Abstract
The dorsomedial hypothalamus (DMH) has been implicated in the coordination of stress responses. Restraint stress or systemic corticosterone (CORT) treatment induces a rapid increase in tissue concentrations of serotonin (5-hydroxytryptamine; 5-HT) in the DMH. Although the mechanism for rapid changes in 5-HT concentrations in the DMH is not clear, earlier results suggest that stress-induced increases in CORT may inhibit 5-HT transport from the extracellular fluid by acting on corticosterone-sensitive organic cation transporters (OCTs). We tested the hypothesis that perfusion of the medial hypothalamus (MH), which includes the DMH, with the OCT blocker decynium 22 (D-22) would potentiate the effects of mild restraint on extracellular 5-HT. Male Sprague-Dawley rats, implanted with a microdialysis probe into the MH, were treated with reverse-dialysis of D-22 (20 μM; 40 min) or vehicle and subjected to either 40 min mild restraint or undisturbed control conditions. Perfusates collected from a separate group of rats were evaluated for the effect of restraint on extracellular CORT concentrations in the MH. Reverse dialysis of D-22 induced an increase (200%) in extracellular 5-HT concentrations in the MH in undisturbed control rats. Restraint in the absence of D-22 did not significantly affect MH CORT or 5-HT concentrations. However, perfusion of the MH with D-22 during restraint led to an increased magnitude and duration of extracellular 5-HT concentrations, relative to D-22 by itself. These results are consistent with the hypothesis that OCTs in the DMH contribute to the clearance of 5-HT from the extracellular fluid under both baseline conditions and mild restraint.
Keywords: 5-hydroxytryptamine, DMH, dorsomedial hypothalamus, organic cation transporter, OCT3, restraint stress
1. Introduction
The dorsomedial hypothalamus (DMH) has been proposed to play an important role in the integration of neuroendocrine, autonomic, and behavioral responses to aversive stimuli (reviewed in DiMicco et al, 2002). For example, changes in DMH neuroactivity alter the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis (Bailey et al, 2003;Bailey and DiMicco, 2001;Keim and Shekhar, 1996), partly through projections to the paraventricular nucleus of the hypothalamus (PVN), a critical neural site for activation of the neuroendocrine stress response (Bailey et al, 2003;ter Horst and Luiten, 1986;Zaretskaia et al, 2008).
These effects may be modulated, in part, through stress-induced activation of the serotonergic system (Li et al, 2004). The DMH receives projections from serotonergic cell bodies in the median raphe nucleus (Vertes et al, 1999) and dorsal raphe nucleus (Commons et al, 2003;Vertes, 1991). Acute exposure to aversive stimuli such as restraint stress, immobilization stress or foot shock, or administration of the stress hormone, corticosterone (CORT), increases tissue concentrations of 5-HT (5-hydroxytryptamine, 5-HT) in the DMH (Culman et al, 1980;Losada, 1988;Lowry et al, 2001;Lowry et al, 2003;Shekhar et al, 1994). Microdissection studies measuring 5-HT concentrations in subdivisions of the DMH indicate that stress-induced increases in tissue concentrations of 5-HT appear to be primarily localized in the dorsal hypothalamic area, including parenchymal, subependymal, and ependymal components (Lowry et al, 2003). It is not clear from these studies if the increased tissue concentrations of DMH 5-HT represent changes in synthesis, storage, release, or metabolism of the neurotransmitter. In addition, microdialyis studies have demonstrated that immobilization stress increases extracellular 5-HT in hypothalamic regions including the anterior and lateral hypothalamus (Shimizu et al, 1992;Shimizu et al, 2000;Shintani et al, 1995). However, actual measurements of extracellular 5-HT concentrations in the DMH in response to acute stress have not been documented; therefore, evidence that stress alters 5-HT signaling in this region is lacking.
In addition to stress-induced increases in DMH 5-HT tissue concentrations, exposure to stress or systemic CORT administration results in highly correlated increases in tissue concentrations of other monoamines, including dopamine (DA) and norepinephrine (NE) (Lowry et al, 2001;Lowry et al, 2003). The co-regulation of these neurotransmitters in the DMH in response to stress or CORT may be related to the presence of a class of polyspecific monoamine transporters, collectively known as organic cation transporters (OCTs), that have been recently identified in the central nervous system (CNS; Amphoux et al, 2006;Baganz et al, 2008;Daws, 2009;Gasser et al, 2006;Gasser et al, 2009;Inazu et al, 2003;Schmitt et al, 2003;Taubert et al, 2007;Vialou et al, 2004;Wu et al, 1998). Originally described in the periphery (for review, see Eisenhofer, 2001;Grundemann et al, 1998;Koepsell et al, 2003), OCTs are bidirectional, low-affinity, high-capacity, carrier-type permeases that function in the clearance of diverse organic cations, including 5-HT, DA, NE, and histamine (Busch et al, 1996;Grundemann et al, 1998). Three subtypes of OCTs have been identified, OCT1, OCT2, and OCT3. All three of these OCT subtypes have been found in the CNS (Gasser et al, 2006;Gasser et al, 2009;Taubert et al, 2007) although the expression of OCT1 mRNA is low (Amphoux et al., 2006). Organic cation transporter 2 is expressed in DA-rich regions of the brain (Taubert et al, 2007) as well as in the choroid plexus, the leptomeninges and the lining of the third ventricle, presumably the ependymal cell layer (Amphoux et al, 2006). In contrast to OCT1 and OCT2, OCT3 appears to have a wide distribution in the brain and is highly expressed in DMH ependymal, subependymal, and parenchymal cells (Gasser et al, 2006;Gasser et al, 2009;Vialou et al, 2008). Organic cation transporter 3 is sensitive to inhibition by CORT, with an estimated IC50 of 30 nM (Gasser et al, 2006;Wu et al, 1998) suggesting a potential role in stress-induced modulation of monoaminergic signaling.
