Abstract
People with schizophrenia consistently report normal levels of pleasant emotion when exposed to evocative stimuli, suggesting intact consummatory pleasure. However, little is known about the neural correlates and time course of emotion in schizophrenia. This study used a well-validated affective picture viewing task that elicits a characteristic pattern of Event Related Potentials (ERP) from early to later processing stages (i.e., P1, P2, P3, and Late Positive Potentials (LPP)). Thirty eight stabilized schizophrenia outpatients and 36 healthy controls viewed standardized pleasant, unpleasant, and neural pictures while ERPs were recorded, and subsequently rated their emotional responses to the stimuli. Patients and controls responded to the pictures similarly in terms of their valence ratings, as well as the initial ERP components (P1, P2, and P3). However, at the later LPP component (500 – 1000 ms), patients displayed diminished electrophysiological discrimination between pleasant versus neutral stimuli. This pattern suggests that patients demonstrate normal self-reported emotional experience and intact initial sensory processing of and resource allocation to emotional stimuli. However, they show a disruption in a later component associated with sustained attentional processing of emotional stimuli.
Keywords: schizophrenia, anhedonia, emotion, pleasure, Event Related Potentials (ERP)
Anhedonia, historically defined as the diminished capacity to experience pleasure, is widely regarded as a core negative symptom of schizophrenia (Kirkpatrick, Fenton, Carpenter, & Marder, 2006). Clinically, anhedonia manifests as a treatment-resistant emotional disturbance that is common throughout the course of illness and a key determinant of poor functional outcome (Horan, Blanchard, & Kring, 2006). Research into anhedonia has expanded considerably over the past decade and has revealed a seemingly paradoxical set of findings. On the one hand, people with schizophrenia report experiencing generally low levels of pleasure based on clinical interviews, self-report trait measures, and naturalistic studies using the experience sampling method (Horan, Blanchard, Clark, & Green, 2008; Horan et al., 2006). On the other hand, they consistently report experiencing levels of pleasant emotions that are similar to non-patients in laboratory studies that use evocative stimuli (Kring & Moran, 2008). The overall pattern suggests that people with schizophrenia are indeed capable of experiencing a normal range and intensity of pleasant emotions to evocative stimuli, yet for some reason experience generally low levels of pleasure in their daily lives.
One promising explanation for this apparent discrepancy is that only certain subcomponents of hedonic experience are disrupted in schizophrenia. Building on affective neuroscience models that distinguish among neurally dissociable hedonic processes (Berridge & Robinson, 1998, 2003; Knutson, Fong, Adams, Varner, & Hommer, 2001; O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001), Kring (Kring, 1999) proposed that schizophrenia may be characterized by intact “consummatory pleasure”, which refers to pleasure derived from engaging in an enjoyable activity, but impaired “anticipatory pleasure”, referring to pleasure derived from anticipating that an activity will be enjoyable (Depue & Iacono, 1989; Gray, 1987; Klein, 1984). In support of this hypothesis, an initial report from our group (Gard, Kring, Gard, Horan, & Green, 2007) found that schizophrenia patients reported comparable levels of consummatory pleasure, but lower levels of anticipatory pleasure, than healthy controls in the course of daily life (using the experience sampling method) and on a self-report trait measure that distinguishes these components of hedonic experience (Gard, Germans-Gard, Kring, & John, 2006).
To date, the vast majority of research into emotional experience in schizophrenia is based on self-report data (Kring & Moran, 2008). However, relying solely on self-reported experience is limiting for several reasons. First, self-report may be subject to potential biases (e.g., fallible memory, demand characteristics, and response biases), though this is equally true for people with and without schizophrenia. Second, emotion is thought to comprise multiple components in addition to self-reported experience, including expressive, autonomic, electrocortical, and cerebral blood flow changes (Mauss & Robinson, 2009). The burgeoning affective neuroscience literature has used neuroimaging and electrophysiology to advance this line of research to the neural level of analysis (Bradley & Lang, 2007; Wager et al., 2008). Affective neuroscientists have developed electrophysiological paradigms to study the time course and topography of neural responses to emotional stimuli. Event Related Potentials (ERPs) provide a direct measure of neural activity on the order of milliseconds and are particularly well suited to investigate temporal aspects of emotion processing. ERP methods isolate early- (i.e., less than about 200 ms), middle- (about 200-300 ms) and late-latency (greater than about 300 ms) components of responses following stimulus onset, thus allowing for careful consideration of time course. Although ERP’s have been extensively used to study various aspects of cognitive and perceptual processing in schizophrenia (e.g., (Jeon & Polich, 2003; Turetsky et al., 2007; Wynn, Lee, Horan, & Green, 2008)), they have rarely been applied to investigate emotional disturbances associated with this disorder.
The current study used a multi-method assessment of emotional responding in schizophrenia by applying a well-validated picture viewing paradigm that robustly elicits characteristic ERPs to emotional versus neutral stimuli in healthy individuals (for a review see (Olofsson, Nordin, Sequeira, & Polich, 2008)). In this passive picture viewing paradigm, participants are shown a series of standardized pleasant, unpleasant, and neutral photos from the International Affective Picture Set (IAPS; (Lang, Bradley, & Cuthbert, 1999)). ERP’s are recorded to picture onset and self-report ratings of emotional experience to the pictures are subsequently collected.
Two late-latency ERP components reliably demonstrate sensitivity to the emotionally arousing nature of pleasant and unpleasant pictures in healthy subjects (see (Olofsson et al., 2008) for a review). First, starting around 300 ms post-onset, the amplitude of an ERP component commonly labeled “P3” is significantly larger for both pleasant and unpleasant pictures as compared to neutral pictures. Subsequently, a slow sustained positivity, termed the “Late Positive Potential” (LPP; beginning about 500 ms), is maintained for pleasant and unpleasant pictures for at least several hundred milliseconds. The P3 and LPP components of the ERP are typically maximal at mid-line and parietal electrodes. The valence-sensitive ERP amplitudes in this paradigm are typically interpreted to reflect “motivated attention”, meaning that basic motivational appetitive and defensive brain systems are preferentially engaged by pleasant and unpleasant pictures, respectively (Bradley & Lang, 2007). According to this account, the P3 and LPP enhancements reflect a relatively automatic increase in allocation of attentional resources to, and sustained attentional processing of, motivationally relevant stimuli (Bradley & Lang, 2007).
In addition to the consistent P3 and LPP enhancements to pleasant and unpleasant stimuli, valence-related effects for some earlier ERP’s and lateralized responding are less frequently reported for picture viewing paradigms in healthy subjects (see (Olofsson et al., 2008)). Regarding early ERP’s, although the short-latency P1 (100-200 ms) response appears primarily sensitive to sensory properties of the stimuli, several studies demonstrate enhanced P2 (200-300 ms) responses to pleasant compared to unpleasant and neutral stimuli. Theoretically, the mid-latency P2 enhancement for pleasant stimuli is believed to reflect selective attention to affective images that are assumed to be of intrinsic relevance. Regarding laterality, some studies have reported either greater overall right hemisphere activation or valence-specific effects (i.e., greater left hemisphere activation for pleasant vs. greater right activation for unpleasant pictures (Cunningham, Espinet, DeYoung, & Zelazo, 2005; Dolcos & Cabeza, 2002)), though laterality effects are inconsistently reported.
