Abstract
Accumulating evidence suggested that an orphan G protein-coupled receptor (GPR)30, mediates nongenomic responses to estrogen. The present study was performed to investigate the molecular mechanisms underlying GPR30 function. We found that knockdown of GPR30 expression in breast cancer SK-BR-3 cells down-regulated the expression levels of estrogen receptor (ER)-α36, a variant of ER-α. Introduction of a GPR30 expression vector into GPR30 nonexpressing cells induced endogenous ER-α36 expression, and cotransfection assay demonstrated that GPR30 activated the promoter activity of ER-α36 via an activator protein 1 binding site. Both 17β-estradiol (E2) and G1, a compound reported to be a selective GPR30 agonist, increased the phosphorylation levels of the MAPK/ERK1/2 in SK-BR-3 cells, which could be blocked by an anti-ER-α36-specific antibody against its ligand-binding domain. G1 induced activities mediated by ER-α36, such as transcription activation activity of a VP16-ER-α36 fusion protein and activation of the MAPK/ERK1/2 in ER-α36-expressing cells. ER-α36-expressing cells, but not the nonexpressing cells, displayed high-affinity, specific E2 and G1 binding, and E2- and G1-induced intracellular Ca2+ mobilization only in ER-α36 expressing cells. Taken together, our results demonstrated that previously reported activities of GPR30 in response to estrogen were through its ability to induce ER-α36 expression. The selective G protein-coupled receptor (GPR)30 agonist G1 actually interacts with ER-α36. Thus, the ER-α variant ER-α36, not GPR30, is involved in nongenomic estrogen signaling.
GPR30 induces expression of a novel variant of ER-α, ER-α36, which is a receptor that mediates non-genomic estrogen signaling.
It is well known that the estrogenic activities are mediated by both genomic and nongenomic signaling (1,2,3,4,5). The genomic estrogen signaling is mediated by direct actions of nuclear-localized estrogen receptors (ERs: ER-α and ER-β) as ligand-induced transcription factors (1,3,4). On the other hand, nongenomic estrogen signaling involves extranuclear events mediated by ERs (6). Although ERs have long been considered as nuclear localized proteins, recent studies have revealed that a small population of ERs is expressed on the plasma membrane that play important roles in some nongenomic estrogen-signaling events (6), such as activation of various protein kinases (7,8).
An early version of ER-α-deficient mice generated by insertion of a Neo cassette in the exon 1 of the mouse ER-α gene that basically knocked out the AF-1 domain of ER-α retains several nongenomic estrogenic responses such as estrogen-induced intracellular calcium mobilization, which could not be blocked by the pure antiestrogen, ICI 182,780 (9). In the same ER-α knockout mice, it was reported that 4-hydroxyestradiol-17β, a catecholestrogen, induced the uterine expression of an estrogen-responsive gene, lactoferrin, which again could not be inhibited by ICI 182, 780 (10). Estrogen still induced Src phosphorylation in the neocortex of the ER-α knockout mice (11). Thus, it was postulate that the AF-1 activation function may be dispensable for these nongenomic estrogen signalings (12). However, because ICI 182,780 inhibits activities mediated by all known ERs, it was also speculated that other ERs or estrogen binders might exist.
Recently, an orphan G protein-coupled receptor, GPR30 was reported to mediate nongenomic estrogen signaling that was insensitive to ICI 182,780; estrogen stimulates changes of Ca2+ currents and cAMP signaling in cells expressing GPR30 (13,14) and activates the MAPK/ERK phosphorylation and the phosphoinositide 3-kinase (PI3K)/ Akt activation via transactivation of the epidermal growth factor (EGF) receptor pathway in ER-negative but GPR30-positive breast cancer cells (15). Thus, GPR30 was considered as a novel type of extranuclear ER that mediates nongenomic estrogen signaling.