In an earlier study, we reported that infusion of the OCT blocker decynium 22 (D-22) directly into the MH in freely moving rats induces a dose-dependent increase in extracellular 5-HT concentrations (Feng et al, 2005). This result is consistent with other studies suggesting that OCTs may play a functional role in clearing extracellular monoamines in the CNS (Baganz et al, 2008; for review, see Daws, 2009;Gasser et al, 2006;Gasser et al, 2009;Kitaichi et al, 2005;Nakayama et al, 2007;Vialou et al, 2004;Vialou et al, 2008). However, since OCTs are low-affinity, high-capacity transporters, it is believed that they will play a more important role in modulation of 5-HT when extracellular 5-HT concentrations are elevated, such as during increased behavioral arousal or aversive stimulation (Rueter et al, 1997), following treatment with selective serotonin reuptake inhibitors, or in individuals with low expression of the high-affinity, low capacity serotonin transporter (SERT) (Baganz et al, 2008; for review, see Daws, 2009). In this study, we tested the hypothesis that OCTs contribute to regulation of extracellular 5-HT concentrations in the MH. The goals of the current study were to: 1) determine if mild restraint increases extracellular 5-HT in the MH and 2) determine if exposure to mild restraint potentiates extracellular increases in 5-HT induced by intra-MH administration of the OCT inhibitor D-22.
2. Results
2.1 Effect of D-22 perfusion into the MH on extracellular 5-HT under baseline conditions and during mild restraint
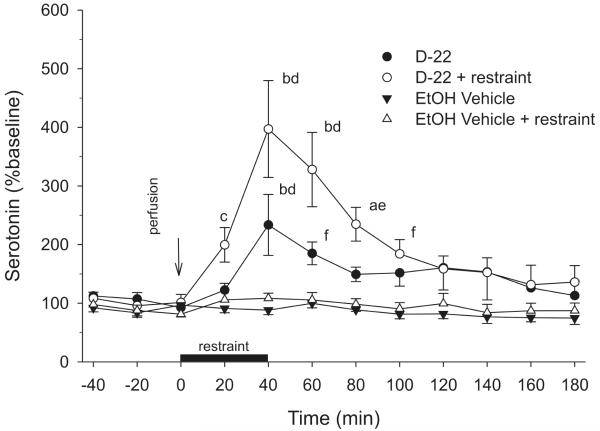
The effects of D-22 and mild restraint on extracellular 5-HT in the MH are summarized in Figure 1. Decynium 22 in unrestrained control rats resulted in a significant increase in extracellular 5-HT concentrations in the MH (approximately 200% above baseline values). Restraint by itself did not alter extracellular 5-HT concentrations at any time point. Decynium 22 also increased extracellular 5-HT concentrations in rats exposed to restraint. Both the magnitude and duration of the effects of D-22 were greater in rats exposed to restraint (reaching approximately 400% above baseline values) relative to the response to D-22 in undisturbed control rats. There were significant effects of treatment (F(3, 11) = 11.99, P < 0.001), time (F(11, 169) = 13.01, P < 0.001) and a treatment × time interaction (F(33, 169) = 4.70, P < 0.001). The reverse-dialysis of 20 μM D-22 into the MH in rats subjected to restraint increased extracellular 5-HT concentrations when compared to pretreatment (−20 min) 5-HT values (F(11, 43) = 8.51, P < 0.001). This effect was detected 40, 60 and 80 min following initiation of the treatment (Bonferroni’s t-test, P < 0.001 at times 40, 60 and P < 0.05 at time 80). In the absence of restraint, intra-hypothalamic perfusion of D-22 induced a more modest increase in extracellular 5-HT concentrations when compared to the −20 min pretreatment value (F(11, 40) = 3.96, P < 0.001). Post hoc analysis with the Bonferonni t-test versus the baseline value showed that this effect was present in the second sample (40 min) following the initiation of D-22 perfusion (P < 0.001). In rats treated with intra-MH vehicle, extracellular 5-HT concentrations were stable with respect to the baseline 5-HT values throughout the duration of the study in the presence or absence of 40 min exposure to mild restraint.
Fig 1.
