Abstract
Epstein-Barr virus has been implicated in the etiology of systemic lupus erythematosus (SLE) through serologic and immunologic studies. A potential mechanism for this influence is through molecular mimicry. The EBV nuclear antigen EBNA-1 contains a region, PPPGRRP, with considerable homology to the initial sequence targeted by antibodies in Sm B’ autoimmunity, PPPGMRPP. This study examined whether immunization of rabbits and mice with peptides containing the PPPGRRP sequence from EBNA-1 constructed on a poly-lysine backbone was able to drive the development of autoantibodies against the Smith antigen (Sm) and the related antigenic complex, the U1 nuclear ribonucleoproteins (nRNP). PPPGRRP immunization, and immunization with an EBNA-1 fragment containing PPPGRRP, led to autoantibodies in both rabbits and mice at high frequency (83% of rabbits and 43% of mice). Five out of six immunized rabbits developed either leucopenia or lymphopenia or both. The fine specificity of antibody binding against the lupus-associated autoantigens Sm B’, nRNP A, and nRNP C after immunization with the EBNA-1-derived peptides was very similar to the early antibody binding patterns against these proteins in human SLE. This similarity, as well as the prevalence of autoimmunity after immunization with these peptides, identifies PPPGRRP as a strong candidate for molecular mimicry in SLE etiology.
Keywords: autoantibodies, molecular mimicry, systemic lupus erythematosus, Epstein-Barr virus
1. Introduction
Systemic lupus erythematosus (SLE) is a prototypic systemic autoimmune disease characterized by the presence of autoantibodies against cellular antigens. Antibodies against the spliceosomal component Smith antigen (Sm) are found nearly exclusively in SLE patient sera [1, 2]. Previous work in our laboratory showed that during the development of anti-Sm B’ in SLE, autoantibodies often first target a repeated, proline-rich motif, PPPGMRPP [3–5]. This epitope occurs three times within the carboxyl-terminus of SmB’ with another similar sequence, PPPGMRPGP also found in the carboxyl-terminus.
Antibody specificity then diversifies through a process of epitope spreading to include other humoral targets of Sm B’, as well as other SLE autoantigens [6–9]. Epitope spreading is also seen in SLE for multiple other antigen systems [10–13]. Immunization of mice and rabbits with synthetic PPPGMRPP peptides constructed on a poly-lysine backbone (MAP™) led to autoantibodies against Sm B’, Sm D, nRNP A, nRNP C and dsDNA. The immunized animals also variably developed lupus-like clinical symptoms, including thrombocytopenia, seizures, and proteinuria [6, 9]. Thus, the PPPGMRPP epitope is critical as the first epitope in Sm B’ autoimmunity and as the foundation for a more diverse autoimmune response.
In addition to Sm B’, other common lupus autoantigens share similar early autoantibody targets. Antibodies against Sm and nRNP often appear almost simultaneously in the development of lupus [1]. Interestingly, the initial epitopes targeted in nRNP A and nRNP C autoimmunity are also very similar to PPPGMRPP [Poole et al. manuscript accepted pending revisions], and antibodies against the initial epitopes of both nRNP A and nRNP C cross-react with PPPGMRPP from Sm B’[14]. Antibodies against the nRNP A and nRNP C components of the nRNP complex are commonly found in SLE, while antibodies against the 70K component are more strongly associated with mixed connective tissue disease. Antibodies against PPPGMRPP also cross-react with other proline rich structures [4, 15].
Such similarity in the initial targets of diverse autoantibodies suggests a common initiating factor. Mounting evidence implicates infection with Epstein-Barr virus (EBV) as an environmental risk factor for SLE. Prior EBV infection has been associated with SLE in both pediatric and adult patients [16–23]. The viral nuclear protein EBNA-1 contains the epitope PPPGRRP (aa #398–404), which is highly similar to, and cross-reactive with, the initial epitope of Sm B’ [14, 24]. Antibodies against EBNA-1 in SLE patients are more diverse than in SLE-unaffected controls and bind to multiple, often cross-reactive regions, including the PPPGRRP sequence [25]. Antibodies in unaffected individuals are primarily directed against a large (226 aa) region consisting entirely of glycine and alanine [25].
Immunization with EBNA-1 or EBNA-1-derived peptides led to the development of autoimmunity in at least two animal models. DNA vaccination of mice with an EBNA-1-expressing plasmid resulted in the production of anti-Sm and anti-dsDNA antibodies [26]. Preliminary studies using immunization of two rabbits with the PPPGRRP sequence from EBNA-1 also led to autoimmunity and epitope spreading [27]. The current work expands on these data by investigating the capacity of immunity against the EBV epitope PPPGRRP to develop into lupus-like autoimmunity in both rabbits and mice. Two distinct antigens containing the PPPGRRP epitope are used. These antigens are the peptide itself constructed on a poly-lysine backbone and a recombinant fragment of EBNA-1 that contains the PPPGRRP sequence. The capacity of immunization with PPPGRRP to produce antibodies against the peptide of immunization, the cross-reactive Sm peptide, and the autoantigens Sm and nRNP was examined. These experiments show that the immune response initiated against the cross-reactive peptide PPPGRRP was sufficient to instigate autoimmunity, providing a proof of concept for a role for EBV in the initiation of lupus.
2. Methods
2.1 Mice and rabbits
Five strains of 6-wk-old, female, inbred mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). These strains include A/J, C57BL/6J (B6), C57BL/J, BALB/c, and SJL/J. Mice were kept in a pathogen-free, American Association for Accreditation of Laboratory Animal Care-accredited facility. Ten New Zealand White, outbred rabbits (Middlefork Kennels, Salisbury, MO) were also used for these experiments. The Institutional Animal Care and Use Committee at OMRF or Handke Farms approved all experiments.
2.2 Human sera
Well-characterized SLE patient sera with precipitating levels of anti-Sm and/or anti-nRNP autoantibodies were chosen from our stored collection of coded patient serum samples and used as positive controls for the solid-phase mapping and ELISA assays. Sera from SLE-unaffected patients stored in the serum collection were used as negative controls. All experiments were approved by the OMRF and OUHSC Institutional Review Boards.
2.3 Antigens
Bulk quantities of the peptides PPPGRRP and PPPGMRPP were constructed on polylysine backbones (MAP™, Applied Biosystems, Foster City, CA) by the Oklahoma University Health Sciences Center Molecular Biology-Proteomics facility. The N-terminal (aa 1–89), C-terminal (aa 331–641), and middle (aa 90–330) fragments of EBNA-1 were generated as 6-His tagged fusion proteins. They were expressed and purified as previously described [25]. Briefly, EBNA-1 DNA was amplified from B95-8 cells using PCR. Amplification products were cloned into the pCR2.1 expression vector (Invitrogen, Carlsbad, CA). The fidelity of the PCR products was verified by sequencing. Cloned EBNA-1 fragments were excised with Eco RI and Hind III and cloned into the pMal-C2 expression vector (New England Biolabs, Beverly, MA). Full-length nRNP A (gene ID 6626) cloned into the pRSET-B vector (Invitrogen, Carlsbad, CA) was received as a gift from C. Lutz (University of Medicine and Dentistry of New Jersey) [28]. The nRNP A construct was then transformed into BL21 gold E. coli (Stratagene, Carlsbad, CA). Recombinant fragments were expressed following the manufacturer's instructions. Full-length EBNA-1 protein was purchased from Biodesign (Carmel, NY).