However, there are some reports that challenge the role of GPR30 as an extranuclear ER. Recent study showed that introduction of GPR30 antisense oligonucleotides failed to block ERK activation and cell growth induced by estrogen in ER-positive breast cancer cells (16). Pedram et al. (17) did not find the cAMP or ERK activation in GPR30-positive, ER-negative breast cancer cells. Another study demonstrated that the GPR30-selective agonist G1 failed to exert estrogenic effect in two classical estrogen target organs, the uterus and the mammary gland (18). More recently, Otto et al. (19) generated GPR30-deficient mice and demonstrated that the development of reproductive organs was unimpaired in these mice, and the estrogenic responses in the uterus and the mammary gland were completely maintained in GPR30-deficient animals. Thus, a role for GPR30 as a membrane-based ER remains controversial because the exact mechanism by which GPR30, a receptor without a ligand-binding domain, acts in response to estrogen remains elusive.
Previously, we identified and cloned a 36-kDa variant of ER-α, ER-α36, which is mainly expressed on the plasma membrane and mediates nongenomic estrogenic signaling (20,21). ER-α36 lacks both transcription activation domains, AF-1 and AF-2, of the 66-kDa ER-α (ER-α66) and possesses a truncated ligand-binding domain and an intact DNA-binding domain, consistent with the fact that ER-α36 has no intrinsic transcriptional activity (20) and suggesting that ER-α36 may have a spectrum of ligand selectivity different from ER-α66. ER-α36 retains the nuclear localization signal and functions as a dominant-negative inhibitor of transcription activities of both ER-α and -β (20). ER-α36 is generated from a promoter located in the first intron of the ER-α66 gene (22), indicating that ER-α36 expression is regulated differently from ER-α66 and consistent with the findings that ER-α36 is expressed in ER-negative breast cancer cells that lack ER-α66 expression (20,23). Here, we present strong evidence to demonstrate that GPR30 induces ER-α36 expression and that ER-α36 is an extranuclear ER that mediates nongenomic estrogen signaling.
Results
GPR30 signaling induces ER-α36 expression
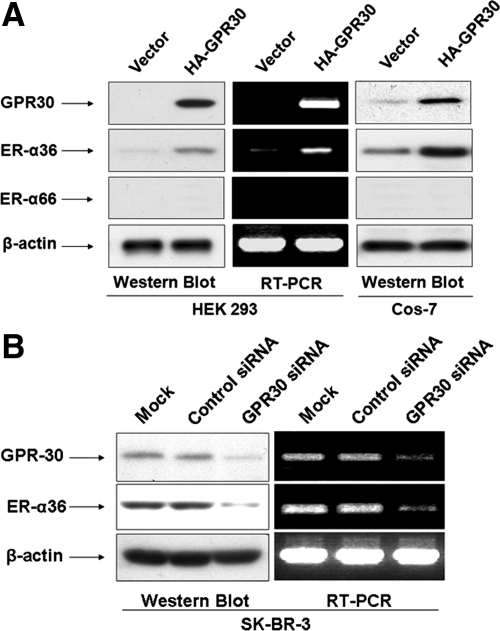
Because ER-α36 and GPR30 share some features (e.g. their activities are not blocked by the pure antiestrogen ICI 182, 780) (15,20), we decided to examine whether ER-α36 is a downstream target gene of GPR30 signaling. Human embryonic kidney (HEK) 293 cells that express no detectable levels of endogenous ER-α36 and GPR30 were transiently transfected with a hemagglutinin (HA)-tagged-GPR30 expression plasmid. Endogenous ER-α36 expression was up-regulated in HEK293 cells transfected with the GPR30-expression vector but not in cells transfected with an empty expression vector (Fig. 1A). However, we did not detect an induction of ER-α66 expression in GPR30 transfected HEK293 cells (Fig. 1A). The same result was obtained when COS-7 cells were transfected with the HA-GPR30 expression vector (Fig. 1A). Furthermore, when endogenous GPR30 expression was knocked down with GPR30-specific small interfering RNA (siRNA) in ER-negative breast cancer SK-BR-3 cells, ER-α36 expression in siRNA-transfected cells was down-regulated (Fig. 1B). RT-PCR analysis indicated that ER-α36 expression is regulated by GPR30 signaling at the transcription level (Fig. 1B). These results suggested that ER-α36 expression is subjected to the positive regulation of GPR30-mediated signaling.