Perfusion of decynium 22 (D-22) into the medial hypothalamus (MH) potentiated extracellular serotonin (5-hydroxytryptamine; 5-HT) concentrations in rats subjected to mild restraint when compared to perfusion of D-22 into the MH in rats that were not restrained. There were no significant changes in extracellular 5-HT concentrations in the MH in unrestrained or restrained rats perfused with vehicle. The dark bar represents the duration of restraint and the simultaneous perfusion of D-22 or dilute ethanol vehicle. The arrow indicates the onset of perfusion (n = 5/group; aP < 0.05 vs −20 min sample; bP < 0.001 vs. −20 min sample; cP < 0.05 vs. all other groups; dP < 0.001 vs. all other groups; eP < 0.001 vs. vehicle-treated groups, P < 0.05 vs D-22; fP < 0.05 vs. vehicle-treated groups).
In comparisons between treatments at each time point, rats subjected to a combination of reverse-dialysis of 20 μM D-22 into the MH and restraint exhibited increases in both the magnitude and duration of MH extracellular 5-HT concentrations when compared to rats treated with intra-MH D-22 in the absence of restraint. In the first post-treatment sample (t = 20 min), restrained rats treated with D-22 had higher 5-HT concentrations than those measured in all other groups (Student Newman Keuls (SNK) test, P < 0.05). This difference was maintained in samples collected at 40, 60 and 80 min after treatment initiation (SNK, P < 0.001 vs all other groups at 40, 60 min; P < 0.001 vs vehicle-treated groups at 80 min, P < 0.05 vs D-22 without restraint at 80 min). At 100 min, restrained rats treated with D-22 had higher MH 5-HT concentrations than the vehicle-treated groups (SNK, P < 0.05) but did not differ statistically from unrestrained D-22-treated rats. Extracellular 5-HT concentrations in rats treated with D-22 and subjected to restraint returned to levels that did not differ from other groups for the remainder of the experiment. In the absence of restraint, intra-hypothalamic D-22 increased extracellular 5-HT when compared to the vehicle-treated groups. This effect was detected 40 and 60 min following the onset of treatment (SNK, P < 0.001, P < 0.05, respectively).
2.2 Effect of mild restraint on extracellular CORT in the MH
The restraint procedure employed in this study did not significantly increase extracellular CORT levels in the MH when compared to pre-restraint values. However, there was a trend for increased MH CORT levels in response to restraint (F(11,77) = 1.68; P = 0.093). CORT levels, uncorrected for probe recovery, for the sample immediately preceding restraint and 20 min following restraint were 1.89 ± 0.67 ng/mL and 4.62 ± 1.13 ng/mL (n = 7), respectively.
3. Discussion
Blockade of OCTs in the MH using reverse-dialysis of D-22 increased extracellular 5-HT concentrations under basal conditions and during a mild restraint procedure. Both the magnitude and duration of the D-22-induced increase in extracellular 5-HT were greater in rats exposed to restraint, suggesting that restraint increased 5-HT release in the DMH. A restraint-induced increase in extracellular 5-HT was not detectable in rats that did not receive D-22, presumably because the restraint-induced increase in extracellular 5-HT was not sufficient to exceed the capacity for rapid 5-HT clearance by SERT and other transporters and therefore was not sufficient to induce subsequent overflow into the pool of extracellular fluid sampled by the microdialysis probe. In drug nal̈ve rats, rapid clearance of 5-HT is probably mediated predominantly by the presynaptic SERT but with contributions from other transporters including OCTs (Daws, 2009). These findings are consistent with the hypothesis that OCTs play a role in clearance of 5-HT from the extracellular fluid within the MH under both basal conditions and during mild restraint.
3.1. Decynium 22 increased MH extracellular 5-HT under baseline conditions
The data in this study replicated our previous finding that local administration of the OCT3 inhibitor D-22 can increase extracellular 5-HT concentrations in the MH under baseline conditions (Feng et al, 2005). In our earlier studies, we showed that intra-hypothalamic administration of 30 and 100 μM D-22 resulted in local increases in extracellular 5-HT concentrations of approximately 450 and 700%, respectively (Feng et al, 2005). The increase in MH 5-HT of approximately 200% in response to reverse-dialysis of 20 μM D-22 is consistent with this dose-response profile and with previous studies demonstrating that a lower concentration of D-22 (10 μM) into rat MH by reverse-dialysis (Feng et al, 2005), or into mouse hippocampus by pressure-ejection (Baganz et al, 2008) has no effect on extracellular 5-HT concentrations under baseline conditions. The ability of higher concentrations of D-22, such as 20 μM used in this study, to elevate extracellular 5-HT concentrations may be due to a more complete blockade of OCT3-mediated transport, particularly at sites distal to the surface of the microdialysis probe where D-22 concentrations would be lower. Organic cation transporter 3 is abundantly expressed in the MH and appears to account for the majority of OCT-mediated uptake in this region (Gasser et al, 2006;Gasser et al, 2009).