2.4 Immunizations
Two groups of rabbit experiments were performed. In the first, rabbits (n=6) were immunized with PPPGRRP-MAP™, or with sterile saline alone. On day 1, 0.5 mg of immunogen (or saline alone) was emulsified with an equal volume of complete Freund's adjuvant (FCA) and injected ½ subcutaneously (sc) and ½ intraperitoneally (ip). Boosting with 0.5 mg of immunogen in incomplete Freund's adjuvant (IFA) into the subcutaneous tissue and intraperitoneal space was performed on days 26, 53 and 99. The final boost (0.5 mg of immunogen or sterile saline) was given intravenously on day 152 without adjuvant. Serial bleeds were collected from each animal prior to immunization and on a weekly basis for the duration of the experiment, at least 100 weeks.
The rabbits in the second group (n=4) were immunized identically to the first, except that instead of PPPGRRP-MAP™ peptide they were immunized with the N-terminal (aa 1–89), C-terminal (aa 331–641), or the middle (aa 90–330) fragments of EBNA-1.
Six mice from each of five different mouse strains were immunized with PPPGRRP (experimental) and two with FCA without peptide. On day 1, FCA was emulsified with an equal volume (0.1 ml/0.1 ml) of sterile saline containing 100 µg of immunogen or saline alone for negative controls and injected in equal portions i.p. and s.c. Boosting with 0.1 mg of immunogen in IFA into the s.c. tissue and i.p. space was conducted on days 10, 29, and 62. Mice were serially bled on days 16, 23, 49, 93, 121, 153, 202, and 240.
2.5 Enzyme-linked immunosorbent assays
Standard solid-phase assays were used to measure the antibody reactivity in mouse or rabbit sera, as described previously [9]. One microgram of the antigen Sm (Immunovision, Springdale, AR), Sm/nRNP (Immunovision), PPPGMRPP-MAP™, PPPGRRP-MAP™ or EBNA-1 (Biodesign, Saco, ME) was coated per well in each of 96 polystyrene wells/plate. Mouse, human, or rabbit sera at a dilution of 1:100, were incubated in each well for 3 hrs. After incubation, plates were washed and incubated with anti-mouse, anti-human, or anti-rabbit alkaline phosphatase-conjugated γ-chain-specific goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA; or Sigma Chemical Co., St. Louis, MO) at a 1/5,000 (for rabbit) or a 1/10,000 (for mouse and human) dilution. Para-nitrophenyl phosphate disodium was used as a substrate for alkaline phosphatase, and plates were read at 405 nm with a micro-ELISA reader (Dynatech, Alexandria, VA).
All ELISA tests were considered positive if the optical density (OD) was at least 2 standard deviations above the negative control mean for rabbits and each mouse strain.
2.6 Immunofluorescence
Rabbit sera were tested for antinuclear Abs (ANA) by a standard ANA test (INOVA Diagnostics, Inc., San Diego, CA). Mice were tested for ANA by immunofluorescence with mouse kidney substrate slides (INOVA diagnostics, San Diego, CA). Mouse and rabbit sera were tested for anti-dsDNA antibodies by immunofluorescence of Crithidia luciliae test (INOVA diagnostics, San Diego, CA), following the manufacturer’s instructions.
2.7 Solid-phase peptide synthesis and autoantibody assays
Decapeptides overlapping by eight amino acids representing the protein sequence of nRNP C (accession #PO9234) [29], and maximally overlapping octapeptides encompassing the sequence of nRNP A (accession #PO 9012) [30] and Sm B’ (accession #P14678) [31] were constructed on the ends of polyethylene pins using Fmoc solid-phase peptide chemistry as previously described [14, 27, 32]. Serum antibody binding to each peptide in the solid-phase assay was performed using a modified ELISA technique. Briefly, individual solid-phase peptides were incubated with a 1:500 dilution of rabbit sera for 2 h at room temperature. Each pin block was washed and incubated with anti-rabbit IgG Fc fragment-specific alkaline phosphatase conjugate or with anti-human IgG alkaline phosphatase conjugate for the positive controls (Jackson Immunoresearch Laboratories), overnight at 4°C. Pin blocks were washed and then incubated at 37°C with para-nitrophenyl phosphate disodium substrate (Sigma-Aldrich, St. Louis, MO) until positive control wells had absorbance readings of 1.0 at 405 nm. A well-characterized human positive control serum was used to normalize the results among multiple plates. Solid-phase epitope mapping results were considered positive if the absorbance was at least four standard deviations above the control mean.
2.8. Hematology
Blood smears from rabbits were examined manually and scored for total leucocytes, segmented neutrophils, banded neutrophils, and lymphocytes. Blood smears from each rabbit prior to immunization and two rabbits immunized with saline in FCA were used as controls. Control white blood cell and lymphocyte counts were averaged, and immunized rabbits were considered leucopenic or lymphopenic if the number of cells became lower than two standard deviations below the control mean.
2.9 Western Blotting
HeLa cell extracts or recombinant nRNP A were subjected to electrophoresis in 12.5% polyacrylamide gels and transferred to a nitrocellulose membrane. Blots were blocked with 3% (w/v) dry milk in TBS. Purified PPPGRRP antibodies were diluted 1:10 and incubated with 40 µg/ml Sm, Sm/nRNP, or U1A proteins for 2 h at room temperature. Antibodies were then incubated with individual membrane strips for 2 h at RT. After thorough washing, alkaline phosphatase goat anti-human IgG conjugate was added for 3 h. Serum antibody binding to specific proteins was visualized by the addition of the nitrotetrazolium blue/5-bromo-4-chloro-3-indolyl phosphate substrate (Fisher Scientific, Waltham, MA).
2.10 PPPGRRP antibody purification and testing for cross-reactivity
PPPGRRP antibodies were purified from the sera of immunized rabbits by using affinity column chromatography. Columns were created by conjugating PPPGRRP-MAP™ to 1 ml of sepharose beads. Sera were passed through the column and the column was washed with 20 column volumes of buffer. Retained antibodies were eluted with 3M NaSCN, dialyzed, and concentrated. Cross-reactivity of purified PPPGRRP antibodies was tested by incubating the antibodies with 40 µg/ml purified Sm or Sm/RNP antigens (Immunovision) for 3 h at room temperature. Cross-reactivity was analyzed by measuring inhibition in binding of these pre-incubated antibodies on both Western blot and ELISA. The peptide pre-absorbed antibodies were incubated with the nitrocellulose-bound antigens, and antibody binding was detected as described above. Blots were scanned and band intensity was quantified by using Scion Image software for Windows (Scion Corporation, Fredrick, MA). Inhibition was measured by comparing intensity of binding between the purified PPPGRRP antibodies and the antibodies that had been pre-adsorbed with nRNP or Sm.
2.11 Statistical analysis
Differences in the proportions of animals making antibodies were analyzed with the Chi-square test. Differences in the optical density of ELISA tests were evaluated for significance using the Kruskal-Wallis test. A Chi-square test was used to evaluate amino acid enrichment in the epitope mapping experiments. P values of less than 0.05 were considered significant for all tests.
