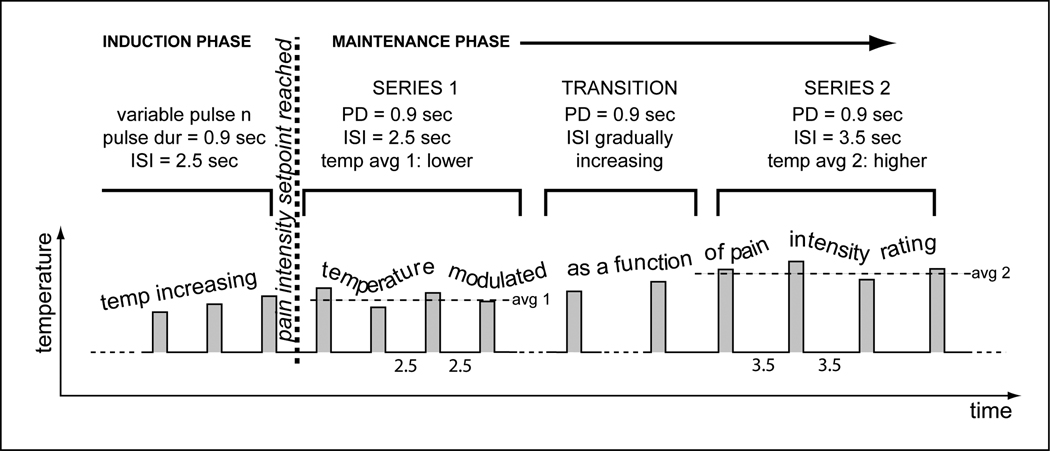
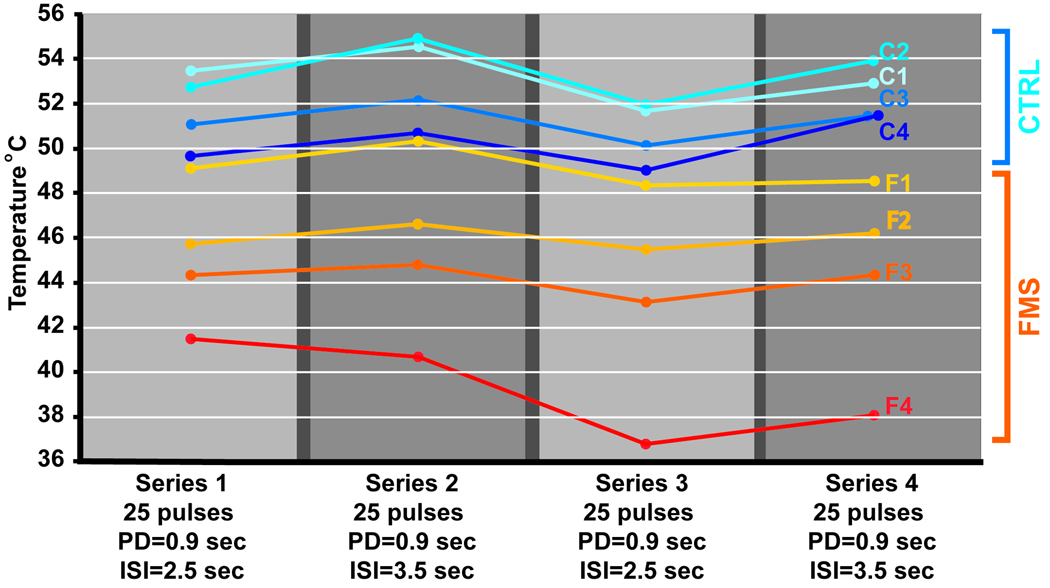
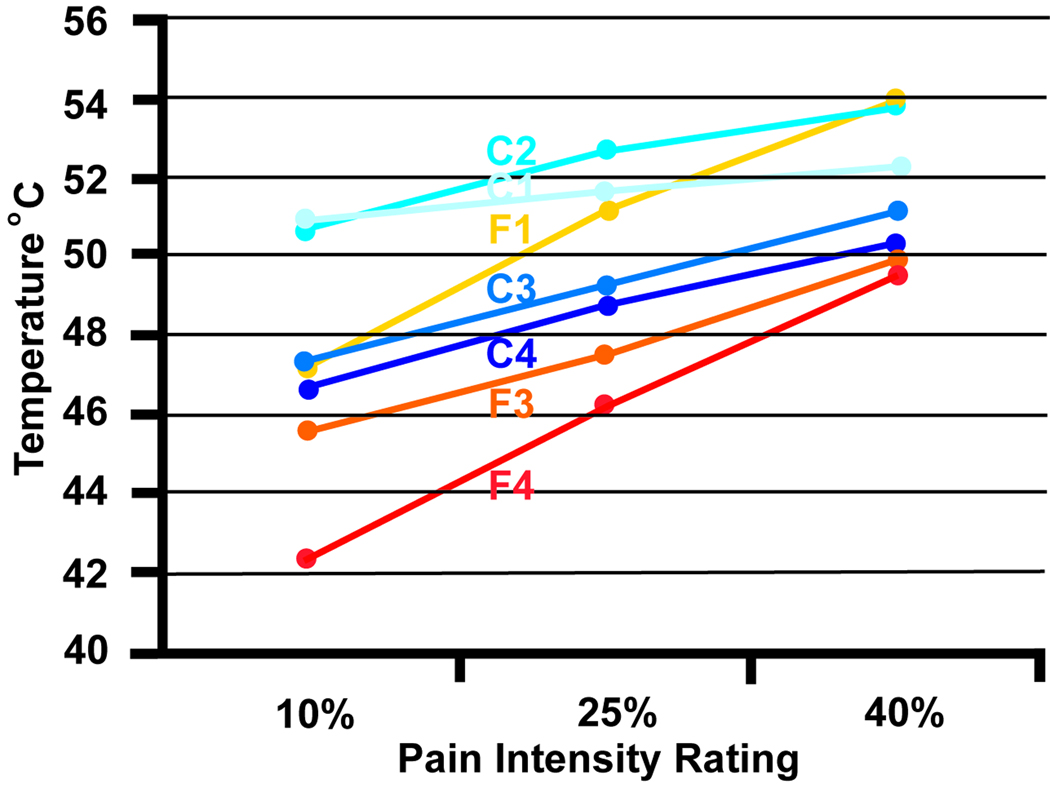
Figure 3 presents data from 4 female healthy controls (age range 22–42 years, mean age 30 years) and 4 female fibromyalgia patients (age range 38–60 years, mean age 51 years). The disease onset of the FMS subjects was between 10 and 17 years ago. Two of the patients reported that the onset coincided with a car accident; in the remaining subjects the onset was unrelated to trauma. One FMS patient (F1) had no pain on the day of testing. The average intensity of spontaneous (clinical) pain of the remaining 3 FMS subjects was 39% in the upper part of the body (arms, chest, head) and 33% in the lower part of the body prior to the start of the testing session. These subjects underwent an experiment that consisted of 4 consecutive series of 25 thermal pulses each. The temperature was modulated to maintain average pain intensity (setpoint of 35%). Overall, the average temperature required to maintain 35% pain intensity was lower for FMS patients than for controls. The same subjects were tested with a conventional protocol that acquired stimulus intensity response curves with short widely-spaced thermal stimuli (Figure 4). Both the conventional and the pain clamping method revealed greater sensitivity for the FMS subjects. However, one FMS subject (F2) produced useable data when tested with prolonged series of stimuli (Figure 3) but was unable to reliably rate the brief individual stimuli of the conventional protocol.
Figure 3.
(A). Pain intensity clamping protocol with pulsed stimulation. Pulse duration (CNT) was the same for all 4 series. ISI was switched between 2.5 and 3.5 sec. from series to series. The thermode temperature was adjusted after each stimulus pulse as a function of the eVAS position to maintain a 35% average pain intensity. (B).The protocol shown in Fig. 3A was used to collect data on 4 female patients with fibromyalgia syndrome and 4 healthy female control subjects. The dots represent the temperature means required for maintaining the 35% pain intensity setpoint for each respective series. The temperatures were lower for the FMS subjects (F1–F4) than for the controls (C1–C4), i.e., the FMS subjects had higher thermal pain sensitivity. The change in ISI required a pronounced compensatory temperature change in the control subjects. For FMS patients the temperature change was small or exhibited a continuous downward trend across all 4 series (progressive sensitization as a result of prolonged stimulation). These data demonstrates that the method is able to discriminate between different pain sensitivities.