3.2. Mild restraint did not alter extracellular MH 5-HT concentrations
Mild restraint, by itself, did not alter extracellular 5-HT concentrations in the MH. This is consistent with our finding that the mild form of restraint used, in which rats were gently coaxed into the restraint tube within the microdialysis chamber during the dark phase of the daily light cycle, did not result in detectable increases in extracellular CORT within the DMH. These findings are also consistent with previous microdialysis studies demonstrating that restraint does not alter extracellular 5-HT concentrations in the hippocampus (Kirby et al, 1997;Muchimapura et al, 2002). In contrast, increases in 5-HT concentrations in subnuclei of the hypothalamus adjacent to the DMH are clearly evident in rats subjected to a more rigorous form of restraint for a longer duration (Shimizu et al., 1992, 2000; Shintani et al., 1995). Interestingly, in the studies in which restraint failed to increase extracellular 5-HT, increases in extracellular concentrations of 5-hydroxyindoleacetic acid (5-HIAA), the major metabolite of 5-HT, were detected in the hippocampus (Kirby et al, 1997). This could result from restraint-induced increases in 5-HT release, re-uptake, and metabolism, with no net increase in extracellular 5-HT in the pool of CSF sampled by the microdialysis probe, due to rapid clearance by SERT and other transporters at the sites of release.
The finding that restraint alone did not increase extracellular 5-HT concentrations in the MH seems at odds with the finding that restraint increased 5-HT tissue concentrations in a restricted region of the DMH (the dorsal hypothalamic area) of female Lewis rats (Lowry et al, 2003). However, extracellular and tissue concentrations of 5-HT may be differentially regulated, particularly if OCT2- (Amphoux et al, 2006) or OCT3-expressing (Gasser et al, 2006;Gasser et al, 2009;Vialou et al, 2008) ependymal or glial cells in the MH accumulate 5-HT. Ependymal cells can metabolize 5-HT via the actions of monoamine oxidase A in mammalian (Verleysdonk et al, 2004) and non-mammalian vertebrates (for review, see Lowry et al, 1996). Therefore, the uptake of 5-HT by ependymal cells may explain why restraint increases tissue concentrations of 5-HT in a microdissected region that includes the ependymal layer (Lowry et al, 2003). The transport of 5-HT by ependymal cells may also explain the concurrent elevation of 5-HT and 5-HIAA tissue concentrations within the DMH of different vertebrate species following restraint (Lowry et al, 2001;Lowry et al, 2003) or administration of CORT (Gasser et al, 2006;Losada, 1988;Lowry et al, 2001). Consistent with the hypothesis that restraint activates serotonergic neuronal firing and 5-HT release, restraint increases c-Fos expression in serotonergic neurons within the dorsal and median raphe nuclei (Takase et al, 2005) and induces modest increases in extracellular 5-HT concentrations in some forebrain structures, including the basolateral nucleus of the amygdala (Mitsushima et al, 2006) and the central nucleus of the amygdala (Mo et al, 2008).
The effect of restraint to increase extracellular 5-HT concentrations in some studies, but not others, could be due to a number of factors, including 1) methodological differences that vary the aversiveness of the restraint, resulting in differential increases in serotonin release or local CORT concentrations, 2) selective activation of a subset of serotonergic neurons projecting to specific forebrain limbic structures including the amygdala (Lowry et al, 2005), 3) regional differences in clearance rates, via SERT, OCTs, or other poorly characterized 5-HT transporters, such as the plasma membrane monoamine transporter (PMAT; also known as equilibrative nucleoside transporter 4 (ENT4) (for review, see Daws, 2009;Zhou et al, 2007), or multidrug and toxin extrusion protein 1 (MATE1) (Hiasa et al, 2006;Otsuka et al, 2005), 4) different effects of restraint on serotonergic systems in male and female rats (Mitsushima et al, 2006), 5) different effects of restraint on serotonergic systems in different rat strains (Lowry et al, 2003), or 6) a combination of these factors.
3.3. D-22 and restraint interacted to increase extracellular 5-HT in the MH
The finding that D-22 and restraint interacted to increase extracellular 5-HT concentrations, to levels above those induced by D-22 or restraint alone, suggests that restraint induced a moderate increase in 5-HT release that does not exceed the capacity for rapid clearance via SERT and other transporters including OCTs, and that extracellular 5-HT concentrations were increased by blockade of 5-HT clearance by D-22. This interpretation is supported by our previous findings that reverse-dialysis of CORT, a selective OCT blocker with high affinity for OCT3 (Gasser et al, 2006), into the MH potentiates the effects of the 5-HT releasing agent D-fenfluramine on extracellular 5-HT concentrations (Feng et al, 2009). Taken together, these data support a functional role for OCTs in modulating monoaminergic neurotransmission by altering 5-HT clearance in the presence of elevated 5-HT release, such as that which occurs during behavioral arousal, or in response to aversive stimuli, 5-HT-releasing drugs such as D-fenfluramine, or under other conditions when extracellular 5-HT concentrations are elevated such as following treatment with selective serotonin reuptake inhibitors, or low expression of SERT (for review, see Daws, 2009).