Figure 1.
GPR30 signaling induces ER-α36 expression. A, GPR30 transfection induced endogenous ER-α36 expression in HEK293 and COS-7 cells. An HA-GPR30 expression vector or an empty expression vector was transiently transfected into HEK293 and COS-7 cells. Western blot and RT-PCR analysis were used to examine the expression levels of GPR30 and endogenous ER-α36 or ER-α66 in transfected cells. B, Knockdown of GPR30 expression in SK-BR-3 cells resulted in down-regulation of endogenous ER-α36 expression. SK-BR-3 cells were transiently transfected with GPR30 siRNA or control siRNA for 48 h. The expression levels of endogenous ER-α36 protein and mRNA in transfected cells were assessed with Western blot and RT-PCR analysis.
GPR30 signaling activates ER-α36 promoter activity
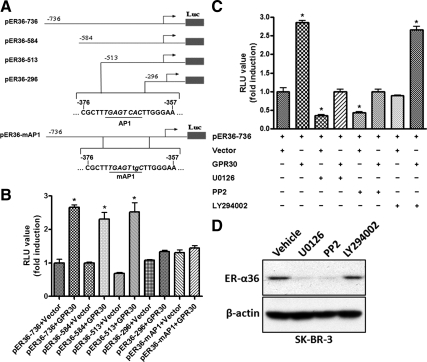
To examine whether GPR30 signaling influences ER-α36 promoter activity, we performed transient cotransfection assay in HEK293 cells with an HA-GPR30 expression vector and a luciferase reporter plasmid driven by the ER-α36 promoter. GPR30 expression resulted in an about 3-fold induction of ER-α36 promoter activity, which was blocked by pretreatment of the MAPK kinase (MEK)1/2 inhibitor U0126, and the Src inhibitor PP2, but not by the PI3K inhibitor LY294002 (Fig. 2C). Corresponding to the effects on the ER-α36 promoter activity, U0126 and PP2 down-regulated the steady state levels of endogenous ER-α36 in SK-BR-3 cells whereas LY294002 was without effect on the ER-α36 expression (Fig. 2D). When a series of 5′ truncated promoter of ER-α36 was analyzed, we found that HA-GPR30 transfection failed to activate the promoter activity of the pER36–296 reporter plasmid (Fig. 2B). Close examination of the DNA sequence in the deleted region revealed an activator protein 1 (AP-1) binding site located between −513 and −296 (relative to the transcription initiation site) residues of the ER-α36 promoter region (Fig. 2A). We then mutated the AP-1 binding site in the pER36-736 plasmid (Fig. 1A) and found that the mutation totally abrogated GPR30-induced promoter activity (Fig. 1B). Taken together, our results demonstrated that GPR30 signaling activated ER-α36 promoter activity presumably through the Src/MEK1/2/AP-1 pathway.
Figure 2.
GPR30 signaling activates ER-α36 promoter activity. A, Schematic structures of luciferase reporter plasmid driven by different 5′-truncated promoters of ER-α36. The −736, −584, −513, and −296 indicate residues upstream of the transcription initiation site, respectively. An AP-1-binding site, which was mutated in pER36-mAP-1, is also indicated. B, HEK293 cells were transfected with different reporter plasmids together with an empty expression vector or the expression vector for HA-GPR30, and luciferase activities were assayed and normalized using a cytomegalovirus-driven Renilla luciferase plasmid. Columns, Means of four independent experiments; bars, se. *, P < 0.05, for cells transfected with GPR30 vs. without GPR30. C, HEK293 cells were transfected with the pER36–736 reporter plasmid with the empty expression vector or the expression vector for GPR30 and then treated with vehicle, 10 μm U0126, 10 μm PP2, or 10 μm LY294002 for 24 h after which luciferase activities were analyzed. Results shown in the graph were mean ± se, and experiments were repeated four times. *, P < 0.05 for cells cotransfected with an empty expression vector and treated with vehicle vs. different conditions. D, SK-BR-3 cells maintained in DMEM supplemented with 10% FBS were treated with vehicle, 10 μm U0126, 10 μm PP2, or 10 μm LY294002 for 24 h. Cells were then harvested and analyzed by Western blot analysis. RLU, Relative light units.