The primary goal of this study was to further evaluate emotional responding in schizophrenia at multiple levels. Specifically, the goal was to determine whether patients’ ERP responses to pleasant versus neutral stimuli match their typically normal self-reported emotional experience. To test the main study hypotheses, we focused on the late-latency P3 and LPP ERP components, which have most consistently demonstrated sensitivity to emotional picture content. The inclusion of unpleasant stimuli permits a comprehensive examination of emotional responding and addresses another discrepancy in the schizophrenia research literature -namely, that patients typically report normal levels of unpleasant emotions in response to evocative stimuli, yet report elevated levels of trait negativity (Horan, Blanchard et al., 2008). We evaluated two competing hypotheses: 1) patients and controls will demonstrate comparable patterns for P3 and LPP (elevated amplitudes for pleasant and unpleasant stimuli compared to neutral stimuli), consistent with laboratory studies showing comparable self-reported emotional responses to stimuli, or 2) patients will show smaller P3 and LPP’s for pleasant stimuli and larger for unpleasant stimuli compared with controls, consistent with their self-reports of low trait positive affectivity and elevated trait negative affectivity. A secondary goal was to examine potential between-group differences in P1, P2, and laterality effects which, as described above, have been less reliably established. We examined whether both groups demonstrated larger P2 responses to pleasant versus neutral stimuli and greater right versus left hemisphere responding. Finally, within the schizophrenia group, we evaluated whether trait anhedonia and clinically rated negative symptoms were related to behavioral and ERP responses in the picture viewing task.
Methods
Participants
Forty-nine stabilized outpatients with schizophrenia and 41 healthy control subjects participated in the study. Eleven patients and 5 controls were excluded from the analyses due to not having a sufficient number of acceptable EEG trials (see below). Thus, the final sample consisted of 38 patients and 36 controls. Schizophrenia patients were recruited from outpatient treatment clinics at the Veterans Affairs (VA) Greater Los Angeles Healthcare System and through presentations in the community. Patients met criteria for schizophrenia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; (First, Gibbon, Spitzer, & Williams, 1996)). Exclusion criteria for patients included: substance abuse or dependence in the last six months; mental retardation; a history of loss of consciousness for more than one hour; an identifiable neurological disorder; or insufficient fluency in English. Thirty-two patients were receiving atypical antipsychotic medications, two patients were receiving typical antipsychotic medications, and two were receiving both types of medication. Four patients were prescribed benzodiazepines, and these patients were asked to refrain from taking these medications on the day of testing (i.e., at least 12 hr before assessment). Ten patients were prescribed anticholinergic medications (of these, three took typical antipsychotics, four took risperidone, 2 took aripiprazole, and 1 took olanzapine).
Nonpatient control participants were recruited through newspaper advertisements and flyers posted in the local community. Control participants were screened with the SCID and SCID II (First, Gibbon, Spitzer, Williams, & Benjamin, 1996) and were excluded if they met criteria for any psychotic disorder; bipolar mood disorder; recurrent depression (defined as more than a single major depressive episode); substance dependence; or paranoid, schizotypal, or schizoid personality disorder. Control participants were also excluded if there was any evidence (according to participant report) of a history of psychotic disorder among their first-degree relatives. Additional exclusion criteria for all patients and control participants included age less than 18 or over 55 years, active substance use disorder in the past 6 months, identifiable neurological disorder, mental retardation, or seizure disorder.
All participants had the capacity to give informed consent and provided written informed consent after all procedures were fully explained in accordance with procedures approved by the Institutional Review Boards at UCLA and the VA Greater Los Angeles Healthcare System.
Clinical Ratings
Brief Psychiatric Rating Scale (BPRS)
For all patients, psychiatric symptoms during the previous month were rated using the expanded 24-item UCLA version of the BPRS (Lukoff, Nuechterlein, & Ventura, 1986; Overall & Gorham, 1962) by a trained rater. Each item is rated on a scale ranging from 1 – 7. Five empirically derived subscales scores were calculated (based on the mean of items comprising the scale (Guy, 1976)).
Scale for the Assessment of Negative Symptoms (SANS)
Negative symptoms during the preceding month were evaluated using the SANS (Andreasen, 1984). Four SANS global scales were used in the current study: Affective flattening, Alogia, Anhedonia-Asociality, and Avolition-Apathy. The SANS Attention scale was not included in the current analyses given findings suggesting that this scale is not conceptually related to the negative symptom complex (Blanchard & Cohen, 2006).
All SCID, BPRS, and SANS interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) based on established procedures (Ventura, Green, Shaner, & Liberman, 1993; Ventura, LIberman, Green, & Shaner, 1998). The process included formal didactics, achieving a minimum level of reliability using an extensive library of videotaped interviews as well as live, co-rated interviews conducted with faculty members. After certification, all raters participated in a continuous quality assurance program that involved periodic reliability checks and co-rated live interview with faculty.
Trait measures
Anhedonia Measures
All participants completed the Revised Social Anhedonia Scale (RSAS; (Eckblad, Chapman, Chapman, & Mishlove, 1982)) and the Physical Anhedonia Scale (PAS; (Chapman & Chapman, 1978)). These scales demonstrate good psychometric properties and have been extensively utilized in schizophrenia (Edell, 1995). For the RSAS, coefficient alphas were .90 for patients and .84 for controls. For the PAS, coefficient alphas were .80 for patients and .84 for controls.
Procedures
Participants performed an affective picture viewing task similar to that described in Cuthbert et al. (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). Twenty pleasant, unpleasant, and neutral pictures were selected from the IAPS (Lang et al., 1999). Local IRB constraints led us to exclude pictures with extremely graphic scenes involving weapons, violence, physical injuries/deformities, or nudity. The IAPS pictures depicted a wide range of social and non-social content, including erotica, people embracing, sports scenes, babies, animals (fierce or cute), upset or ill people, car accidents, pollution, storms, and common household objects1. The three types of pictures differed on mean normative ratings (9-point scales, pleasant high) of valence (pleasant: M = 7.65, [SD = .34]; neutral: 4.92 [.22]; unpleasant: 2.95 [.62]). In addition, the emotional pictures were higher on normative arousal ratings (pleasant: 5.09 [.81]; unpleasant: 5.83 [.78]; neutral: 3.07 [.59]). Each picture was shown in a randomized order and was presented twice, for a total of 40 trials for each valence. Stimuli were presented on a 17” CRT monitor positioned 1 m in front of the participant. Stimuli presentation and synchronization was administered through E-Prime v1.1 (PST Technologies, Pittsburgh, PA).
Each trial began with a 500 ms fixation screen followed by a blank, white screen presented for 300 ms. A picture was then shown for 6 s. The intertrial interval ranged from 8 – 10 s. The total recording session lasted for approximately 30 minutes. Participants were instructed to simply view the pictures.
After the EEG recording session, participants re-viewed the pictures and reported on their emotional experience. Each picture was presented on the screen for a maximum of 10 s. Participants were instructed to view the pictures and press the spacebar on a keyboard when they were ready to make their experience rating; if the participant did not press the spacebar within 10 s the picture ended and the participant was prompted to make their experience rating. The number of timed out trials was low for both patients (M = .86, SE = .28) and controls (M = .31, SE = .29); preliminary analyses revealed no group, picture type, or interaction effects (all F’s < 1.84, p’s > .05). Time to pressing the spacebar was computed as picture viewing time. Separate valence and arousal ratings were collected using a computerized modification of the Self Assessment Manikin (Lang, 1985). Ratings were performed on a 1-9 Likert scale, with scores ranging from very unpleasant/unarousing (a score of 1) to very pleasant/arousing (a score of 9). After the participant made his/her rating, the next picture was presented.