3. Results
3.1 Immunization with the EBNA- derived peptide PPPGRRP results in autoimmunity
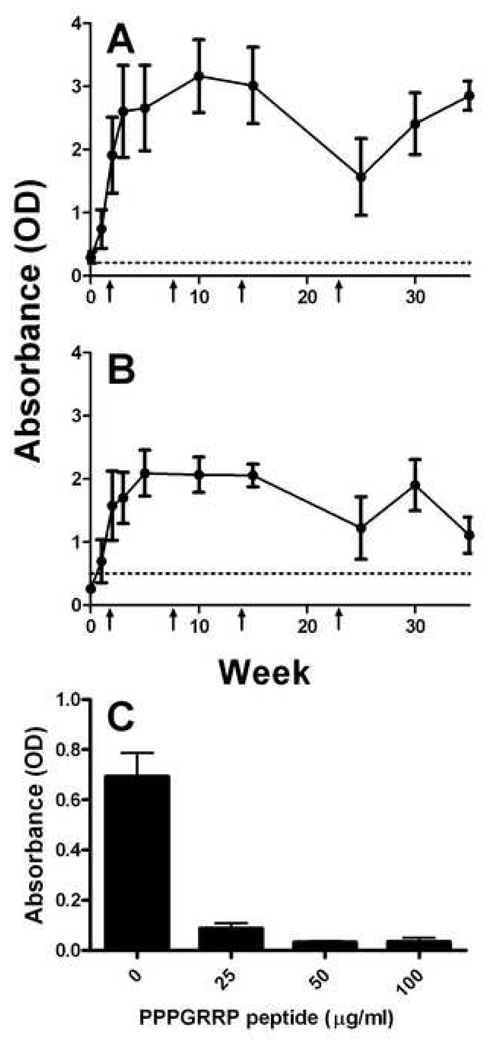
All PPPGRRP-immunized rabbits developed antibodies against the peptide of immunization by week 5, after 1 immunization and 1 boost. Antibody titers were high; detectable antibodies were at 1:2,000 (1 rabbit) 1:4,000 (2 rabbits) and greater than 1:8000 (1 rabbit) dilutions. All four rabbits also developed anti-PPPGMRPP antibodies by week 5 (Figure 1); detectable antibodies dilutions ranged from 1:100 to more than 1:8,000. Inhibition with PPPGRRP linear peptide demonstrated that the binding to PPPGMRPP was almost entirely cross-reactive; 97% of the binding to the PPPGMRPP-MAP™ peptide was eliminated after incubation with PPPGRRP linear peptide (Figure 1C).
Figure 1. PPPGRRP-immunized rabbits make cross-reactive anti-peptide antibodies.
Four rabbits were immunized with PPPGRRP-MAP™ and two with FCA alone. (A) Serial serum samples were examined for antibodies against PPPGRRP. Mean antibody binding of all four PPPGRRP-immunized rabbits at each bleed is shown. The dashed horizontal line shows two standard deviations above the negative control mean. Arrows indicate times of booster immunizations. (B) Serum antibody binding to the Sm-derived peptide PPPGMRPP was observed. Means of all four rabbits at each time point are shown as in panel A. (C) Antibodies to PPPGMRPP are primarily cross-reactive. Serum from each PPPGRRP-immunized rabbit at week 5 was incubated with 0–100 µg/ml of the peptide PPPGRRP and tested for binding to PPPGMRPP. Error bars represent standard error of the mean for each experiment.
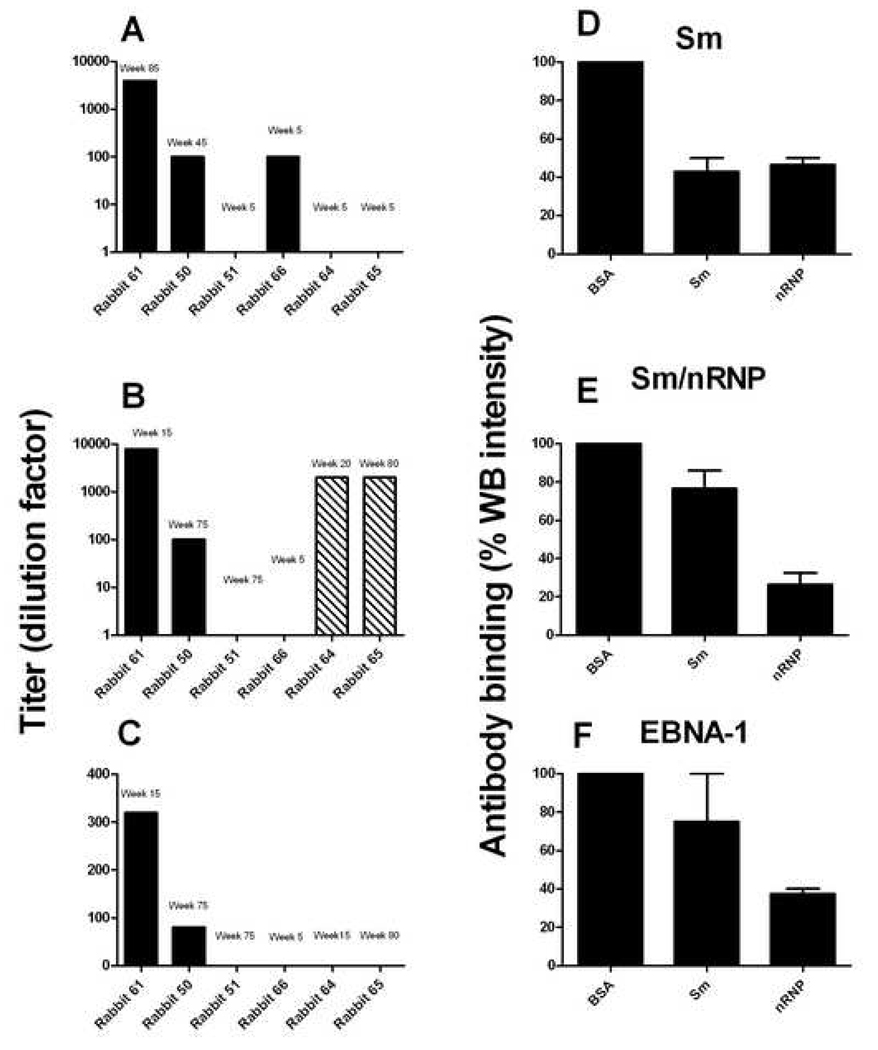
Three out of four PPPGRRP-immunized rabbits developed antibodies against purified Sm. One rabbit had anti-Sm antibodies as early as week 5 (Figure 2). The anti-Sm antibodies were long-lasting. In 2/3 rabbits that developed anti-Sm, the antibodies persisted for at least 95 weeks post-immunization. In the other, the anti-Sm antibodies remained until week 35. Half of the PPPGRRP-immunized rabbits developed antibodies against the Sm/nRNP complex (Figure 2). One of these rabbits exhibited dramatic early production of autoantibodies by week 15. The other began producing autoantibodies by week 45. In both of these rabbits, antibodies were long-lasting, with one rabbit having detectable anti-nRNP antibodies at week 75, and the other having antibodies detected past week 85. The negative control rabbits did not develop any detectable anti-spliceosomal autoantibodies.
Figure 2. Rabbits immunized with EBNA-1 peptides containing PPPGRRP develop autoantibodies.
(A) Immunized rabbits were tested for serum antibody binding to purified Sm by ELISA. The antibody titer in the sample date with the highest binding for each rabbit is shown. Rabbits 50, 51, 61 and 66 were immunized with PPPGRRP-MAP peptide (solid bars) and rabbits 64 and 65 were immunized with the EBNA-1 C-terminal fragment (striped bars). (B) Four out of six rabbits were positive for anti-Sm/nRNP by ELISA, including 50% of the PPPGRRP-immunized animals and both EBNA-1 C-terminal fragment-immunized rabbits. The antibody titer at the sample with the maximum absorbance value for each rabbit is shown. (C) Anti-nuclear antibodies (ANA) were detected using immunofluorescence. The maximum ANA titer for each PPPGRRP-immunized rabbit is shown. (D–F) Antibodies from immunized rabbits exhibited characteristics of molecular mimicry. Antibodies binding to PPPGRRP were purified from PPPGRRP and EBNA-1 C-terminal fragment-immunized rabbits and pre-incubated with purified Sm and nRNP before testing for binding to purified Sm (D), Sm/nRNP (E) and EBNA-1 (F). Binding of these antigens by PPPGRRP-specific antibodies, as well as inhibition of binding after pre-incubation demonstrates cross-reactivity between PPPGRRP antibodies and autoantibodies.