Figure 4.
The stimulus intensity-response relationships were acquired with conventional methodology for the same subjects as in figure 4. A series of brief (3 sec) widely spaced (ISI=30sec) thermal pulses of stepwise increasing temperature was administered to the palmar surface of the hand. The sensitivity ranking order obtained with this method was the same as the order obtained with the pain intensity clamping protocol. One subject (F2) was unable to reliably rate the 3 sec thermal pulses and is not represented in this graph.
Oscillations
During a prolonged sequence of response-dependent stimulation, pain intensity is not stable; it oscillates around the setpoint as illustrated in figure 2. The modulated stimulus parameter, e.g., the thermode temperature, exhibits an oscillation of identical wavelength but of opposing phase angle. In autopilots of aircraft and in other control systems oscillations are undesirable and the control algorithms are carefully tuned to obtain optimal damping of the wave. The oscillations observed in pain intensity clamping experiments thus far have resisted damping by tuning the control parameters and training the subjects in the eVAS rating task. The primary determinant of this behavior appears to be modulation by systems that respond to changes in the threat level (stimulus intensity trend detectors). “Offset analgesia” (Grill and Coghill, 2002), which renders subjects relatively insensitive when stimulus intensity is trending down, illustrates this point. During descending series the pain system recognizes that the driving force for protective behavior (pain) can be scaled back because the stimulus (threat level) is receding. When pain intensity ratings fall below the setpoint the stimulus magnitude increases, but the subject is slow to perceive this increase but eventually overshoots the setpoint. Upward-trend detectors accelerate the increase in pain intensity. Oscillations are pronounced during continuous stimulation and are less so during pulsed stimulation series, because the intervals between pulses allow partial recovery from trend effects. Oscillations blind the subject regarding the setpoint and may be sensitive to the presence of chronic pain, which often waxes and wanes in intensity. Future investigations are needed to characterize oscillations as indicators of central nociceptive trend detectors.
Reliability Test of Pain Rating
The new method, unlike most traditional pain tests, provides the opportunity to monitor the subject’s rating reliability. In our experience, the average pain intensity rating across 20 samples is within < 4% of the setpoint.
Interactions between Stimulus Parameters
High temperatures, short ISIs and long pulse durations all lead to a high pain intensity. It may be of interest for the investigator to measure the relative contribution of these parameters to pain intensity. For instance, exposure to nociceptive stimulation can lead to pronounced and longer lasting sensitization in subjects with fibromyalgia sy ndrome when compared to healthy controls (Staud, Price et al., 2004;Wong F, 2010). The decay characteristics of sensitization induced by stimulus pulses can be studied by changing ISI in the middle of a pulse series while measuring the automatic temperature adjustment that is required to maintain pain intensity at the setpoint. For instance, for subjects with slowly decaying sensitization, lengthening ISI will result in a smaller temperature increase than in individuals where sensitivity normalizes rapidly after each stimulus.
Transition Phase
In experiments where temperature is the dependent variable and a change in ISI or pulse duration is introduced, it is advisable to introduce a transition period to make the change less noticeable and prevent sudden large swings in pain intensity. For instance, if ISI is to be switched from 2.5 to 3.5 sec, it is useful to distribute the change over 4 pulses: ISI increases by 0.25 sec from one pulse to the next until it reaches the new setting of 3.5 sec.
In experiments where it is deemed important to describe a stimulus-response function, the setpoint can be changed during a testing session or between sessions. For example, setpoints of 20 and 50 would dictate average temperatures associated with mild and moderate pain, respectively.
Discussion
The pain intensity clamping method differs from most traditional stimulation methods: (a) It finds the stimulus magnitude that leads to a predetermined pain intensity, regardless of how sensitive or insensitive the subject is. This eliminates the need for time-consuming pilot sessions to find the appropriate level of stimulation for each subject or sub-group. (b) It allows prolonged stimulation, because there is no risk that pain intensity will escalate to intolerable levels due to progressive sensitization. (c) It blinds the subject regarding the physical magnitude of the stimulus and the effects of drugs or other interventions on pain sensitivity.