G1, a previously reported GPR30-specific agonist, specifically recognizes ER-α36 and activates the phosphorylation of ERK1/2 mediated by ER-α36
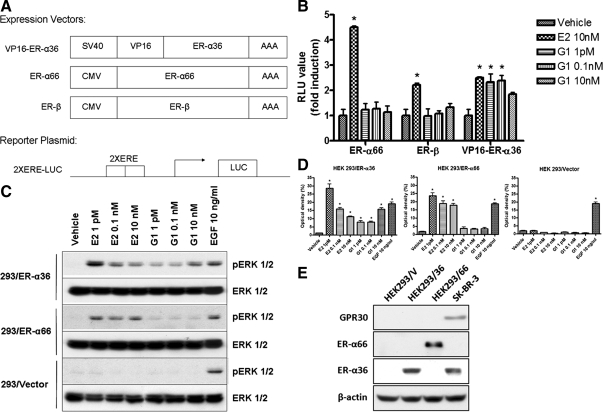
Recently, a compound named G1 was reported to induce various activities in GPR30-expressing cells and was unable to bind to both ER-α66 and -β (24,25). To test the possibility that G1 may recognize ER-α36 that possesses a truncated ligand-binding domain lacking helices 9–12 of the ligand-binding domain of ER-α66, we first tested the ability of G1 to activate transactivation activity of a VP16-ER-α36 fusion protein in a cotransfection assay with a luciferase reporter gene driven by two estrogen-response elements (EREs) and a minimal promoter, 2×ERE-Luc. Because ER-α36 lacks intrinsic transcription transactivation activity but retains the DNA- binding domain, we constructed a VP16-ER-α36 fusion protein by fusing in-frame the transcription activation domain of the VP16 transcription factor with the full-length ER-α36 (Fig. 3A). HEK293 cells were transiently cotransfected with the 2×ERE-Luc reporter vector and the expression vectors for the VP16-ER-α36, ER-α66, or ER-β, respectively. E2 treatment effectively induced the transcription activities of all three proteins, VP16-ER-α36, ER-α66, and ER-β, suggesting that this VP16-ER-α36 fusion protein was able to activate luciferase activity of the 2×ERE-Luc vector in response to estrogen. G1 treatment, however, only induced luciferase activity in cells transfected with the VP16-ER-α36 expression vector (Fig. 3B), consistent with the previous report that G1 failed to bind to both ER-α66 and ER-β (24) and suggesting that G1 may recognize ER-α36 specifically.
Figure 3.
G1 specifically induces ER-α36 functions. A, Schematic drawing of the expression vectors for VP16-ER-α36, ER-α66, and ER-β, and of a luciferase reporter gene driven by two EREs and a minimal promoter, 2×ERE-Luc. B, G1 activates transcriptional activity mediated by a VP-16-ER-α36 fusion protein. HEK293 cells were cotransfected with expression vectors for ER-α66, ER-β, or VP16-ER-α36 together with 2×ERE-Luc and then treated with indicated concentrations of E2 or G1. After normalization, luciferase activities were assessed. The columns represent means ± se of three experiments. *, P < 0.05 for vehicle control vs. different treatments. C and D, G1 induces ERK1/2 phosphorylation in 293/ER-α36 cells. The 293/Vector, 293/ER-α36, and 293/ER-α66 cells in serum-free medium were treated with vehicle and indicated concentrations of E2, G1, or EGF for 10 min. Western blot analysis was performed to assess induction of ERK1/2 phosphorylation. The columns represent three studies combined. *, P < 0.05 for vehicle control vs. different treatments. The representative results are shown in panel C. E, The GPR30, ER-α36, and ER-α66 expression status in HEK293/Vector (HEK293/V), HEK293/ER-α36 (HEK293/36), and HEK293/ER-α66 (HEK293/66) cells.