Rabbits were assayed for ANA and dsDNA antibodies by immunofluorescence. Both of the PPPGRRP-immunized rabbits that tested positive for nRNP antibodies by ELISA also had positive ANAs. The rabbit that developed anti-nRNP autoantibodies by week 15 was ANA positive from weeks 15–35, and from week 85–95, with titers as high as 1:320 and a speckled pattern. The rabbit that developed autoantibodies at bleed 45 tested positive for ANA at week 65, with a nuclear/nucleosomal pattern and a titer of 1:80. The dates when the rabbits were ANA positive closely correlated with the dates when Sm/nRNP antibodies were detected by ELISA. Neither of the other PPPGRRP-immunized rabbits, nor the FCA-immunized rabbits had detectable ANA. No anti-dsDNA antibodies were observed.
Purified PPPGRRP antibodies were used to assay for cross-reactivity and potential molecular mimicry. PPPGRRP antibodies were purified by using affinity chromatography and were tested for binding to Sm, nRNP and EBNA-1 by using ELISA. All three antigens were bound by the purified PPPGRRP antibodies. Pre-incubation of the purified antibodies with Sm/nRNP inhibited binding to nRNP (76.3% inhibition), EBNA-1 (62.6% inhibition), and Sm (55.6% inhibition), and pre-incubation with Sm inhibited binding to Sm (57.1%). Inhibition of antibody binding to nRNP and EBNA-1 were 23.4% and 25.0%, respectively (Figure 2 D–F) after pre-incubation with purified Sm. Purified PPPGRRP antibodies also bound to denatured Sm from HeLa cell extracts and to recombinant nRNP A in Western blot assays. Inhibition of binding occurred after incubation with Sm and nRNP A proteins (Data not shown).
Rabbit sera that were positive for Sm or Sm/nRNP antibodies were tested for antibodies against the nRNP subunits nRNP A and nRNP C by Western blot. Both rabbits that made Sm/nRNP antibodies were also positive for nRNP A and Sm autoantibodies. The rabbit that was positive for anti-Sm but not anti-Sm/nRNP did not have serum antibodies that bound to nRNP A. None of these rabbits made detectable levels of nRNP C antibodies.
3.2 Peptide specificity of autoantibody binding in PPPGRRP-immunized rabbits
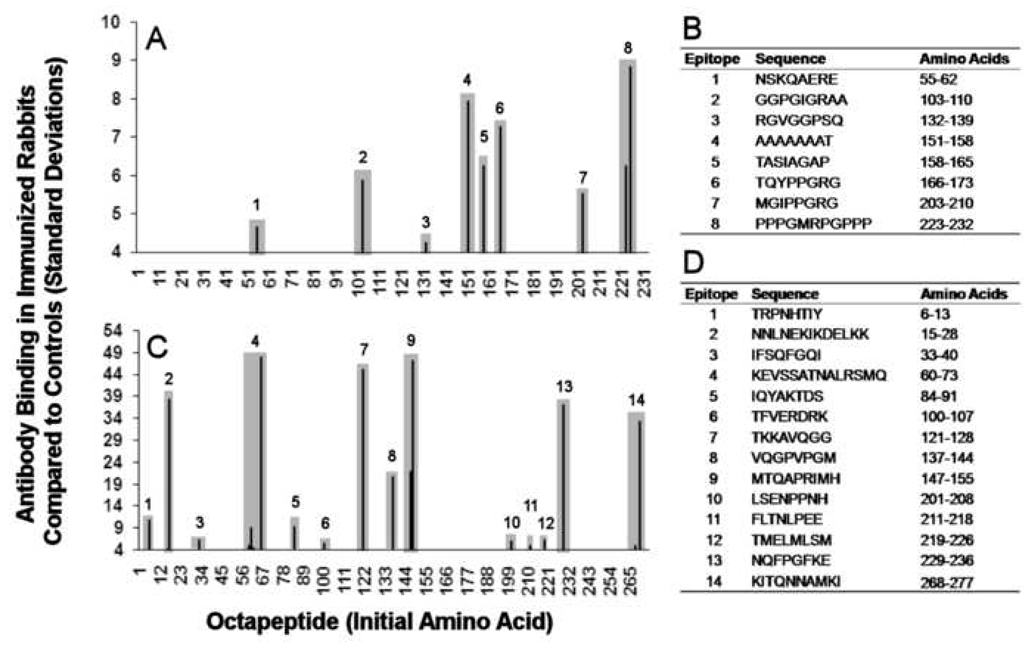
Sera that were positive for antibody binding to nRNP A or Sm were analyzed for antibody fine specificity using the solid-phase peptide assay. Eight epitopes of SmB’ were commonly bound by antibodies from the PPPGRRP-immunized, Sm-positive rabbits (Figure 3A). These antibodies included the peptides PPPGMRGPPP (aa #223–232), which is similar to the peptide of immunization, TQYPPGRG (aa #166–173) and MGIPPGRG (aa #203–210) which both share a 4-amino acid sequence with PPPGRRP, and five other sequences with little to no homology with PPPGRRP (Figure 3B). Antibodies from the two nRNP A antibody-positive rabbits bound to 14 epitopes of nRNP A in the solid-phase peptide assay (Figure 3C,D). No clear patterns of homology with PPPGRRP were discernable with the antibody binding pattern for nRNP in these rabbits.
Figure 3. Epitope mapping of anti-Sm B’ and anti-nRNP A antibodies in PPPGRRP-immunized rabbits.
(A) Antibody binding in the sera from rabbits with anti-Sm antibodies was mapped using solid-phase peptide analysis. Eight epitopes were significantly bound, including one that contains the peptide PPPGMRGPPP (*) (n=3). (B) Epitopes recognized by anti-SmB’ sera are listed. (C) Antibody binding to sequential peptides of the nRNP A proteins was mapped for the two nRNP A antibody-positive rabbits. Fourteen epitopes were identified (D).
3.3 Autoantibody production after immunization with EBNA-1 fragments containing the PPPGRRP sequence
Rabbits were immunized with purified recombinant EBNA-1 fragments to determine whether autoimmunity similar to that seen in the PPPGRRP-immunized rabbits would develop when the immunogenic peptide was found within a larger fragment of the protein. Two rabbits were immunized with the C-terminal fragment of EBNA-1, which contains the PPPGRRP epitope, one rabbit was immunized with the middle fragment, which contains the glycine-alanine-rich repeat portion of EBNA-1, and one rabbit was immunized with the N-terminal fragment (aa 1 – 89).
Both of the rabbits immunized with the C-terminal EBNA-1 fragments, as well as the rabbit immunized with the middle EBNA-1 fragment, produced antibodies against full length recombinant EBNA-1. The C-terminal fragment-immunized rabbits both developed autoantibodies against the Sm/nRNP complex (Figure 2B) while neither of the rabbits immunized with either the N-terminal or middle fragments of EBNA-1 made antibodies directed against this complex. Anti-Sm/nRNP antibodies were detectable at a dilution of 1:2,000 in both rabbits immunized with the EBNA-1 C-terminal fragment. Rabbit sera were tested for antibodies against the nRNP subunits nRNP A and nRNP C using Western blot, and for antibodies against Sm by ELISA. Both of the C-terminal fragment-immunized rabbits that developed Sm/nRNP antibodies were positive for anti-nRNP A. One rabbit was also positive for antibodies against nRNP C and Sm.