In addition to the practical considerations listed above, the intent of the response-dependent evaluation of pain sensitivity described here is to increase the sensitivity of psychophysical methodology to mechanisms for chronic pain. The pain intensity oscillations are useful for assessing trend-dependent modulation. Pulsed stimulation with a preheated thermode preferentially excites unmyelinated nociceptors (Vierck, Jr., Cannon et al., 1997) which are selectively inhibited by opioid analgesics (Cooper and Vierck, Jr., 1986). Also, prolonged repetitive stimulation can reveal properties of temporal summation of pain that are abnormal for some chronic pain patients (Staud, Price et al., 2004;Wong F, 2010). Continuous stimulation has similar benefits. The ability to reveal abnormal pain processing with parametric variations that are unknown to the subjects should greatly increase the analytic power of psychophysical testing for different populations of subjects who report chronic pain.
Acknowledgements
Funding: NIH NINDS 1K23NS43435
NIH NIDCR U24 DE016509
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J. Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Byas-Smith MG, Bennett GJ, Gracely RH, Max MB, Robinovitz E, Dubner R. Tourniquet constriction exacerbates hyperalgesia-related pain induced by intradermal capsaicin injection. Anesthesiology. 1999;91:617–625. doi: 10.1097/00000542-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Chen AC, Niddam DM, rendt-Nielsen L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci. Lett. 2001;316:79–82. doi: 10.1016/s0304-3940(01)02374-6. [DOI] [PubMed] [Google Scholar]
- Cooper BY, Vierck CJ., Jr Measurement of pain and morphine hypalgesia in monkeys. Pain. 1986;26:361–392. doi: 10.1016/0304-3959(86)90064-3. [DOI] [PubMed] [Google Scholar]
- Donaldson GW, Chapman CR, Nakamura Y, Bradshaw DH, Jacobson RC, Chapman CN. Pain and the defense response: structural equation modeling reveals a coordinated psychophysiological response to increasing painful stimulation. Pain. 2003;102:97–108. doi: 10.1016/s0304-3959(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best. Pract. Res. Clin. Rheumatol. 2003;17:593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J. Neurophysiol. 2002;87:2205–2208. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- Hardy JD, Goodell H, Wolff HG. Influence of skin temperature upon the pain threshold as evoked by thermal radiation. AMA. Arch. Neurol. Psychiatry. 1952;67:689–690. [PubMed] [Google Scholar]
- Hardy JD, Wolff HG, Goodell H. Studies on pain: measurements of aching pain threshold and discrimination of differences in intensity of aching pain. J. Appl. Physiol. 1952;5:247–255. doi: 10.1152/jappl.1952.5.6.247. [DOI] [PubMed] [Google Scholar]
- Mayer EBM. Functional Pain Syndromes: Presentation and pathophysiology. Seattle: IASP Press; 2009. Functional pain disorders: Time for a paradigm shift? pp. 531–565. [Google Scholar]
- Niddam DM, Graven-Nielsen T, rendt-Nielsen L, Chen AC. Non-painful and painful surface and intramuscular electrical stimulation at the thenar and hypothenar sites: differential cerebral dynamics of early to late latency SEPs. Brain Topogr. 2001;13:283–292. doi: 10.1023/a:1011180713285. [DOI] [PubMed] [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Sang CN, Gracely RH, Max MB, Bennett GJ. Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories. Evidence for a central mechanism. Anesthesiology. 1996;85:491–496. doi: 10.1097/00000542-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Bailin D, Grant M, Gracely RH. Competition alters the perception of noxious stimuli in male and female athletes. Pain. 1998;76:231–238. doi: 10.1016/s0304-3959(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Jr., Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J. Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- Wong F, Rodrigues A, Schmidt S, Vierck C, JR, Mauderli AP. Extreme thermal sensitivity and pain-induced sensitization in a fibromyalgia patient. Pain Research and Treatment (in press) 2010 doi: 10.1155/2010/912513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann. N. Y. Acad. Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]