To further confirm this, we also examined the activation of the ERK1/2 in response to E2 and G1 in HEK293 cell lines stably transfected with the expression vector of ER-α36 or ER-α66 (293/ER-α36 or 293/ER-α66), respectively. E2 induced phosphorylation of the MAPK/ERK1/2 in both 293/ER-α36 and 293/ER-α66 cell lines but not in the control cells transfected with the empty expression vector (293/Vector) (Fig. 3, C and D). EGF, however, was able to induce ERK phosphorylation in control HEK293/Vector cells, indicating there is no global defect in the MAPK/ERK1/2 signaling of 293/Vector cells (Fig. 3, C and D). G1 induced the phosphorylation levels of ERK1/2 in 293/ER-α36 cells but not in 293/ER-α66 cells (Fig. 3, C and D), consistent with the report by Bologa et al. (24) that G1 is unable to bind ER-α66. These results again indicated that G1 is able to specifically induce the ERK activation mediated by ER-α36 because we did not detect GPR30 expression in these cell lines (Fig. 3E).
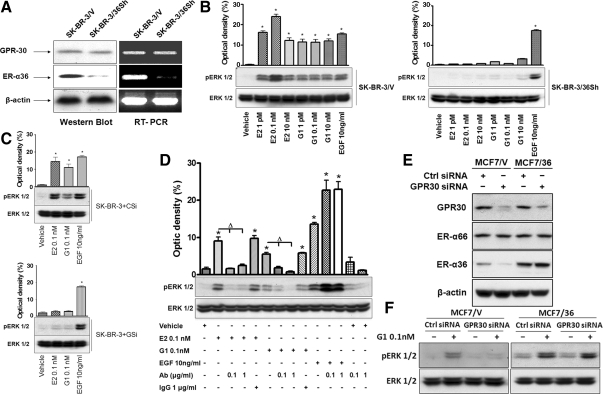
To further establish that G1 is a selective ER-α36 agonist, we generated a stable cell line from breast cancer SK-BR-3 cells (SK-BR-3/36Sh) that express small hairpin RNA (shRNA) specifically for ER-α36 and found that ER-α36 expression was down-regulated at both protein and mRNA levels and GPR30 expression remained intact in the cell line (Fig. 4A). Treatment of both E2 and G1 induced ERK activation in SK-BR-3 cells transfected with the empty expression vector (SK-BR-3/V), but not in SK-BR-3 cells transfected with an ER-α36 shRNA expression vector (SK-BR-3/36Sh) in which the levels of GPR30 expression were intact (Fig. 4B). In addition, both E2 and G1 failed to activate ERK phosphorylation in SK-BR-3 cells transiently transfected with GPR30 siRNA (SK-BR-3+GSi), in which expression levels of both ER-α36 and GPR30 were down-regulated (Fig. 1B), whereas in SK-BR-3 cells transiently transfected with control siRNA (SK-BR-3+CSi), E2 and G1 potently activated ERK phosphorylation (Fig. 4C). The phosphorylation of ERK1/2 induced by E2 and G1 was abrogated in SK-BR-3 cells pretreated with 0.1 or 1 μg/ml of an anti-ER-α36-specific antibody (Fig. 4D) that was raised against the C-terminal ligand-binding domain of ER-α36, whereas the control rabbit IgG was without any effect (Fig. 4D). In addition, the anti-ER-α36 antibody failed to block the EGF-activated phosphorylation of ERK1/2 in SK-BR-3 cells (Fig. 4D), indicating the selective activity of the anti-ER-α36 antibody.
Figure 4.