PPPGRRP antibodies were purified from the autoantibody-positive sera of the C-terminal fragment-immunized rabbits and tested for binding to EBNA-1 and Sm/nRNP antigens by using ELISA. Binding of the purified antibodies was seen against each antigen. Pre-incubation of purified PPPGRRP antibodies with Sm/nRNP or Sm antigens inhibited binding to nRNP by 70.9% and 23.4%, respectively. EBNA-1 binding by purified PPPGRRP antibodies was also inhibited by pre-incubation with Sm/nRNP (42.2%) and Sm (20.7%) (Figure 2 D–F). Purified PPPGRRP antibodies from one rabbit bound to denatured Sm from HeLa cell extracts and to recombinant nRNP A in Western blot assays (data not shown). Binding was inhibited after incubation with the respective proteins. The finding that purified PPPGRRP-specific antibodies bind to these autoantigens, and that incubation with the autoantigens can inhibit binding to EBNA-1 is evidence of cross-reactivity and molecular mimicry.
3.4 Autoantibody fine specificity in EBNA-1 fragment-immunized rabbits
Solid-phase epitope mapping of the anti-nRNP A, nRNP C or Sm B’ response was performed on sera from each antibody-positive rabbit. The antibody binding patterns of the sera from the EBNA-1 C-terminal fragment-immunized animals were compared to the pre-immune sera. Each of the rabbits that tested positive for binding to nRNP A, nRNP C or Sm by either ELISA or Western blot also made antibodies that bound to epitopes in the solid-phase peptide assay.
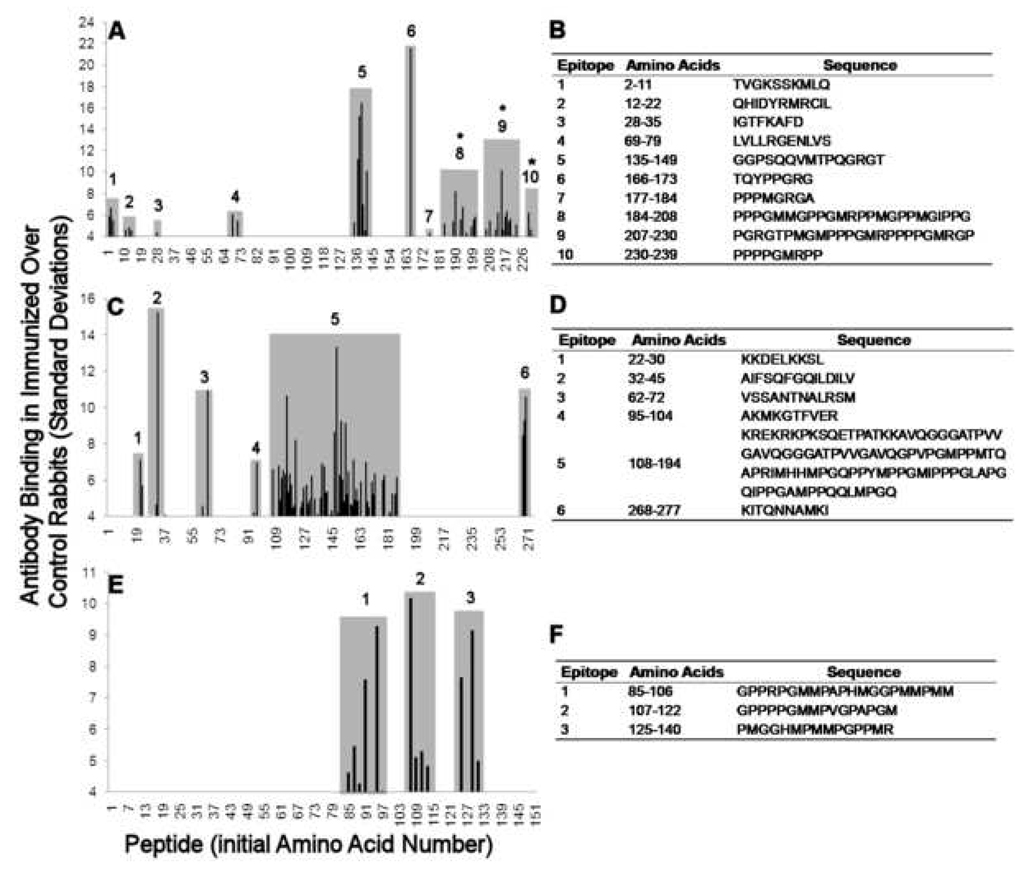
Epitope mapping of the antibodies against Sm B’ revealed binding to 41 octapeptides, organized into 10 epitopes (Figure 4A–B). The amino acids proline (χ2=4.3, p=0.04) and methionine (χ2=3.9, p=0.045) were significantly over-represented in the peptides that were bound by antibodies compared to the peptides that were not bound by antibodies. The majority of the octapeptides (64.4.%) recognized by antibodies were in a proline-rich region from aa 166–239, which is remarkable since this region comprises only 29.6% of the sequence. This area of the protein sequence contains the initial Sm B’ humoral epitope in human SLE, PPPGMRPP, as well as the second sequence targeted in the development of Sm B’ autoimmunity, PPPGMRGP [4]. Both of these sequences were bound by antibodies in the serum from the C-terminal fragment-immunized rabbit.
Figure 4. Rabbit humoral epitope profile for spliceosomal antigens.
Serum antibody binding from rabbits immunized with the EBNA-1 C-terminal fragment and positive for the respective antigens was mapped by solid-phase epitope analysis for (A, B) SmB’ (n=1), (C, D) nRNP A (n=2) and (E, F) nRNP C (n=1). Epitopes are shown as shaded areas. The same rabbit was positive for both Sm and nRNP C. Sera that had the highest titers of anti Sm/nRNP by ELISA were selected for analysis, and the antibody binding profiles were compared to the pre-immunized sera. (A) Ten epitopes were found for Sm B’. These epitopes include the regions containing the sequence PPGMRPP (*). Epitopes are listed (B). (C) Antibodies bound to six epitopes of nRNP A (D). (E) Three humoral epitopes were found for nRNP C. Sequences and amino acid numbers are listed (F).
Anti-nRNP A antibodies were organized into six epitopes (Figure 4B–C). One major region of nRNP A contained most of the antibody-bound peptides, spanning aa #108–194. This epitope included aa #140–146 PVPGMPP, aa #161–171 PPYMPPGMIPPP, and aa #181–187 PPGAMPP, all of which are similar to PPPGRRP of EBNA-1 or PPPGMRPP of SmB’.
Antibodies against nRNP C were found in one C-terminal fragment-immunized rabbit. Three dominant regions of antibody binding to nRNP C were found using solid-phase epitope mapping. The sequences were aa #85–106 GPPRPGMMPAPHMGGPMMPMM, aa 107–122 GPPPPGMMPVGPAPGM and aa #125–140 PMGGHMPMMPGPPMMR (Figure 4C–D). The bound sequences are significantly enriched for proline (χ2=6.0 p=0.014), glycine (χ2=8.8 p=0.003) and methionine (χ2=13.9 p<0.001) compared to the unbound regions of the protein sequence.