The rapid time course of the D-22-induced increase in extracellular 5-HT concentrations in restrained rats is consistent with the rapid but moderate increases in extracellular 5-HT observed in other brain regions such as the basolateral amygdala and central nucleus of the amygdala following exposure of rats to restraint (Mitsushima et al, 2006;Mo et al, 2008). In those studies, elevated 5-HT concentrations were detected in the first dialysate sample collected following initiation of restraint (Mitsushima et al, 2006;Mo et al, 2008). In the amygdala, these increases are likely due to increased release of 5-HT that exceeds the capacity of local clearance mechanisms to remove the 5-HT from the synapse, leading to diffusion of 5-HT into extra-synaptic compartments (for review, see Daws, 2009). In rats treated with intra-hypothalamic D-22, increases in extracellular 5-HT were detected in the first 20 min sample after the initiation of the restraint and peak concentrations coincided with the final 20 min of D-22 infusion and restraint. Extracellular 5-HT remained elevated for up to 60 min after the termination of restraint and the removal of D-22 from the reverse-dialysis perfusate, consistent with other studies demonstrating persistent effects of D-22 on 5-HT clearance in vivo (Baganz et al, 2008). Together, these findings are consistent with the demonstration that inhibition of OCT-mediated transport by D-22 inhibits the overall rate of clearance of 5-HT from the extracellular fluid (Baganz et al, 2008). The consequences of inhibition of OCT-mediated transport by D-22, or by other high-affinity OCT3 inhibitors such as CORT, remain to be determined. However, recent reports that OCT3 has anxiolytic effects in rats (Vialou et al, 2008) and D-22 has antidepressant-like effects in mice (Baganz et al, 2008) suggest that OCT3-mediated 5-HT transport, and its regulation by CORT or other mechanisms, may have important consequences for the regulation of emotional states and emotional behavior.
4. Experimental Procedure
4.1 Animals
Male Sprague-Dawley rats (250-300 g), obtained from the University of South Dakota Laboratory Animal Services, were maintained in a temperature-controlled room with a reverse 12L:12D photoperiod (lights off at 10:00 A.M.). Food and water were available to the animals ad libitum. Care for the animals followed NIH Guidelines for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with the University of South Dakota Institutional Animal Care and Use Committee.
4.2. Drug Treatments
Decynium 22 (1,11-diethyl-2,22-cyanine iodide, D-22; Sigma-Aldrich Chemical Co., St. Louis, MO) was dissolved in 100% ethanol and diluted to 20 μM in artificial cerebrospinal fluid (aCSF) with a final ethanol concentration of 0.2 %. The choice of the 20 μM dose of D-22 as a means of providing submaximal increases in extracellular 5-HT in the MH was based on our previous dose-response curve generated for the drug (Feng et al, 2005). Control animals were perfused with aCSF containing 0.2 % of the ethanol vehicle.
4.3 Surgical Procedures
Stereotaxic surgeries were performed aseptically under xylazine-ketamine anesthesia (Ketoset, Fort Dodge Labs Inc., Fort Dodge, IA; xylazine, Vedco, Inc., St. Joseph, MO). Following anesthesia, the rats were mounted in a stereotaxic frame in the flat-skull position and implanted with a unilateral guide cannula (20-G, cut to project approximately 4 mm dorsal to the DMH; Plastics One, Inc., Roanoke, VA), directed towards the DMH. Coordinates for the guide cannula implantation, relative to Bregma, were: AP −3.3 mm; ML ± 0.7 mm and DV −4.6 mm from the cortical surface (Paxinos and Watson, 1986). The pedestal was initially fixed to the skull using super glue; it was then permanently fixed to the skull with a combination of glass ionomer cement (GC Corp., Alsip, IL) and cranioplastic acrylic (Plastics One, Inc.) using surgical screws for support. After surgery, the rats were housed individually and allowed to recover for 3 to 5 days before undergoing further experimental procedures.
4.4. Microdialysis procedures and 5-HT analysis
The night before experimentation, rats were lightly anesthetized with ketamine (80 mg)-xylazine (10 mg) and implanted with a concentric dialysis probe to a depth of −8.6 mm from the cortical surface (Paxinos and Watson, 1986). The dialysis membrane had a molecular weight cut-off of 5 kDa, and a 2.2 mm working membrane length (Farmer et al, 1996;Feng et al, 2005). Under these conditions, the probes sampled from the DMH as well as the dorsal portion of the ventromedial hypothalamus and dorsally, the dorsal hypothalamic area. Therefore, the probes sampled from a large region of the MH, including the DMH. The probes were attached to a liquid swivel (Instech Laboratories, Plymouth Meeting, PA), which allowed the animals freedom of movement in 10-gallon aquaria. Artificial cerebrospinal fluid was perfused through the probes overnight to allow for equilibration at a rate of 0.1 μL/min using a microinfusion pump (CMA, North Chelmsford, MA) and a 1 mL glass syringe. The experiments were initiated at 10:00 AM during the dark phase of the rat’s photoperiod under dim diffuse lighting. The perfusion rate was increased to 0.42 μL/min and dialysates were collected at 20 min intervals. Extracellular 5-HT concentrations were measured by high performance liquid chromatography (HPLC) with electrochemical detection as previously described (Farmer et al, 1996;Feng et al, 2005). Briefly, 8.4 μL samples were injected into a rheodyne injector using a 5 μL loop to insure that each sample over-filled the injection loop. Serotonin separation was accomplished by reverse phase liquid chromatography using a Sepstick 3 μm C-18 microbore column (Bioanalytical Systems, West Lafayette, IN) and a pneumatic displacement pump with nitrogen gas pressure to produce a pulse-free flow (Bradberry et al, 1991). Following separation, 5-HT was detected with a glassy carbon electrode (Bioanalytical Systems, West Lafayette, IN) at an oxidation potential of +0.6 V relative to an Ag/AgCl2 reference electrode using an LC-4C potentiostat (Bioanalytical Systems) set at 0.5 nA/V. The mean baseline 5-HT concentration in the MH for all rats was 0.87 ± 0.13 pg/5 μL (uncorrected for probe recovery) with a 2:1 signal to noise ratio of 0.06 ± 0.01 pg.