EEG Recording and processing
Participants’ EEG activity was continuously recorded during the picture viewing task. EEG activity was collected using a 64-channel Neuroscan SynAmps2 amplifier and a Neuroscan 64-channel QuickCap (Compumedics USA, Charlotte, NC). Data were sampled at 500 Hz with filter settings of 0 to 100 Hz in DC acquisition mode. 64 cap-mounted, equidistant sintered Ag-AgCl electrodes were positioned in the QuickCap using the 10-20 international placement system. Additionally, four electrodes were used to measure horizontal electrooculogram (EOG; placed on the outer canthus of the left and right eye) and vertical EOG (placed above and below the left eye). All electrodes were referenced to a point halfway between electrodes Cz and CPz and a forehead ground was employed. All electrodes were re-referenced offline to the left and right mastoids.
All data were processed offline using Neuroscan Scan 4.3 software. Eyeblinks were removed from the data using established mathematical procedures (Semlitsch, Anderer, Schuster, & Presslich, 1986). Data were low-pass filtered at 20 Hz and then epoched to 100 ms pre- and 1000 ms post-stimulus. Baseline correction on the 100 ms prior to stimulus presentation was applied. Artifact rejection was performed for any trial that exceeded +/- 100 μV at electrode sites FP1, FPz, FP2, AF3, AF4, F7, F3, Fz, F4, F8, FT7, FCz, FT8, T7, C3, Cz, C4, T8, CPz, P7, P8, P3, Pz, P4, O1 and O2. Participants with more than 50% of the total trials rejected using these criteria were excluded from the analyses. Eleven patients and five controls met these criteria and were not included in the analyses. For the remainder of participants, a mean (SE) of 78.2 (2.0) % of trials were accepted for schizophrenia patients and 82.4 (2.0) % for controls. Preliminary analyses revealed no group, picture type, or interaction effects for number of acceptable trials (all F’s < 2.23, p’s > .05).
Guided by prior studies (e.g., (Cuthbert et al., 2000; Olofsson et al., 2008)), ERP waveforms were created by averaging all accepted trials separately for each picture valence at electrodes F3, Fz, F4, C3, Cz, C4, P3, Pz and P4. Four ERP components were identified (based on visual inspection of the waveforms) in the following time intervals: 1) P1: mean amplitude in the range of 60-120 ms; 2) P2: mean amplitude in the time range of 150-250 ms; 3) P3: mean activity in the time range of 250-500 ms; 4) LPP: mean amplitude in the range of 500-1000 ms.
Data Analysis
For demographic and self-report trait data, group differences for continuous variables were evaluated with t-tests and for categorical variables with chi-square tests. For behavioral data from the picture viewing task, separate 2 (group: patients, controls) × 3 (picture type: pleasant, unpleasant, neutral) ANOVAs were used to examine valence ratings, arousal ratings, and viewing times. Follow-up contrasts for each analysis were Bonferroni-corrected for multiple comparisons. Descriptive data are presented in the text as means with corresponding standard errors.
Analyses of the ERP data were conducted in two phases. The primary analyses focused on the three midline electrodes (Fz, Cz, Pz), which are the sites where ERP responses are typically maximal in this paradigm. For each ERP component (i.e., P1, P2, P3, LPP), a 2 (group) × 3 (picture type: pleasant, unpleasant, neutral) × 3 (caudality: Fz, Cz, Pz) repeated measures ANOVA was run. The secondary analyses examined laterality effects, which have been less consistently identified in this paradigm. These analyses used the six off-mid-line electrode sites. For each ERP component (i.e., P1, P2, P3, LPP), a 2 (group) × 3 (picture type) × 2 (laterality: left [average across F3, C3, P3], right [average across F4, C4, P4]) repeated measures ANOVA was run. For both ERP data analysis phases, Greenhouse-Geisser epsilon corrections were used for repeated-measures analyses with more than one degree of freedom. In these cases, we report the uncorrected degrees of freedom and the corrected p values. Follow-up contrasts for each ERP analysis were Bonferroni-corrected for multiple comparisons. Descriptive data are presented as means with corresponding standard errors. Effect sizes are presented as Cohen’s partial eta squared (ηp2), which corresponds to the following conventions: small (.01), medium, (.06) and large (.14) (J. Cohen, 1988).
Finally, within the schizophrenia group, exploratory correlational analyses examined relations between self-reported trait anhedonia, negative symptoms, and the behavioral and ERP variables from the picture viewing task with Pearson correlation coefficients. From the picture viewing task, the nine behavioral variables (valence ratings, arousal ratings, and viewing time for each picture type) were included. In addition, 12 ERP variables derived from the primary analyses that focused on midline electrodes were included (i.e., average across electrodes Pz, Cz, Fz for pleasant, unpleasant, and neutral picture types for P1, P2, P3, and LPP). All correlation analyses used a two-tailed significance level of 0.05.
Results
Group characteristics
Demographic information and trait anhedonia scores for both groups and symptom ratings for the schizophrenia patients are presented in Table 1. The groups did not significantly differ in sex composition, marital status, or parental education level. However, the patients were older and had higher education levels than controls. (This project attempted to match subjects on parental education, not personal education). On the trait measures, patients reported higher social and physical anhedonia than controls. Finally, the patients had a typical age of onset and showed mild to moderate levels of clinical symptoms at the time of testing.
Table 1.
Demographic, Trait, and Clinical information
| Patients | Controls | Statistic | |
|---|---|---|---|
| Sex (% male) | 81.6 | 74.3 | χ2 (1,73) = .45 |
| Age (SD) | 44.5 (10.6) | 38.5 (10.3) | t(72) = 2.44* |
| Marital status | |||
| Never married | 60.5 | 71.4 | χ2 (2, 73) = 3.21 |
| Currently married | 5.3 | 11.4 | |
| Ever married | 34.2 | 17.1 | |
| Education (SD) | 12.8 (1.5) | 14.7 (1.5) | t(72) = -5.43*** |
| Parental education (SD) | 14.7 (3.3) | 14.2 (2.5) | t(72) =.67 |
| Physical Anhedonia Scale (SD) | 19.1 (9.4) | 9.8 (6.0) | t(72) = 5.04*** |
| Social Anhedonia Scale (SD) | 17.0 (8.7) | 8.0 (5.2) | t(72) = 5.32*** |
| Age of onset (SD) | 21.6 (10.4) | ||
| BPRS | |||
| Thought Disturbance (SD) | 2.7 (1.3) | ||
| Anxiety/Depression (SD) | 2.5 (0.9) | ||
| Anergia (SD) | 2.1 (0.8) | ||
| Hostility (SD) | 1.8 (0.8) | ||
| Activation (SD) | 1.2 (0.4) | ||
| SANS | |||
| Affective Flattening (SD) | 2.2 (1.4) | ||
| Alogia (SD) | 1.0 (1.2) | ||
| Avolition (SD) | 3.1 (1.1) | ||
| Anhedonia (SD) | 2.9 (1.4) |
Notes: Means are presented with accompanying SD ’s.
p < .05;
p < .001
In light of group differences in age and education, preliminary analyses examined whether these demographic characteristics significantly correlated with the nine behavioral variables or the 12 primary ERP variables from the picture viewing paradigm within each group. For the behavioral data across groups, one of the 18 correlations was significant in the patients (neutral picture viewing times correlated -.33 with age [p < .05]) and two of the 18 were significant in controls (arousal ratings for unpleasant pictures correlated -.39 with age and .36 with education [p’s < .05]). For ERP data across groups, one of the 24 correlations was significant in the patients (LPP in the unpleasant condition correlated .34 with education [p < .05] and two of the 24 correlations were significant in the controls (age correlated .46 [p < .01] with P2 in the pleasant condition and education correlate .37 [p < .05] with P1 in the neutral condition). Given these generally negligible relations and the fact that the groups did not differ in parental education, age and education were not considered further in the analyses.