3.5. Clinical manifestations of autoimmunity in the PPPGRRP and EBNA-1 C terminal-immunized rabbits
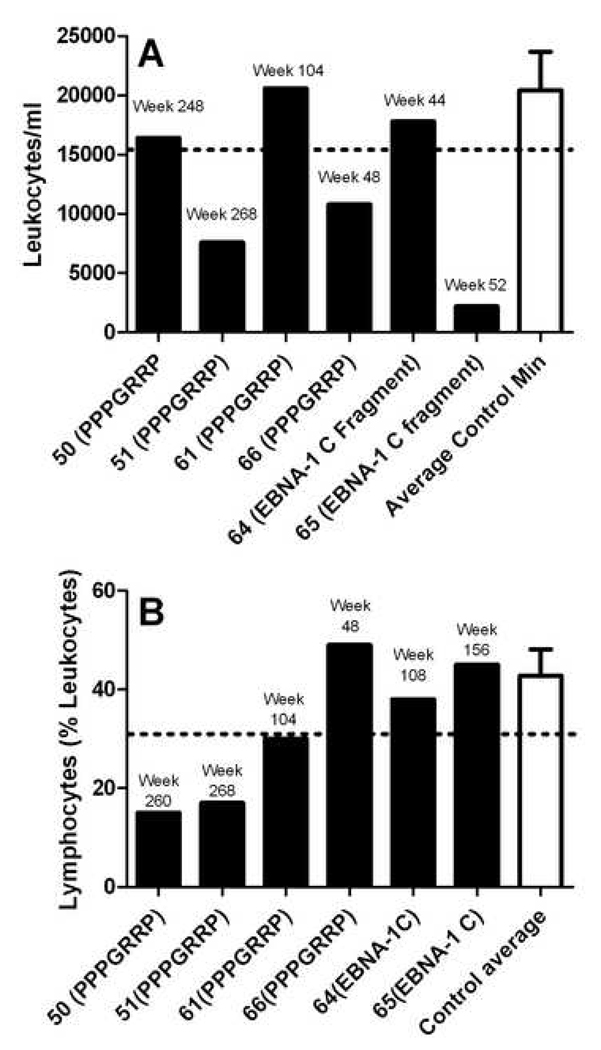
Five out of six rabbits immunized with PPPGRRP-MAP™ or the C-terminal EBNA-1 fragment developed either leucopenia or lymphopenia. Leukopenia was found in 2/4 rabbits immunized with PPPGRRP-MAP™, and 1/2 rabbits immunized with the C-terminal fragment of EBNA-1. One control, the EBNA-1 middle-fragment-immunized rabbit, also developed leucopenia, although the degree was substantially less than the test rabbits. Lymphopenia occurred in 3/4 PPPGRRP-immunized rabbits. None of the controls or the C-terminal fragment-immunized rabbits developed lymphopenia. One of the PPPGRRP-MAP™-immunized rabbits developed early neutropenia, which resolved but was followed by lymphopenia (Figure 5).
Figure 5. Rabbits immunized with PPPGRRP or EBNA-1 C terminal fragments develop hematologic abnormalities.
A. Two rabbits immunized with PPPGRRP-MAP™ developed leucopenia, as did one rabbit immunized with the EBNA-1 C terminal fragment. B. Three out of four PPPGRRP-immunized rabbits developed lymphopenia. Lymphopenia was not found in the EBNA-1-immunized or negative control rabbits. Values are shown for the sample from each rabbit with the minimum number of leucocytes (A) or lymphocytes (B) and the week post-immunization at which the sample was drawn is shown. Controls bars represent the average of the sample with the lowest number of relevant cells from each control animal (n=4). The dashed horizontal line represents two standard deviations below the negative control mean. Error bars indicate standard error.
3.6 Antibody production after immunization of mice with the peptide PPPGRRP
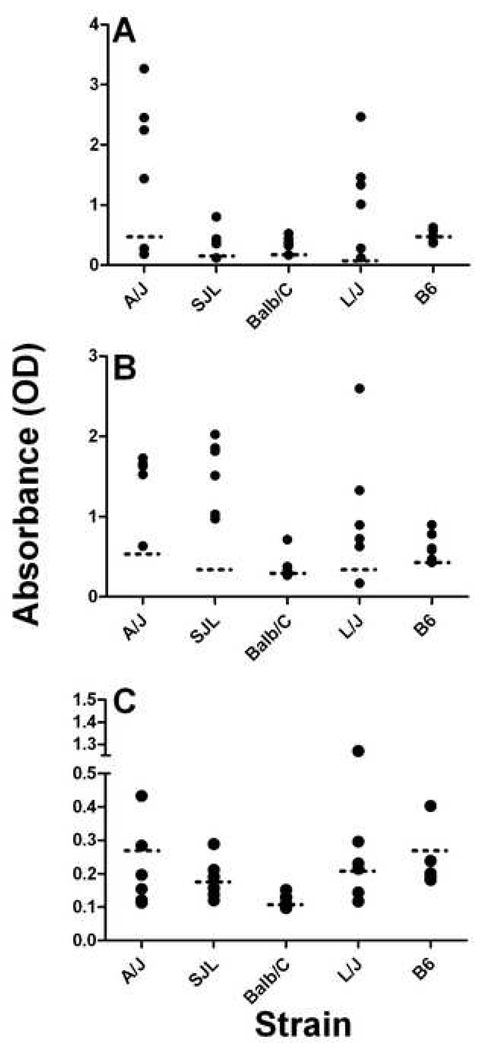
To examine the ability of PPPGRRP immunization to induce autoimmunity in an additional animal model, five strains of mice were immunized with PPPGRRP-MAP™ and tested for the development of autoantibodies. Mice were assayed for antibodies against the peptide of immunization, PPPGRRP, and for cross-reactive antibodies and autoantibodies. The mice developed strain-specific responses to PPPGRRP, ranging from 100% of L/J mice (mean max OD=1.1±0.86) to two of the B6 mice (mean max OD= 0.47±0.11) making significant levels of antibodies (Figure 6A). No mouse had antibodies against this peptide prior to immunization (mean OD=0.10).
Figure 6. Mouse autoantibodies after immunization with PPPGRRP.
(A) Mice were immunized with PPPGRRP-MAP™, and serum antibodies were tested for binding to the peptide of immunization by ELISA. The date of maximum antibody binding to PPPGRRP of sera is shown for each mouse (A). (B) Mice from all strains developed antibodies against the Sm-derived peptide PPPGMRPP after immunization with PPPGRRP. The absorbance of the sample with maximum binding to PPPGMRPP from each mouse is shown. (C) Maximal antibody binding to purified Sm/nRNP in PPPGRRP-immunized mice is shown. Dashed horizontal lines indicate two standard deviations above the control mean for each strain. All values are shown for a 1:100 dilution of mouse serum.
Analysis of antibodies against the cross-reactive, Sm-derived peptide PPPGMRPP revealed that nearly all PPPGRRP-immunized mice made antibodies that bound this spliceosomal peptide (Figure 6B). The Balb/C mice (mean max OD=0.39±0.16) had significantly lower mean antibody binding (as measured by ELISA) than all other strains except L/J (mean max OD=1.1±0.84) (p=0.03 for each strain). The B6 mice (mean max OD =0.63±0.18) had significantly lower binding than the A/J (mean max OD=1.47±0.41) or the SJ/L mice (mean max OD= 1.53±0.44) (P=0.009 for each strain) (Figure 6B).
To ascertain whether immunization with PPPGRRP could lead to the development of autoantibodies, antibody binding to the Sm/nRNP complex was assessed by ELISA for each strain. Mice from every strain made autoantibodies against this complex in response to immunization with PPPGRRP. As would be expected from the antibody response to the peptide of immunization, a higher proportion of mice made antibodies against Sm/nRNP in the L/J strain (4/6) than any other. Half (3/6) of the Balb/c and the SJL mice made antibodies against Sm/nRNP, while 2/6 A/J mice and only one B6 mouse made significant levels of antibodies against this autoantigen complex (Figure 6C). One SJL mouse made anti-Sm/nRNP antibodies that were detectable at a dilution of 1:1000, and one L/J mouse had these antibodies at a dilution of 1:500. All other antibodies were detectable at a maximum dilution of 1:100.
ANA testing was performed on each mouse (Figure 6C). At least one mouse from all strains except the A/J mice tested positive for ANA. There was one positive mouse for the B6 strain and also the Balb/C strain and 2 positive mice for the L/J strain and also the SJL/J strain. One of the SJL mice had the highest titer ANA at 1:80. No dsDNA antibodies were detected in these mice.