4.5. Reverse-dialysis of D-22 and restraint
After confirming a stable 5-HT baseline consisting of three consecutive dialysate samples that deviated less than 10% in peak height, the experiments were initiated. All subsequent 5-HT values, beginning at −40 min, are expressed as a percentage change calculated from the mean 5-HT value obtained from the 3 baseline samples. Following collection of three samples after the 5-HT baseline was established (time = 0), rats received one of the following treatment combinations: 1) reverse-dialysis of 20 μM D-22 into the MH combined with restraint (n = 5); 2) reverse-dialysis of 20 μM D-22 into the MH without restraint (n = 5); 3) reverse-dialysis of 0.2 % ethanol vehicle into the MH combined with restraint (n = 5); or 4) reverse-dialysis of 0.2 % ethanol vehicle into the MH without restraint (n = 5). Restraint was accomplished by placing a polyvinyl chloride (PVC) tube (6 cm i.d., 27 cm long) with a 2 cm channel cut in the top that allowed passage of the lines attached to the microdialysis probe (Mo et al, 2008) on the floor of the aquarium and coaxing the rat to enter the tube. These tubes, which were sealed using #11 rubber stoppers, prevented movement in any direction but did not fully immobilize the rats. Perfusions of D-22 or vehicle and restraint, when applied, were initiated at time 0 and continued for 40 min (two sampling periods). The estimated lag time for displacement of aCSF in the tubing leading to the microdialysis probe before the onset of drug infusion into the MH was 20 min and the perfusions were timed so that the drug delivery to the MH and the initiation of restraint occurred at the same time. At the conclusion of the 40 min restraint period the stoppers were removed and the rats were released back into the testing chamber. Dialysates were collected and analyzed for 3 h following the onset of the treatment.
4.6. Measurement of corticosterone
Microdialysis samples were assayed for CORT using a commercially available 125I radioimmunoassay (RIA) kit (Cat. No. 07120103, MP Biomedicals, LLC, Irvine, CA). Methods used were based on previous studies (Droste et al, 2008; Linthorst et al, 1994). Dialysates were diluted (1:200) with steroid diluent according to the standard RIA kit protocol. A standard curve was generated with standards provided by the kit with the addition of 2 standards made with steroid diluent (2.5 ng/mL and 10 ng/mL) to allow for detection of dialysate concentrations of CORT. Standards and dialysate samples were run in duplicate. Both 125I tracer and anti-serum were added to all standard and sample tubes (glass, 12 × 75 mm). All tubes were then vortexed briefly and incubated at room temperature for 2 h. Following incubation, a precipitant solution was added to all tubes and then tubes were vortexed thoroughly. All assay tubes were then centrifuged for 15 minutes at 2500 r.p.m. (1000 x g). Once centrifuged, all tubes were decanted and allowed to drain for 10 min. The precipitate was counted in a gamma counter (COBRA II auto-gamma, Packard Bioscience Company, Shelton, CT). The inter-assay variance was 15% and the intra-assay variance was 12% with a detection limit of 0.33 ng/mL. Concentrations of CORT from microdialysis samples were not corrected for probe recovery.
4.7 Histological verification
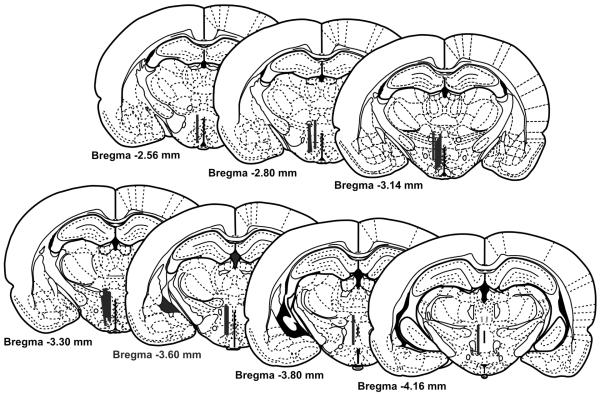
Rats were killed by an injection of Fatal Plus (0.5 mL i.p.; Vortech, Dearborn, MI). The brains were removed, fixed in 10% formalin (Fisher Scientific, Pittsburgh, PA) and serially sectioned into 60 μm sections at −15 °C using a cryostat (Leica Instruments, Heidelberg, Germany). Probe placement was verified under a microscope by an investigator blind to treatment using the Paxinos and Watson atlas (1986). Animals were excluded from the study when the probe tracts missed the DMH, punctured the ventricle or penetrated the base of the brain. The locations of the microdialysis probe placements within the DMH are illustrated in Figure 2.
Fig 2.