G1 is a selective ER-α36 agonist. A, Western blot and RT-PCR analysis of SK-BR-3/V and SK-BR-3/36Sh cells for GPR30 and ER-α36 expression. SK-BR-3/V, SK-BR-3 cells transfected the empty expression vector. SK-BR-3/36Sh, SK-BR-3 cells transfected with an ER-α36-specific shRNA expression vector. B, SK-BR-3/V and SK-BR-3/36Sh cells maintained in serum-free medium were treated with vehicle and indicated concentrations of E2, G1, or EGF for 10 min. Cells were then harvested and lysed for Western blot analysis to assess phosphorylation levels of ERK1/2. The columns represent three experiments combined. *, P < 0.05 for vehicle control vs. different treatments. The representative results are also shown. C, Both E2 and G1 failed to induce ERK phosphorylation in GPR30 expression knocked down SK-BR-3 cells. SK-BR-3+Csi, SK-BR-3 cells transfected with control siRNA. SK-BR-3+Gsi, SK-BR-3 cells transfected with GPR30 siRNA. The columns represent three combined experiments. *, P < 0.05 for vehicle control vs. different treatments. The representative results are also shown. D, An anti-ER-α36-specific antibody blocked induction of ERK1/2 phosphorylation by both E2 and G1. SK-BR-3 cells maintained in serum-free medium were treated with PBS, anti-ER-α36 antibody at 0.1 or 1 μg/ml, or 1 μg/ml of rabbit IgG 1 h before being treated with vehicle and indicated concentrations of E2, G1, or EGF for 10 min. Western blot analysis was used to assess the levels of ERK1/2 phosphorylation. The columns represent the mean ± se from three experiments. The representative results are also shown. *, P < 0.05 for cells treated with vehicle vs. different treatments; Δ, P < 0.05, for cells treated with antibody vs. without antibody; the representative results are also shown. E, The expression levels of GPR30, ER-α36, and ER-α66 in MCF7/V and MCF7/36 cells transiently transfected with control siRNA or GPR30 siRNA, respectively. F, The phosphorylation levels of the MAPK/ERK1/2 after the treatment of G1 (0.1 nm) in MCF7/V and MCF7/36 cells transfected with control siRNA or GPR30 siRNA. Ab, Antibody; Ctrl, control.
ER-α36 functions independently of GPR30
To determine whether GPR30 is required for ER-α36 function, we used a stable cell line, MCF7/36, that constitutively expresses high levels of recombinant ER-α36 driven by a cytomegalovirus (CMV) promoter. Transient transfection of GPR30 siRNA down-regulated GPR30 expression in MCF7 control cells transfected with the empty vector, MCF7/V and MCF7/36 cells (Fig. 4E). However, GPR30 siRNA efficiently down-regulated ER-α36 expression in MCF7/V cells but failed to do so in MCF7/36 cells. When G1-induced ERK phosphorylation was examined in the cells transfected with or without GPR30 siRNA, we found that G1 still induced ERK phosphorylation in GPR30 siRNA-transfected MCF7/36 cells that expressed down-regulated levels of GPR30 but intact levels of ER-α36 (Fig. 4F). These results indicated that ER-α36 functions to induce ERK phosphorylation independently of GPR30.
E2 and G1 bind to ER-α36 in HEK293/36 and SK-BR-3 cells
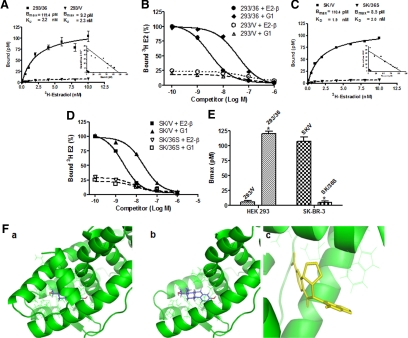
Saturation analysis and Scatchard plotting of [3H]E2 binding to HEK293/36 cells (293/36) and SK-BR-3/Vector cells (SK/V) showed the presence of a single, high-affinity [dissociation constant (KD) = 2.2 or 1.9 nm], saturable, low-capacity (Bmax = 119.4 or 108.4 pm) specific estrogen-binding site in both cell lines, respectively (Fig. 5, A and C). Competitive binding assays showed that G1 is an effective competitor of E2 (Fig. 5, B and D). HEK293 transfected with the empty expression vector (293/V) and SK-BR-3 cells that express shRNA specifically for ER-α36 (SK/36Sh) exhibited a dramatic decrease of specific [3H]-E2 binding (Fig. 5, B, D, and E), suggesting that both E2 and G1 specifically recognize ER-α36.