4. Discussion
The results of this study help to explain and expand on the growing body of evidence associating EBV infection with the development of SLE. In both the rabbit and mouse immunization models, antibody specificity directed against the EBNA-1 regions containing the peptide PPPGRRP also encompassed the cross-reactive Sm peptide PPPGMRPP and resulted in humoral autoimmunity against numerous components of the Sm/nRNP complex. Immunization with PPPGRRP or with the EBNA-1 fragment containing the PPPGRRP sequence led to Sm or nRNP autoimmunity in 5/6 rabbits and 13/30 mice, primarily in 3 of 5 inbred strains in the mice. Such frequency of autoimmunity indicates that the promotion of autoantibodies resulting from immunity to the cross-reactive EBNA-1 region is not a rare or unlikely event; rather, PPPGRRP immunity is a potent impetus for autoantibody development. Furthermore, the autoantibodies persist in the long-term. In the rabbits, the highest titers of autoantibodies are present more than 70 weeks after the initial immunization for 4/5 rabbits that developed autoantibodies. Similarly, in the mice, autoantibodies generally increase up to the end of the experiment at 240 days after the initial immunization.
Cross-reactivity of the PPPGRRP antibodies with spliceosomal autoantigens was established by using purified PPPGRRP antibodies, suggesting molecular mimicry as a starting point for humoral autoimmunity. The diverse antibody binding pattern found by the epitope mapping experiments, however, indicated that the antibodies against Sm B’ and nRNP A are not merely cross-reactive with the peptide of immunization, although epitopes similar to the immunizing peptides are recognized in each of the three proteins studied. Cross-reactivity is likely to initiate self-binding, with subsequent epitope spreading and maturation of the humoral response leading to true autoimmunity against a broad panel of unrelated sequences. As has been recently observed, the epitope targeted by the initial immune response may be only transiently affected, and the subsequent spreading is likely to produce stronger antibody binding to other regions [13].
Our experiments show patterns of autoantibody specificity that are strikingly consistent with studies examining the development of human SLE. The Sm-directed autoantibody response initiated by EBNA-1 peptide immunization is very similar in the epitopes recognized in the development of Sm autoimmunity in human lupus. Our study found antibodies in the immunized animals against both the first (PPPGMRPP) and second (PPPGMRGP) epitopes that were earlier seen to be targeted in the development of Sm autoantibodies in human patients [4, 5].
We have recently identified epitopes bound by the earliest occurring nRNP A and nRNP C antibodies in human lupus [Poole et al. manuscript in preparation]. Nine out of 12 of the initial human epitopes targeted in nRNP A humoral autoimmunity were either identical or adjacent to epitopes that were recognized by antibodies from the immunized rabbits in the current study. The epitopes in common included both proline-rich sequences, such as HMPGQPPYMPPPGM (aa #156–169), MPPPGMIPPPGLA (aa #164–169), PPPGLAPGQI (aa #171–180) and GQIPPGAMP (aa #178–186), which are similar to the peptide of immunization. Dissimilar epitopes such as KMKGTFVERDRK (aa #96–107), QETPATKKAVQGGG (aa #116–129), VPGMPPMTQAPRIMH (aa #137–148), AMPPQQLM (aa #184–191), and PPQQLMPGQ (aa #186–194) are also targeted. A similar pattern was seen in nRNP C, where 2/3 epitopes identified as common early epitopes in human lupus were shared with or adjacent to those bound by the immunized rabbit [Poole, et al. manuscript in preparation].
All of these epitopes were proline-rich and likely cross-reactive with the PPPGRRP peptide of immunization. Such a striking commonality in the epitopes targeted by autoantibodies between PPPGRRP-immunized animals and the beginnings of human SLE suggests that similar mechanisms are driving autoantibody development in both the human patients and the immunized rabbits, and demonstrates that PPPGRRP immunity has the capacity to initiate a pattern of autoantibody production similar to what is seen in human SLE. Proline-rich epitopes also commonly occur in polyreactive natural antibodies [33]. These epitopes are the targets of molecular mimicry, and may additionally be targeted by early autoreactive antibodies because they normally occur on the surface of the protein, because antibodies against these regions are naturally somewhat promiscuous in binding, or for other reasons that have not yet been defined. It is apparent that such proline-rich regions are often key in the development of autoantibodies.
In a previous report, two rabbits were immunized with PPPGRRP and demonstrated the development of lupus-like autoimmunity [27]. The results of the present study confirmed the autoimmune-potentiating characteristics of the EBNA-1 peptide PPPGRRP using two immunization strategies for the rabbits. The antibody-inducing potential of PPPGRRP immunization was also seen in multiple strains of mice. Mice immunized with PPPGRRP developed antibodies against both the peptide of immunization and the Sm-derived peptide PPPGMRPP. A subset of mice developed antibodies against the Sm/nRNP complex in a strain-dependent manner. The development of antibodies against Sm/nRNP in multiple strains after immunization indicates that immunity against the EBV-derived peptide PPPGRRP is sufficient to drive cross-reactive, and eventually autoreactive humoral immunity in several mouse models.
EBV infection may precipitate SLE through a variety of mechanisms, including molecular mimicry, immortalization of autoreactive B cells, and/or inappropriate immune stimulation [34–36]. An important consideration concerning a putative role for EBV in the development of SLE is that very few EBV-infected individuals go on to develop SLE. The involvement of humoral immunity against PPPGRRP in the development of autoimmunity provides a partial explanation for this difficulty with the model, since humoral immunity against the cross-reactive regions of EBNA-1 is normally restricted, but is common in SLE patients [25]. These findings demonstrate that immunity to the EBNA-1 peptide PPPGRRP is not only sufficient, and indeed likely, to precipitate lupus-like autoimmunity in 2 different animal models, but also generates antibodies that are similar to those generated in the natural process of autoimmune development in human SLE.