Illustrations showing microdialysis probe placements within the medial hypothalamus (MH) for individual rats used for analysis of extracellular serotonin (5-hydroxytryptamine; 5-HT) concentrations. Black bars, indicating the working surface of the microdialysis probe (0.2 mm wide, 2.2 mm long), are drawn to scale. The figures are adapted from the atlas by Paxinos and Watson (1986).
4.8 Statistics
Data from the 5-HT analysis were analyzed by two-way ANOVA with repeated measures (SigmaStat version 2.03, Systat Software Inc., San Jose, CA) with treatment as a between-subjects factor and time as a within-subjects factor. In the presence of a significant treatment x time interaction, the effects of treatment across time were compared to pretreatment values using Bonferroni’s t-test, where the sample preceding treatment initiation (−20 min) served as the control value. Differences in means of extracellular 5-HT concentrations between groups at individual time points were tested using the SNK test. Data from the CORT measurements were analyzed using a one-way ANOVA with repeated measures, with restraint as a between-subjects factor and time as a within-subjects factor. All data were tested for the presence of outliers using the Grubbs’ test (Rohlf and Sokal, 2009). There were three outliers detected among the 240 samples evaluated for 5-HT (1 in the Vehicle, no restraint group and 2 in the D-22, no restraint group; P < 0.05). An additional three 5-HT measurements that exceeded 600% changes in 5-HT during the later sampling periods (140 – 180 min) were treated as missing values. There were five outliers detected among the 96 samples evaluated for CORT (P < 0.05). Significance levels for all statistical tests were set at P ≤ 0.05.
Acknowledgements
This work was supported by NIH grants R01 DA019921 (G.L.F.) and COBRE P20 RR15567 which is designated a Center for Biomedical Research Excellence, and NSF grants 0921969 (C.A.L.), 0921874 (K.J.R.). C.A.L is a recipient of a NARSAD 2007 Young Investigator Award, and an NSF CAREER Award (NSF-IOS #0845550).
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine; serotonin
- aCSF
artificial cerebrospinal fluid
- CNS
central nervous system
- CORT
corticosterone
- DA
dopamine
- D-22
1, 11-diethyl-2,22-cyanine iodide; decynium 22
- DMH
dorsomedial hypothalamus
- ENT4
equilabrative nucleoside transporter 4
- HPA
hypothalamic-pituitary-adrenal
- HPLC
high performance liquid chromatography
- MATE1
multidrug and toxin extrusion protein 1
- MH
medial hypothalamus
- NE
norepinephrine
- OCT1
slc22a1, solute carrier family 22 (organic cation transporter), member 1; organic cation transporter 1
- OCT2
slc22a2, solute carrier family 22 (organic cation transporter), member 2; organic cation transporter 2
- OCT3
slc22a3, solute carrier family 22 (organic cation transporter), member 3; organic cation transporter 3; extraneuronal monoamine transporter (EMT)
- PMAT
plasma membrane monoamine transporter
- PVC
polyvinyl chloride
- PVN
paraventricular nucleus of the hypothalamus
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SERT
slc6a4, solute carrier family 6 (neurotransmitter transporter, serotonin), member 4; serotonin transporter
- SNK
Student Newman Keuls
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Regulatory Systems
Reference List
- 1.Amphoux A, Vialou V, Drescher E, Bruss M, Mannoury La CC, Rochat C, Millan MJ, Giros B, Bonisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R8–15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- 4.Bailey TW, Nicol GD, Schild JH, DiMicco JA. Synaptic and membrane properties of neurons in the dorsomedial hypothalamus. Brain Res. 2003;985:150–162. doi: 10.1016/s0006-8993(03)03047-6. [DOI] [PubMed] [Google Scholar]
- 5.Bradberry CW, Sprouse JS, Aghajanian GK, Roth RH. Sub-picogram determination of serotonin using HPLC with electrochemical detection for microdialysis studies of serotonin release. Adv. Exp. Med. Biol. 1991;294:81–89. doi: 10.1007/978-1-4684-5952-4_7. [DOI] [PubMed] [Google Scholar]
- 6.Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 1996;395:153–156. doi: 10.1016/0014-5793(96)01030-7. [DOI] [PubMed] [Google Scholar]
- 7.Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- 8.Culman J, Kvetnansky R, Torda T, Murgas K. Serotonin concentration in individual hypothalamic nuclei of rats exposed to acute immobilization stress. Neuroscience. 1980;5:1503–1506. doi: 10.1016/0306-4522(80)90012-3. [DOI] [PubMed] [Google Scholar]
- 9.Daws LC. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol. Ther. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 12.Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: Relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- 13.Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063:69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Feng N, Telefont M, Kelly K, Orchinik M, Forster GL, Renner KJ, Lowry CA. Local perfusion of corticosterone in the rat medial hypothalamus potentiates D-fenfluramine-induced elevations of extracellular 5-HT concentrations. Horm. Behav. 2009;56:149–157. doi: 10.1016/j.yhbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J. Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- 17.Grundemann D, Koster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, Obermuller N, Schomig E. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J. Biol. Chem. 1998;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- 18.Hiasa M, Matsumoto T, Komatsu T, Moriyama Y. Wide variety of locations for rodent MATE1, a transporter protein that mediates the final excretion step for toxic organic cations. Am. J Physiol Cell Physiol. 2006;291:C678–C686. doi: 10.1152/ajpcell.00090.2006. [DOI] [PubMed] [Google Scholar]