Figure 5.
Saturation and competitor binding assay of E2 and G1. A and B, Saturation and competitor binding assay in HEK293/vector (293/V) and HEK293/ER-α36 (293/36) cells. C and D, Saturation and competitor binding assay in SK-BR-3/V (SK/V) and SK-BR-3/36Sh (SK/36S) cells. The spots represent the means from three experiments. E, The difference of Bmax between 293/V and 293/36, and SK/V and SK/36S. F, Both ER-α36 (a) and ER-α66 (b) are colored green for side-by-side comparison. The ligand E2 is colored blue. The conformation is derived from cocrystallization data for ER-α66 agonist conformation (Protein Data Bank Code: 1ERE). c, Docking of G-1 into the ER-α36 homology model. The optimal ER-α36 docked structures of G1 based on protein interactions as computed using AutoDock is shown in yellow, and complementary residues on ER-α36 receptor model are shown in green.
ER-α36 lacks the helices 9–12 of the ligand-binding domain of ER-α66 (20). Based on a computer model of ER-α36 built with a homology-modeling method with ER-α66 agonist-bound crystallographic conformation (Protein Data Bank accession code 1ERE), we found that the ligand-binding domain of ER-α36 possesses a conformation different from that of ER-α66 with a more open ligand-binding pocket (Fig. 5F, a and b), suggesting that ER-α36 has a ligand-binding spectrum different from ER-α66. We then conducted molecular docking studies [with AutoDock (www.scripps.edu/mb/olson/doc/autodock)]. When docked into the homology conformation of ER-α36, we found that G1 was able to fit into the altered ligand-binding pocket of ER-α36 (Fig. 5F, panel c). The polar contacts deep within the pocket and the hydrophobic interactions at the outer rim of the ligand-binding cavity brought a strong binding of G1 to ER-α36. Taken together, our data strongly demonstrated that G1 is an agonist selective for ER-α36, not for GPR30, as claimed previously (24).
ER-α36 mediates intracellular Ca2+ mobilization induced by E2 and G1
Previously, it was reported that both E2 and G1 induced a rapid calcium mobilization in GPR30-transfected cells (24), which was considered as a strong evidence for GPR30 to mediate nongenomic estrogen signaling. We decided to examine whether ER-α36 mediates Ca2+ mobilization induced by E2 and G1.
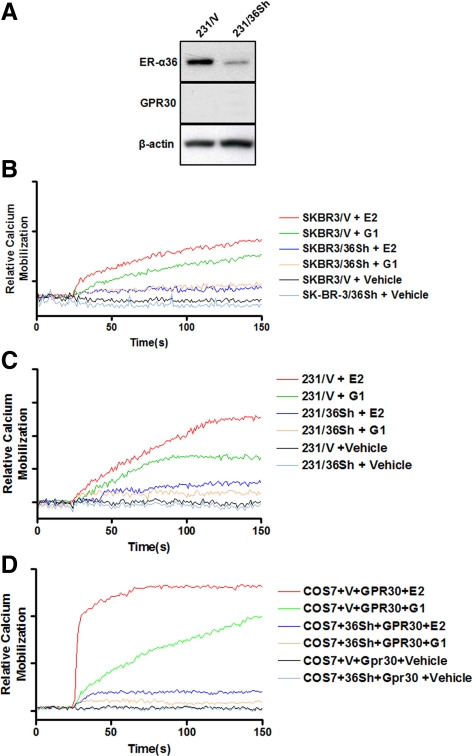
We performed experiments to measure Ca2+ mobilization in SK-BR-3/Vector (SK-BR-3/V) and SK-BR-3/ER-α36 shRNA (SK-BR-3/36Sh) cells. In SK-BR-3/V cells, intracellular Ca2+ concentration was increased after being treated with both E2 and G1 (1 nm) compared with vehicle-treated cells. However, both E2 and G1 failed to induce changes of intracellular Ca2+ concentration in SK-BR-3/36Sh cells (Fig. 6B). These results indicated that ER-α36 is involved in estrogen-induced intracellular Ca2+ response. We also performed Ca2+ mobilization measurements in ER-negative breast cancer MDA-MB-231 cells that express undetectable levels of GPR30 (Ref. 15; Fig. 6A). We used two cell lines: MDA-MB-231 cells stably transfected with an ER-α36 shRNA expression vector (231/36Sh) and MDA-MB-231 control cells stably transfected with an empty expression vector (231/V). Both E2 and G1 (1 nm) induced Ca2+ mobilization in 231/V cells that express undetectable levels of GPR30 whereas failing to do so in 231/36Sh cells (Fig. 6C), consistent with the previous report that estrogen was able to induce Ca2+ mobilization in ER-negative breast cancer MDA-MB-231 and SK-BR-3 cells (26). We consistently observed a modest increase of Ca2+ mobilization in estrogen-treated ER-negative breast cancer cells, a pattern similar to that reported previously (17,26). We decided to measure Ca2+ mobilization in transiently transfected COS-7 as reported (24). Both E2 and G1 (1 nm) induced a strong Ca2+ mobilization in COS-7 cells transiently transfected with a GPR30 expression vector (Fig. 6D), consistent with the previous report (24). However, cotransfection of an ER-α36 shRNA expression vector abrogated the effects of both E2 and G1 (Fig. 6D).