Picture viewing paradigm
Behavioral data
Descriptive information and results of statistical tests are presented in Table 2. For valence ratings, there was a significant main effect of valence but no group or interaction effects. Across groups, there were differences between each valence condition, with mean valence ratings highest for pleasant pictures, followed by neutral and unpleasant pictures. For arousal, there were significant effects for valence and for the valence × group interaction, but a non-significant group effect. Controls reported higher arousal experience in response to pleasant pictures than unpleasant pictures, and higher arousal in response to unpleasant pictures than neutral pictures. However, patients reported higher arousal experience in response to pleasant pictures than to unpleasant and neutral pictures, but patients did not report higher arousal in response to unpleasant compared to neutral pictures. For viewing time, there was a significant valence effect but no significant group or interaction effects. Across groups, there were differences between each condition, with viewing times longest for unpleasant pictures, intermediate for pleasant pictures, and shortest for neutral pictures.
Table 2.
Descriptive Information and F Values for Group × Picture Type ANOVA’s for Behavioral Data During the Picture Viewing Task
| Patients |
Controls |
F value [ηp2] |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pleasant | Unpleasant | Neutral | Pleasant | Unpleasant | Neutral | Group (df = 1,72) | Picture type (df = 2,144) | Group × Type (df = 2,144) | |
| Valence | |||||||||
| M | 7.16 | 2.41 | 5.14 | 7.15 | 2.57 | 5.05 | .02 [.001] | 767.67*** [.92] | .53 [.007] |
| SE | (.16) | (.11) | (.12) | (.16) | (.11) | (.12) | |||
| Arousal | |||||||||
| M | 6.81 | 5.12 | 4.82 | 6.57 | 4.52 | 5.73 | .02 [.000] | 35.15*** [.33] | 5.66** [.07] |
| SE | (.17) | (.15) | (.34) | (.17) | (.15) | (.34) | |||
| Viewing time | |||||||||
| M | 3.28 | 2.81 | 3.66 | 2.68 | 2.33 | 3.23 | 1.08 [.015] | 13.59*** [16] | .15 [.002] |
| SE | (.38) | (.32) | (.40) | (.27) | (.23) | (.32) | |||
Notes:
p < .01;
p < .001
ERP Analyses
Midline electrodes
Figure 1 shows the overall waveforms for each valence at each midline electrode for both groups. Results of the 2 (group) × 3 (picture type) × 3 (caudality: Fz, Cz, Pz) repeated measures ANOVA’s for each ERP component (P1, P2, P3, LPP) are summarized in Table 3. As detailed in the following sections, there were no significant group main effects or interactions involving group except for the late-latency LPP component.
Figure 1.
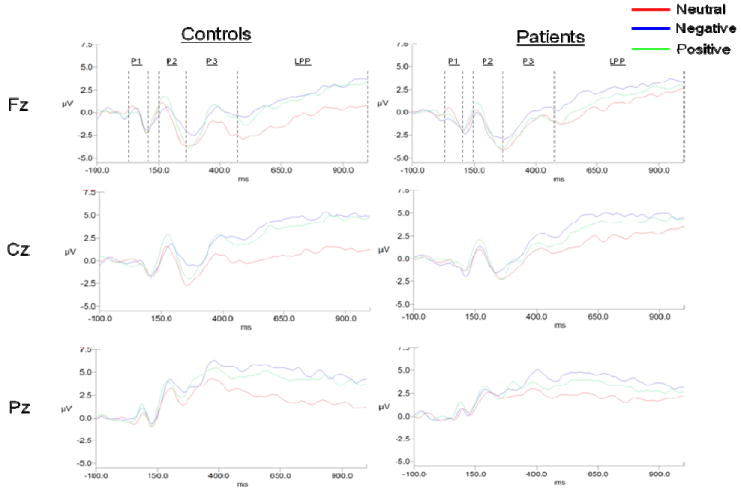
Overall waveforms for each valence at each midline electrode for control and schizophrenia patient groups.
Table 3.
F Values and [Partial Eta Squared] from Group × Valence × Caudality ANOVA’s for ERP’s at Mid-line Electrodes During the Picture Viewing Task
| P1 | P2 | P3 | LPP | |
|---|---|---|---|---|
| Valence (df = 2,72) | .42 [.006] | 3.76* [.050] | 12.54*** [.15] | 22.22*** [.238] |
| Caudality (df = 2,72) | 37.90*** [.345] | 58.00*** [.446] | 86.12*** [.545] | 23.77*** [.248] |
| Group (df = 1,72) | .11 [.002] | 1.81 [.025] | .68 [.009] | .08 [.001] |
| Valence × Caudality (df = 4,72) | 2.91* [.039] | .57 [.008] | .94 [.013] | 2.27† [.031] |
| Valence × Group (df = 2,72) | .58 [.008] | 1.03 [.014] | 1.87 [.025] | 3.28* [.044] |
| Caudality × Group (df = 2,72) | .12 [.002] | .14 [.002] | 1.93 [.026] | 2.99† [.040] |
| Valence × Caudality × Group (df = 4,72) | .42 [.006] | .67 [.009] | .42 [.006] | 1.74 [.024] |
Notes:
p < .05;
p < .01;
p < .001;
= p < .10
P1
There was a significant main effect for caudality, as well as a significant valence × caudality interaction. Follow-up comparisons for the caudality effect revealed larger amplitude responses at Pz (.35 [SE = .19]) than at Cz (-1.00 [.18]) and Fz (-1.22 [.22]), which did not differ from each other. The picture type × caudality interaction was due to the following: for the pleasant and unpleasant conditions, the amplitude at Pz was greater than Fz; for the neutral condition, the amplitude at Pz was greater than both Cz and Fz.
P2
There were significant main effects for valence and caudality. For the valence effect, P2 was significantly larger in the pleasant (1.05 [0.32]) than the neutral (0.43 [0.33]) condition, while neither differed from the negative condition (0.81 [0.32]). The caudality effect revealed significant differences among all three regions with amplitudes largest at Pz (2.68 [.30]), intermediate at Cz (.31 [.31]) and smallest at Fz (-.87 [.39]).