- 19.Inazu M, Takeda H, Matsumiya T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J. Neurochem. 2003;84:43–52. doi: 10.1046/j.1471-4159.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 20.Keim SR, Shekhar A. The effects of GABAA receptor blockade in the dorsomedial hypothalamic nucleus on corticotrophin (ACTH) and corticosterone secretion in male rats. Brain Res. 1996;739:46–51. doi: 10.1016/s0006-8993(96)00810-4. [DOI] [PubMed] [Google Scholar]
- 21.Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- 22.Kitaichi K, Fukuda M, Nakayama H, Aoyama N, Ito Y, Fujimoto Y, Takagi K, Takagi K, Hasegawa T. Behavioral changes following antisense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett. 2005;382:195–200. doi: 10.1016/j.neulet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev. Physiol. Biochem. Pharmacol. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J. Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losada MEO. Corticosterone-induced acute changes on biogenic-amines’ levels in some areas of the rat brain. Biogenic Amines. 1988;5:239–247. [Google Scholar]
- 26.Lowry CA, Burke KA, Renner KJ, Moore FL, Orchinik M. Rapid changes in monoamine levels following administration of corticotropin-releasing factor or corticosterone are localized in the dorsomedial hypothalamus. Horm. Behav. 2001;39:195–205. doi: 10.1006/hbeh.2001.1646. [DOI] [PubMed] [Google Scholar]
- 27.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 28.Lowry CA, Plant A, Shanks N, Ingram CD, Lightman SL. Anatomical and functional evidence for a stress-responsive, monoamine-accumulating area in the dorsomedial hypothalamus of adult rat brain. Horm. Behav. 2003;43:254–262. doi: 10.1016/s0018-506x(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 29.Lowry CA, Renner KJ, Moore FL. Catecholamines and indoleamines in the central nervous system of a urodele amphibian: a microdissection study with emphasis on the distribution of epinephrine. Brain Behav. Evol. 1996;48:70–93. doi: 10.1159/000113187. [DOI] [PubMed] [Google Scholar]
- 30.Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur. J. Neurosci. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 31.Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res. Bull. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muchimapura S, Fulford AJ, Mason R, Marsden CA. Isolation rearing in the rat disrupts the hippocampal response to stress. Neuroscience. 2002;112:697–705. doi: 10.1016/s0306-4522(02)00107-0. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama H, Kitaichi K, Ito Y, Hashimoto K, Takagi K, Yokoi T, Takagi K, Ozaki N, Yamamoto T, Hasegawa T. The role of organic cation transporter-3 in methamphetamine disposition and its behavioral response in rats. Brain Res. 2007;1184:260–269. doi: 10.1016/j.brainres.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition Academic Press; Sydney: 1986. [Google Scholar]
- 36.Rohlf FJ, Sokal RR. Statistical Tables. WH Freeman and Company; San Francisco: 2009. [Google Scholar]
- 37.Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev. Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J. Neurosci. Res. 2003;71:701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- 39.Shekhar A, Katner JS, Rusche WP, Sajdyk TJ, Simon JR. Fear-potentiated startle elevates catecholamine levels in the dorsomedial hypothalamus of rats. Pharmacol. Biochem. Behav. 1994;48:525–529. doi: 10.1016/0091-3057(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu N, Hori T, Ogino C, Kawanishi T, Hayashi Y. The 5-HT(1A) receptor agonist, 8-OH-DPAT, attenuates stress-induced anorexia in conjunction with the suppression of hypothalamic serotonin release in rats. Brain Res. 2000;887:178–182. doi: 10.1016/s0006-8993(00)03031-6. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu N, Take S, Hori T, Oomura Y. In vivo measurement of hypothalamic serotonin release by intracerebral microdialysis: significant enhancement by immobilization stress in rats. Brain Res. Bull. 1992;28:727–734. doi: 10.1016/0361-9230(92)90252-s. [DOI] [PubMed] [Google Scholar]
- 42.Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J. Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav. Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Taubert D, Grimberg G, Stenzel W, Schomig E. Identification of the endogenous key substrates of the human organic cation transporter OCT2 and their implication in function of dopaminergic neurons. PLoS. ONE. 2007;2:e385. doi: 10.1371/journal.pone.0000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res. Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 46.Verleysdonk S, Hamprecht B, Rapp M, Wellard J. Uptake and metabolism of serotonin by ependymal primary cultures. Neurochem. Res. 2004;29:1739–1747. doi: 10.1023/b:nere.0000035810.08543.97. [DOI] [PubMed] [Google Scholar]
- 47.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 48.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 49.Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J. Neurosci. 2004;24:2846–2851. doi: 10.1523/JNEUROSCI.5147-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 52.Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, DiMicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008 doi: 10.1016/j.brainres.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou M, Engel K, Wang J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem. Pharmacol. 2007;73:147–154. doi: 10.1016/j.bcp.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]