Acknowledgements
We thank Handke Farms for animal care, as well as Lauren Curley for technical assistance and Kristina Wasson-Blader, PhD, for review of the manuscript. We are grateful for the kind gift of the expression plasmid for nRNP A (U1A) from Carol Lutz, PhD (University of Medicine and Dentistry of New Jersey). We also thank the Oklahoma University Health Sciences Center Molecular Biology-Proteomics facility for peptide synthesis. This work was supported by grants from the National Institutes of Health (AR48940, AR049084, RR015577, AR053483, AI007633, AR42460, AI31584, AR12253, RR020143, and RR020143), and the Lou Kerr Chair in Biomedical Research at OMRF (JAJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Doll NJ, Wilson MR, Salvaggio JE. Prevalence of Sm antibody in family members of Sm positive patients with systemic lupus erythematosus. J Rheumatol. 1980;7:334–338. [PubMed] [Google Scholar]
- 3.James JA, Mamula MJ, Harley JB. Sequential autoantigenic determinants of the small nuclear ribonucleoprotein Sm D shared by human lupus autoantibodies and MRL lpr/lpr antibodies. Clin Exp Immunol. 1994;98:419–426. doi: 10.1111/j.1365-2249.1994.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbuckle MR, Reichlin M, Harley JB, James JA. Shared early autoantibody recognition events in the development of anti-Sm B/B' in human lupus. Scand J Immunol. 1999;50:447–455. doi: 10.1046/j.1365-3083.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 5.Arbuckle MR, Schilling AR, Harley JB, James JA. A limited lupus anti-spliceosomal response targets a cross-reactive, proline-rich motif. J Autoimmun. 1998;11:431–438. doi: 10.1006/jaut.1998.0227. [DOI] [PubMed] [Google Scholar]
- 6.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B'-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshmukh US, Kannapell CC, Fu SM. Immune responses to small nuclear ribonucleoproteins: antigen-dependent distinct B cell epitope spreading patterns in mice immunized with recombinant polypeptides of small nuclear ribonucleoproteins. J Immunol. 2002;168:5326–5332. doi: 10.4049/jimmunol.168.10.5326. [DOI] [PubMed] [Google Scholar]
- 8.James JA, Harley JB. B-cell epitope spreading in autoimmunity. Immunol Rev. 1998;164:185–200. doi: 10.1111/j.1600-065x.1998.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 9.James JA, Harley JB. A model of peptide-induced lupus autoimmune B cell epitope spreading is strain specific and is not H-2 restricted in mice. J Immunol. 1998;160:502–508. [PubMed] [Google Scholar]
- 10.McCluskey J, Farris AD, Keech CL, Purcell AW, Rischmueller M, Kinoshita G, Reynolds P, Gordon TP. Determinant spreading: lessons from animal models and human disease. Immunol Rev. 1998;164:209–229. doi: 10.1111/j.1600-065x.1998.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 11.Pal R, Deshmukh US, Ohyama Y, Fang Q, Kannapell CC, Gaskin F, Fu SM. Evidence for multiple shared antigenic determinants within Ro60 and other lupus-related ribonucleoprotein autoantigens in human autoimmune responses. J Immunol. 2005;175:7669–7677. doi: 10.4049/jimmunol.175.11.7669. [DOI] [PubMed] [Google Scholar]
- 12.Monneaux F, Muller S. Key sequences involved in the spreading of the systemic autoimmune response to spliceosomal proteins. Scand J Immunol. 2001;54:45–54. doi: 10.1046/j.1365-3083.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh US, Bagavant H, Sim D, Pidiyar V, Fu SM. A SmD peptide induces better antibody responses to other proteins within the small nuclear ribonucleoprotein complex than to SmD protein via intermolecular epitope spreading. J Immunol. 2007;178:2565–2571. doi: 10.4049/jimmunol.178.4.2565. [DOI] [PubMed] [Google Scholar]
- 14.James JA, Harley JB. Linear epitope mapping of an Sm B/B' polypeptide. J Immunol. 1992;148:2074–2079. [PubMed] [Google Scholar]
- 15.Mahler M, Stinton LM, Fritzler MJ. Improved serological differentiation between systemic lupus erythematosus and mixed connective tissue disease by use of an SmD3 peptide-based immunoassay. Clin Diagn Lab Immunol. 2005;12:107–113. doi: 10.1128/CDLI.12.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James JA, Neas BR, Moser KL, Hall T, Bruner GR, Sestak AL, Harley JB. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44:1122–1126. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Harley JB, James JA. Epstein-Barr virus infection may be an environmental risk factor for systemic lupus erythematosus in children and teenagers. Arthritis Rheum. 1999;42:1782–1783. doi: 10.1002/1529-0131(199908)42:8<1782::aid-anr36>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Incaprera M, Rindi L, Bazzichi A, Garzelli C. Potential role of the Epstein-Barr virus in systemic lupus erythematosus autoimmunity. Clin Exp Rheumatol. 1998;16:289–294. [PubMed] [Google Scholar]
- 20.Lu JJ, Chen DY, Hsieh CW, Lan JL, Lin FJ, Lin SH. Association of Epstein-Barr virus infection with systemic lupus erythematosus in Taiwan. Lupus. 2007;16:168–175. doi: 10.1177/0961203306075800. [DOI] [PubMed] [Google Scholar]
- 21.Parks CG, Cooper GS, Hudson LL, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS, Pandey JP. Association of Epstein-Barr virus with systemic lupus erythematosus: effect modification by race, age, and cytotoxic T lymphocyte-associated antigen 4 genotype. Arthritis Rheum. 2005;52:1148–1159. doi: 10.1002/art.20997. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, Lin KH, Lin SC, Tsai WC, Yen JH, Chang SJ, Lu SN, Liu HW. High prevalence of immunoglobulin A antibody against Epstein-Barr virus capsid antigen in adult patients with lupus with disease flare: case control studies. J Rheumatol. 2005;32:44–47. [PubMed] [Google Scholar]
- 23.Yu SF, Wu HC, Tsai WC, Yen JH, Chiang W, Yuo CY, Lu SN, Chiang LC, Chen CJ. Detecting Epstein-Barr virus DNA from peripheral blood mononuclear cells in adult patients with systemic lupus erythematosus in Taiwan. Med Microbiol Immunol. 2005;194:115–120. doi: 10.1007/s00430-004-0230-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman KM, Kirby MY, Harley JB, James JA. Peptide mimics of a major lupus epitope of SmB/B'. Ann N Y Acad Sci. 2003;987:215–229. doi: 10.1111/j.1749-6632.2003.tb06051.x. [DOI] [PubMed] [Google Scholar]
- 25.McClain MT, Poole BD, Bruner BF, Kaufman KM, Harley JB, James JA. An altered immune response to Epstein-Barr nuclear antigen 1 in pediatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:360–368. doi: 10.1002/art.21682. [DOI] [PubMed] [Google Scholar]
- 26.Sundar K, Jacques S, Gottlieb P, Villars R, Benito ME, Taylor DK, Spatz LA. Expression of the Epstein-Barr virus nuclear antigen-1 (EBNA-1) in the mouse can elicit the production of anti-dsDNA and anti-Sm antibodies. J Autoimmun. 2004;23:127–140. doi: 10.1016/j.jaut.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 27.James JA, Scofield RH, Harley JB. Lupus humoral autoimmunity after short peptide immunization. Ann N Y Acad Sci. 1997;815:124–127. doi: 10.1111/j.1749-6632.1997.tb52054.x. [DOI] [PubMed] [Google Scholar]
- 28.Lutz CS, Murthy KG, Schek N, O'Connor JP, Manley JL, Alwine JC. Interaction between the U1 snRNP-A protein and the 160-kD subunit of cleavage-polyadenylation specificity factor increases polyadenylation efficiency in vitro. Genes Dev. 1996;10:325–337. doi: 10.1101/gad.10.3.325. [DOI] [PubMed] [Google Scholar]
- 29.Sillekens PT, Beijer RP, Habets WJ, van Venrooij WJ, Nelissen RL, Geurts van Kessel AH. Human U1 snRNP-specific C protein: complete cDNA and protein sequence and identification of a multigene family in mammals. Nucleic Acids Res. 1988;16:8307–8321. doi: 10.1093/nar/16.17.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelissen RL, Sillekens PT, Beijer RP, Geurts van Kessel AH, van Venrooij WJ. Structure, chromosomal localization and evolutionary conservation of the gene encoding human U1 snRNP-specific A protein. Gene. 1991;102:189–196. doi: 10.1016/0378-1119(91)90077-o. [DOI] [PubMed] [Google Scholar]
- 31.van Dam A, Winkel I, Zijlstra-Baalbergen J, Smeenk R, Cuypers HT. Cloned human snRNP proteins B and B' differ only in their carboxy-terminal part. EMBO J. 1989;8:3853–3860. doi: 10.1002/j.1460-2075.1989.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James JA, Harley JB. Peptide autoantigenicity of the small nuclear ribonucleoprotein C. Clin Exp Rheumatol. 1995;13:299–305. [PubMed] [Google Scholar]
- 33.Tchernychev B, Cabilly S, Wilchek M. The epitopes for natural polyreactive antibodies are rich in proline. Proc Natl Acad Sci U S A. 1997;94:6335–6339. doi: 10.1073/pnas.94.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 35.Poole BD, James JA. Infection and Autoimmunity. In: Tsokos GC, Gordon C, Smolen JS, editors. Systemic Lupus Erythematosus, a Companion to Rheumatology. Philadelphia: Elsevier Press; 2007. pp. 143–155. [Google Scholar]
- 36.Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003;24:584–588. doi: 10.1016/j.it.2003.09.005. [DOI] [PubMed] [Google Scholar]