P3
There were also significant valence and caudality main effects for P3. For the valence effect, activity during the pleasant (1.14 [0.46]) and unpleasant (1.59 [0.46]) conditions, which did not differ from each other, were both significantly larger than the neutral (0.21 [0.45]) condition. The caudality effect revealed differences among all three regions, with amplitudes largest for Pz (3.65 [.39]), intermediate for Cz (.78 [.51]), and smallest for Fz (-1.49 [.54]).
LPP
There were significant main effects of valence and caudality, as well as a significant valence × group interaction. For the valence effect, the amplitude was larger during the pleasant (3.06 [0.47]) and unpleasant (3.72 [0.46]) conditions than the neutral condition (1.44 [0.38]). The caudality effect reflected significantly greater activity at Cz (3.53 [.45]) and Pz (3.42 [.36]) than at Fz (1.28 [.51]).
The valence × group effect is displayed graphically in Figure 2. The significant interaction was due to the following: within the control group, LPP’s were larger for the pleasant and unpleasant pictures as compared to the neutral pictures; within the patient group, LPP’s were larger for the unpleasant than the neutral pictures, but LPP’s during the pleasant and neutral pictures did not differ. However, there were no significant between-group LPP differences for pleasant, unpleasant, or neutral pictures (all t’s < 1.05, p’s > .05).
Figure 2.
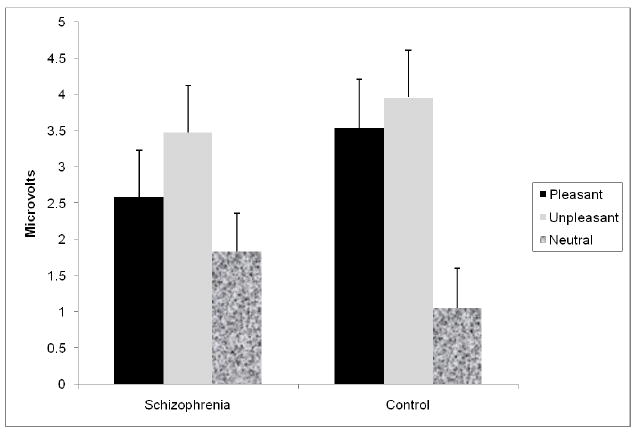
Group × valence interaction for mean amplitude LPP (+SE) across midline electrodes.
Since each IAPS picture was presented twice, a supplemental analysis examined whether the apparent group differences in LPP might be attributable to differential group LPP responses to the first picture block as compared to the second picture block. The analyses were re-run with picture block (1 vs. 2) as a factor in the repeated measures ANOVA. There was a significant overall block effect, F(1,72) = 4.89, p < .05, ηp2 = .06), with LPPs smaller for block 1 (M = 2.43 SE = .45) than block 2 (M = 3.13, SE = .39). However, there were no significant higher-order interactions between picture block and either picture type or group (all F’s < 3.86; p’s < .05, ηp2 < .02), indicating that the group differences in LPP were not attributable to differential group responses across picture blocks.
In summary, both groups demonstrated a similar pattern for midline P1, P2, and P3 responses, including generally maximal responses at electrode Pz, larger P2 amplitudes for pleasant than neutral pictures, and larger P3 amplitudes for pleasant and unpleasant than neutral pictures. However, the groups differed for the latest ERP component. Whereas controls showed larger LPP amplitudes for both pleasant and unpleasant than neutral pictures, patients showed larger LPP amplitudes to unpleasant, but not pleasant, pictures compared to neutral pictures.
Laterality analyses
Results of the 2 (group) × 3 (valence) × 2 (laterality: left, right) ANOVA’s for each ERP component (P1, P2, P3, LPP) are summarized in Table 4. There were no significant group main effects or interactions with group except for the LPP, consistent with midline electrode analyses.
Table 4.
F Values and [Partial Eta Squared] from Group × Valence × Laterality ANOVA’s for ERP’s at Left and Right Hemisphere Electrodes During the Picture Viewing Task
| P1 | P2 | P3 | LPP | |
|---|---|---|---|---|
| Valence (df = 2,72) | .50 [.007] | 4.61* [.060] | 13.04*** [.153] | 20.57*** [.222] |
| Laterality (df =1,72) | 5.62* [.070] | 4.14* [.054] | 7.87*** [.098] | .01 [.000] |
| Group (df =1,72) | .48 [.007] | 2.01 [.027] | .36 [.005] | .12 [.002] |
| Valence × Laterality (df = 2,72) | 2.73† [.037] | 1.36 [.019] | 5.82*** [.075] | 12.06*** [.143] |
| Valence × Group (df = 2,72) | 1.04 [.014] | 1.00 [.014] | 1.54 [.021] | 2.95† [.039] |
| Valence × Laterality × Group (df = 2,72) | .55 [.008] | .74 [.010] | 2.24 [.030] | 3.14* [.042] |
Notes:
p < .05;
p < .01;
p < .001;
= p < .10
P1
The significant laterality main effect reflected larger amplitude responses in the right (-.13 [.14]) than the left (-.37 [.14]) hemisphere.
P2
The laterality main effect reflected larger responses in the right (1.00 [.25]) than left (.75 [.23]) hemisphere. The valence main effect reflected larger amplitude responses in the pleasant (1.12 [.26]) and unpleasant (.95 [.25]) conditions than the neutral condition (.54 [.27])
P3
The laterality main effect reflected larger responses in the right (1.29 [.36]) than left (.86 [.32]) hemisphere. The valence main effect indicated P3’s were larger in the pleasant (1.19 [.37]) and unpleasant (1.62 [.37]) conditions than the neutral condition (.42 [.35]). The valence × laterality effect was due to the following: for the unpleasant condition, right amplitudes were greater than left; for pleasant there was trend in the same direction; for neutral there was no difference.
LPP
The valence main effect again reflected larger responses for the pleasant (2.57 [.40]) and unpleasant (3.15 [.42]) conditions than the neutral condition (1.30 [.32]). The valence × laterality interaction was embedded in a three-way interaction involving group, which is presented graphically in Figure 3. The interaction was due to the following: for the pleasant condition, controls demonstrated greater left than right responses but patients showed no laterality difference; for unpleasant and neutral, there were no laterality differences in either group.
Figure 3.
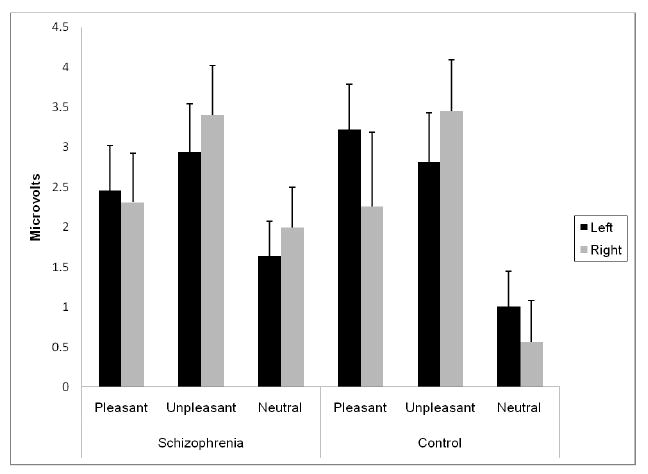
Group × valence × laterality interaction for mean amplitude LPP (+ SE) for left and right hemisphere electrodes.
Thus, in line with analyses of the midline electrodes, the laterality analyses indicated a disturbance in schizophrenia only for LPP, the latest ERP component examined. Specifically, patients failed to show the lateralized responding to pleasant pictures (left > right) that was present for controls.
Correlational analyses within the schizophrenia group
Within the schizophrenia group, exploratory analyses examined correlations between the trait anhedonia scales and SANS negative symptoms on the one hand, and the behavioral and ERP variables from the picture viewing task on the other. We were particularly interested in whether trait anhedonia and clinically rated negative symptoms correlated with valence ratings and ERPs in the pleasant condition. Only a few of the large number of correlations were statistically significant (and medium in magnitude).
Table 5 summarizes the main results for correlations with behavioral data from the picture viewing task. Higher trait physical anhedonia was correlated with less pleasant valence ratings for pleasant and neutral pictures, and with lower arousal ratings for unpleasant pictures. Higher trait social anhedonia was also correlated with less pleasant valence ratings for pleasant pictures. For SANS ratings, higher Anhedonia/Asociality and Alogia were related to longer viewing times for pleasant and unpleasant pictures.
Table 5.
Correlations Among Trait Anhedonia, Negative Symptoms, and Behavioral Data from the Picture Viewing Task Within the Schizophrenia Group
| Social Anhedonia Scale | Physical Anhedonia Scale | SANS Anhedonia/Asociality | SANS Alogia | SANS Affective flattening | SANS Avolition/Apathy | |
|---|---|---|---|---|---|---|
| Valence ratings | ||||||
| Pleasant | -0.32* | -0.35* | -0.29 | -0.18 | -0.25 | 0.04 |
| Unpleasant | 0.00 | -0.21 | 0.04 | 0.28 | 0.19 | 0.15 |
| Neutral | -0.13 | -0.33* | -0.12 | 0.18 | 0.20 | 0.09 |
| Arousal ratings | ||||||
| Pleasant | -0.16 | -0.22 | -0.29 | -0.18 | -0.13 | -0.08 |
| Unpleasant | -0.18 | -0.34* | -0.12 | 0.06 | 0.11 | -0.02 |
| Neutral | -0.08 | -0.22 | 0.14 | 0.31 | 0.31 | 0.49*** |
| Viewing times | ||||||
| Pleasant | -0.04 | -0.11 | 0.35* | 0.44** | 0.21 | 0.24 |
| Unpleasant | 0.14 | 0.12 | 0.39* | 0.34* | 0.09 | 0.22 |
| Neutral | -0.02 | -0.09 | 0.30 | 0.29 | 0.10 | 0.17 |
Notes: SANS = Scale for the Assessment of Negative Symptoms.
p < .05;
p < .01;
p < .005.
Correlations with ERP variables indicated that trait physical anhedonia correlated only with smaller P2 responses during the unpleasant condition (-.35). Scores on the trait social anhedonia and SANS subscales were not significantly correlated with ERP variables
Discussion
This study evaluated competing hypotheses about emotional responding in schizophrenia using a well-validated ERP picture viewing paradigm that permits a fine-grained analysis of the temporal course of emotional responding. Patients demonstrated a pattern of intact behavioral responses (i.e., reported experience, viewing time) and initial ERPs. Despite reporting elevated trait social and physical anhedonia, patients demonstrated valence experience ratings and P1, P2, and P3 responses that were similar to controls for emotional versus neutral stimuli. These similarities indicate that when patients are exposed to evocative stimuli, their self-reported experience and initial electrocortical processing are intact. However, the patients’ neural response to pleasant stimuli appears to go awry rather quickly, as they displayed diminished differentiation between pleasant versus neutral stimuli during the late-latency (500 – 1000 ms) LPP interval. This difference suggests a disturbance in the time course of responding to pleasant stimuli in schizophrenia.
Behavioral data
The similarity in valence experience ratings between patients and controls converges with many prior studies demonstrating comparable emotional experience during exposure to evocative stimuli in schizophrenia (A. S. Cohen & Minor, in press; Kring & Moran, 2008). Although schizophrenia patients consistently report diminished positive affect (particularly anhedonia) and elevated negative affect on trait measures (Horan, Blanchard et al., 2008), they do not report hypo- or hyper-responsivity when directly exposed to pleasant and unpleasant stimuli, respectively. Within the schizophrenia group, self-reported and clinically-rated anhedonia (and other negative symptoms) showed generally small correlations with valence ratings, as has been previously reported (Horan, Blanchard et al., 2008).
The patients were also similar to controls in terms of their viewing times of the IAPS stimuli, with both groups viewing emotional pictures longer than neutral pictures. We are not aware of prior studies that examined picture viewing times in schizophrenia, and hence, do not have a basis for comparison. Longer viewing time for emotional pictures has been interpreted to indicate that participants find emotional pictures to be more interesting than neutral pictures (Cuthbert, Bradley, & Lang, 1996; Lang, Greenwald, Bradley, & Hamm, 1993). Alternatively, this may simply indicate that it takes longer to identify and rate the feelings that emotional picture elicit. Regarding clinical correlates, it is interesting that higher levels of clinically-rated anhedonia and alogia correlated with longer viewing times for pleasant and unpleasant pictures. These findings, if replicated, support the notion that patients with these negative symptoms engage in processing emotional stimuli when they are directly confronted with such stimuli, even more so that patients with fewer negative symptoms. Speculatively, these relations might reflect factors such as unusual scanning of visual stimuli or difficulty in labeling emotional valence (e.g. alexithymia), both of which have been reported in schizophrenia (Quirk & Strauss, 2001; van ’t Wout, Aleman, Bermond, & Kahn, 2007).
ERP’s
The current study provides new evidence on the time course of emotional response in schizophrenia by showing for the first time that the initial neural processing of evocative stimuli, as reflected in the P1, P2, and P3 ERP’s, is also intact in schizophrenia. ERP methods, which provide temporal resolution on the order of milliseconds, are particularly well-suited to investigate the early stages of neural responding to emotional stimuli. Neither group demonstrated valence-specific amplitude differences in the early-latency P1, which is sensitive to physical stimulus factors and indexes early sensory processing (particularly within extrastriate visual cortex). However, in line with prior studies, the middle-latency P2 and P3 ERP’s demonstrated sensitivity to valence in both groups. According to Lang’s motivated attention framework (Bradley & Lang, 2007), the P2 response reflects early stimulus discrimination and response selection processes, with the enhanced response to emotional pictures reflecting a relatively automatic attentional capture by emotionally arousing stimuli. The enhanced P3 amplitude to emotional stimuli is thought to reflect greater allocation of attentional resources to emotionally relevant stimuli. As with the valence ratings, there was no evidence of hypo- or hyper-responsivity to pleasant or unpleasant stimuli in the schizophrenia group among these three ERP components. The topography of P1, P2, and P3 responses was also similar across groups, with maximal responses in central and parietal regions, and generally larger responses in the right than left hemisphere. Overall, the ERP data for these components suggest that patients’ reports of pleasure and displeasure experience are accompanied by normal early stimulus processing, attentional capture, and initial resource allocation processes in response to emotional stimuli.
Despite these similarities in initial processing, the patients differed from controls in terms of valence-related amplitude differences and topography during the late-latency LPP interval. As in prior studies (Olofsson et al., 2008), controls showed clearly enhanced LPP amplitudes for pleasant and unpleasant as compared to neutral stimuli. However, the patients showed significantly less LPP differentiation between pleasant versus neutral stimuli. Theoretically, LPP enhancement is believed to index sustained attentional processing of motivationally relevant stimuli (Bradley & Lang, 2007). For example, a recent study in healthy people used a passive picture viewing task and found that heightened LPP for emotional stimuli persisted 800 – 1000 msec after picture offset, suggesting that the LPP was associated with a natural inclination to continue processing the stimulus even when it was no longer displayed (Hajcak & Olvet, 2008). Hence, the pattern of results for LPP suggests a disturbance in sustained attentional processing of pleasant stimuli in schizophrenia.
Secondary analyses indicated that there was also a group difference in laterality during the LPP interval. Since laterality effects have not been consistently reported in ERP picture viewing paradigms, these findings should be interpreted cautiously. Controls demonstrated greater left than right hemisphere responses for pleasant stimuli whereas patients did not demonstrate any laterality effect. Similar laterally effects have only occasionally been reported (e.g., (Cunningham et al., 2005; Dolcos & Cabeza, 2002)). Although not a common finding, the left > right asymmetry of LPP in controls is consistent with neuropsychological models that propose relative left hemisphere specialization for pleasant emotion/approach motivational processing (particularly in frontal regions; (Davidson & Irwin, 1999; Heller, Nitschke, & Miller, 1998)). In addition, the absence of such laterality in schizophrenia patients is consistent with a wide variety of laterality studies (Green, Sergi, & Kern, 2003).
Drawing on the affective neuroscience literature, the schizophrenia patients’ pattern of intact ERP’s during initial processing stages but diminished differentiation between pleasant versus neutral stimuli during the LPP points toward a disturbance in “affective chronometry”. Affective chronometry refers to parameters that vary over the time course of emotional responding, including the rise time to peak magnitude and duration of emotion response following emotional provocation (Davidson, 1998). The task used in this study is particularly well suited to studying affective chronometry. The earlier components involve relatively more stimulus-driven processes, and the later components involve relatively more cognitive evaluation and controlled resources. Consistent with this view, recent studies have shown that these later ERP components (>500 ms) are susceptible to top-down attentional and emotion regulation influences (Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006; Moser, Hajcak, Bukay, & Simons, 2006). In schizophrenia, two preliminary reports also support the current study’s findings. In one study, schizophrenia patients showed the same linear modulation of the eyeblink component of the startle reflex as did healthy controls while viewing emotional pictures, with responses biggest to unpleasant pictures and smallest to pleasant pictures. However, controls maintained this pattern of responding 2.5 s after the picture was removed from view, whereas schizophrenia patients did not (Kring, Germans-Gard, & Gard, in preparation). In a second study, schizophrenia patients and healthy controls showed comparable BOLD responses in orbitofrontal cortex (OFC) to pleasant versus neutral pictures during picture viewing. However, healthy controls demonstrated sustained activation in OFC as well as in dorsolateral prefrontal cortex (DLPFC) once the picture was removed from view up to 12.5 s later, but schizophrenia patients failed to do so (Ursu et al., in preparation). Taken together, these findings support intact in-the-moment responses among people with schizophrenia but a disturbance in sustained emotional responding.
The current findings are broadly consistent with prior studies demonstrating disconnects among emotional response components in schizophrenia. For example, it is well documented that individuals with schizophrenia are markedly less expressive than healthy controls, yet do not differ as much, or as consistently, with respect to reported emotional experience or autonomic physiology (see (Kring & Moran, 2008)). In line with the current study, a handful of ERP and fMRI studies indicate that normal self-reported emotional responses to evocative stimuli are not accompanied by fully normal central nervous system responses (e.g., (Crespo-Facorro et al., 2001; Morris, Heerey, Gold, & Holroyd, 2008; Paradiso et al., 2003; Waltz et al., 2009). Theoretically, coherence among emotional response components is believed to facilitate adaptive responses to environmental demands, although basic affective science research indicates that relations among components are complex (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005; Mauss & Robinson, 2009). The construct of “emotion” cannot be captured with any one measure alone, and additional studies that assess multiple components will help clarify emotional response abnormalities in schizophrenia.
The current findings also converge with studies demonstrating that clinical ratings of negative symptoms are only weakly or non-significantly related to laboratory measures and self-report trait measures of emotional responding in schizophrenia. In looking at emotional responding in schizophrenia, one gets different answers when one uses different methods. For example, studies using affective neuroscience paradigms in schizophrenia challenge longstanding clinical perspectives on negative symptoms. In contrast to a historical definition that has viewed anhedonia as a global incapacity to experience pleasure, studies from affective neuroscience reliably indicate that some aspects of emotional responding to pleasant stimuli are intact (Gold, Waltz, Prentice, Morris, & Heerey, 2008; Kring & Moran, 2008). There is a consensus that new clinical rating scales of negative symptoms in schizophrenia that are informed by affective neuroscience are needed for making progress in understanding and treating negative symptoms (Kirkpatrick et al., 2006). A recently funded NIMH multi-site study “Collaboration to Advance Negative Symptom Assessment in Schizophrenia” is designed to develop a new assessment instrument that addresses such conceptual limitations of current available rating scales.
Additional considerations and implications
Some methodological issues and sample limitations should be considered. The range of arousal reflected in the stimuli used in this study is somewhat truncated compared to most prior studies due to the exclusion of some of the more highly arousing IAPS pictures. While the selected pictures might more closely approximate the emotional experiences that patients are likely to encounter in their daily lives, it possible that a different pattern of responses would be found for more highly arousing stimuli. Along these lines, there was an unexpected between-group difference in arousal ratings; controls reported higher arousal for unpleasant versus neutral pictures but patients did not. Among the few prior studies that examined arousal ratings for IAPS stimuli in schizophrenia, most found normal arousal ratings (see (Herbener, Song, Khine, & Sweeney, 2008)). Although Burbridge and Barch (Burbridge & Barch, 2007) also found lower arousal ratings for unpleasant stimuli, this finding should be interpreted with caution and further evaluated. Studies that assess both self-report and psychophysiology (e.g., heart rate, skin conductance) could shed light on this issue. An additional limitation concerns the correlational analyses with symptoms and trait anhedonia. Only a few of the many correlations were significant, which could reflect a Type 1 error. As noted above, we regard these as exploratory analyses that require replication.
Regarding sample characteristics, the patients were taking various antipsychotic medications at clinically determined dosages. The effects of antipsychotic medications on emotional experience are not clear, although evidence suggests such effects are minimal (Berenbaum & Oltmanns, 1992; Kring & Earnst, 1999; Kring & Neale, 1996). In addition, the patients were older than controls, though age showed generally negligible correlations with the behavioral and ERP variables. Finally, participants were predominantly male, which precluded a meaningful examination of potential sex differences in emotional responding.
Clinically, patients’ normal self-reported experiences of pleasure may be regarded as a relative strength to build upon in psychosocial interventions to address negative symptoms. For example, it may be useful to encourage patients to attend to their feelings of pleasure, providing a basis for engaging in increasingly complex behavioral activation exercises and building personal resources (Beck, Rector, Stolar, & Grant, 2009; Fredrickson, Cohn, Coffey, Pek, & Finkel, 2008). If maintaining positive emotions over time is a difficulty, interventions aimed at building skills to sustain positive feelings may be useful (e.g., (Johnson et al., 2009)). In addition, building skills aimed at emotion identification and differentiation among emotional states (Barrett, Gross, Christensen, & Benevenuto, 2001; Horan et al., 2009) will also be important for psychosocial interventions for negative symptoms.
The ability to sufficiently sustain emotional processing over time is critical for guiding future behavioral choices, and failure to sufficiently process motivationally relevant stimuli could have maladaptive functional consequences for people with schizophrenia. For example, sustained processing reflected in the LPP has been linked to enhanced encoding and retention of motivationally relevant stimuli (e.g., (Dolcos & Cabeza, 2002)). Diminished attentional processing reflected in the LPP could help explain recent findings that schizophrenia patients fail to show the typical memory enhancements for emotional stimuli, particularly over longer delay intervals (Herbener, 2008). Suboptimal encoding of emotional stimuli could also contribute to documented deficits in the ability to anticipate future emotional experiences based on past experiences and to effectively use emotional feedback to guide learning and decision making (Gard et al., 2007; Gold et al., 2008; Horan, Green, Knowlton, Wynn, & Nuechterlein, 2008). Further research using ERP methods can help clarify the potential contribution of disturbances in encoding and maintaining emotions to the hedonic deficit of schizophrenia.
Acknowledgments
This research was supported by Research Grants MH077141 (to WPH), MH43292 and MH65707 (to MFG) from the National Institute of Mental Health, and by the Department of Veterans Affairs, Veterans Integrated Service Network 22, Mental Illness Research Education and Clinical Center. We thank Bruce Cuthbert and Michelle Roth for assistance with the picture rating program.
Footnotes
The following IAPS pictures were used: Pleasant: 1340, 1710, 2040, 2070, 2071, 2150, 2165, 2170, 2208, 2260, 2299, 2345, 4622, 5470, 8370, 8380, 8470, 8496, 8497, 8502; Unpleasant: 1050, 1052, 1120, 1205, 1220, 1525, 1930, 2095, 2750, 3160, 5971, 9300, 9341, 9342, 9495, 9520, 9600, 9620, 9910, 9911; Neutral: 2191, 2200, 2214, 2381, 2393, 2440, 2480, 2487, 2595, 2880, 7002, 7009, 7010, 7020, 7025, 7030, 7040, 7211, 7233.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
Contributor Information
William P. Horan, VA Greater Los Angeles Healthcare System, University of California, Los Angeles
Jonathan K. Wynn, VA Greater Los Angeles Healthcare System, University of California, Los Angeles
Ann M. Kring, University of California, Berkeley
Robert F. Simons, University of Delaware
Michael F. Green, University of California, Los Angeles VA, Greater Los Angeles Healthcare System
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Barrett LF, Gross JJ, Christensen TC, Benevenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition and Emotion. 2001;15:713–724. [Google Scholar]
- Beck AT, Rector NA, Stolar N, Grant P. Schizophrenia: Cognitive Theory, Research, and Therapy. New York: The Guilford Press; 2009. [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116(1):30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Revised Physical Anhedonia Scale. University of Wisconsin; Madison: 1978. [Google Scholar]
- Cohen AS, Minor KS. Emotional Experience in Patients with Schizophrenia Re-revisited: Meta-analysis of Laboratory Studies. Schizophrenia Bulletin. doi: 10.1093/schbul/sbn061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. Jama. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Espinet SD, DeYoung CG, Zelazo PD. Attitudes to the right- and left-: Frontal ERP assymmetries associated with stimulus valence and processing goals. Neuroimage. 2005;4:827–834. doi: 10.1016/j.neuroimage.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annu Rev Psychol. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. University of Wisconsin; Madison: 1982. [Google Scholar]
- Edell WS. The psychometric measurement of schizotypy using the Wisconsin Scales of Psychosis Proneness. In: Miller GA, editor. The Behavioral High-Risk Paradigm in Psychopathology. New York: Springer-Verlag; 1995. pp. 3–46. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition. New York: Biometrics Research; 1996. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95(5):1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Germans-Gard M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Personality. 2006;40:1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1-3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Green MF, Sergi MJ, Kern RS. The laterality of Schizophrenia. In: Hugdal K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge: MIT Press; 2003. pp. 743–772. [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8(2):250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Miller GA. Lateralization in emotion and emotional disorders. Current Directions in Psychological Science. 1998;7:26–32. [Google Scholar]
- Herbener ES. Emotional memory in schizophrenia. Schizophr Bull. 2008;34(5):875–887. doi: 10.1093/schbul/sbn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES, Song W, Khine TT, Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophrenia Research. 2008;98:239–246. doi: 10.1016/j.schres.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin. 2008;34:856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Kring AM. Anhedonia in schizophrenia: A review of assessment strategies. Schizophrenia Bulletin. 2006;32(2):359–373. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Knowlton B, Wynn JK, Nuechterlein KH. Implicit Cognitive Learning in Schizophrenia. Neuropsychology. 2008;22:606–615. doi: 10.1037/a0012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107(1):47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Johnson DJ, Penn DL, Fredrickson BL, Meyer PS, Kring AM, Brantley M. Loving-kindness meditation to enhance recovery from negative symptoms of schizophrenia. Journal of Clinical Psychology. 2009;65:499–509. doi: 10.1002/jclp.20591. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH- MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and affect deficit states. New York: PMA Publishing; 1984. [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kring AM. Emotion in schizophrenia: Old mystery, new understanding. Current Directions in Psychological Science. 1999;8:160–163. [Google Scholar]
- Kring AM, Earnst KS. Stability of emotional responding in schizophrenia. Behavior Therapy. 1999;30:373–388. [Google Scholar]
- Kring AM, Germans-Gard MK, Gard DE. Deficits in the time course of emotion in schizophrenia. in preparation. [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The Cognitive Psychophysiology of Emotion: Anxiety and the Anxiety Disorders. Hillside, NJ: Lawrence Erlbaum; 1985. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4, The Center for Research in Psychophysiology. University of Florida; 1999. International Affective Picture System: Instruction manual and affective ratings. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded Brief Psychiatric Rating Scale. Schizophrenia Bulletin. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: a review. Cognition & Motivation. 2009;23:209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99(1-3):274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43(3):292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160(10):1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Quirk SW, Strauss ME. Visual exploration of emotion eliciting images by patients with schizophrenia. J Nerv Ment Dis. 2001;189(11):757–765. doi: 10.1097/00005053-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Germans-Gard MK, Watrous AJ, Firl A, Minzenberg MJ, et al. Altered affective processing in schizophrenia: The role of cognition-emotion interactions. in preparation. [Google Scholar]
- van ’t Wout M, Aleman A, Bermond B, Kahn RS. No words for feelings: alexithymia in schizophrenia patients and first-degree relatives. Compr Psychiatry. 2007;48(1):27–33. doi: 10.1016/j.comppsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: ‘The Drift Busters’. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Ventura J, LIberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Wager TD, Feldman Barrett L, Bliss-Moreau L, Lindquist K, Duncan S, Kober H, et al. The Neuroimaging of Emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3. New York: Guilford Press; 2008. [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34(6):1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophr Bull. 2008;34(4):679–687. doi: 10.1093/schbul/sbn047. [DOI] [PMC free article] [PubMed] [Google Scholar]