Figure 6.
ER-α36 mediates intracellular Ca2+ mobilization induced by E2 and G1. A, The expression status of ER-α36 and GPR30 in MDA-MB-231 cell variants: MDA-MB-231 cells transfected with an empty expression vector (231/V) and with an ER-α36 shRNA expression vector (231/36Sh). B and C, Intracellular Ca2+ mobilization was measured in SK-BR-3cell variants (B) and MDA-MB-231 cell variants (C). Experiments were performed as described in detail in Materials and Methods after being treated with vehicle or indicated concentrations of E2 or G1. D, Intracellular Ca2+ mobilization was measured in COS-7 cells transiently transfected with the GPR30 expression vector together with the empty expression vector or the ER-α36 shRNA expression vector. Results shown are means of 20 cells emission. Experiments were repeated three times and the representative data are shown.
Discussion
Previously, it was proposed that an orphan G protein-coupled receptor, GPR30, acts as a novel ER that mediates nongenomic estrogen signaling (13,14,15). There are now many subsequent publications that recorded different activities of GPR30 in different systems in response to estrogen or the GPR30-selective agonist G1 (27). Here, we sought to elucidate the molecular mechanism underlying GPR30 activities. Our main findings were as follows: 1) GPR30 signaling induces ER-α36 expression through its ability to activate ER-α36 promoter activity; 2) the GPR30-selective agonist G1 specifically binds ER-α36 and induces ER-α36 activities; 3) ER-α36 mediates nongenomic estrogen signaling independent of GPR30 in ER-α66 negative but ER-α36-positive breast cancer cells. These findings thus indicate that previously reported activities of GPR30 in response to estrogen were actually through its ability to induce expression of ER-α36, a novel variant of ER-α, and ER-α36, in turn, acts as an extranuclear ER to mediate nongenomic estrogen signaling.
ER-α36 is expressed in specimens from a subset of ER-negative patients and a number of established ER-negative breast cancer cells such as MDA-MB-231 that lack the expression of the full-length ER-α66 (20,23). In addition, the partial antiestrogen tamoxifen and the pure antiestrogen ICI 182,780 were unable to block the nongenomic estrogen signaling mediated by ER-α36 (20). These features of ER-α36 are in good agreement with the features of GPR30 reported previously (15). Like GPR30 (28), ER-α36 expression is also frequently associated with HER2 expression in breast cancer specimens (29). Early reports to demonstrate GPR30 function as a novel ER often used cells with high levels of endogenous GPR30 expression, GPR30 expression knocked down with different methods, or introduction of recombinant GPR30 into GPR30-negative cells (13,14,15,24). As demonstrated here, these manipulations of GPR30 expression in cells also influenced endogenous ER-α36 expression. It is also worth noting that COS-7 cells that were used to demonstrate GRR30 function and G1 activities before (13,24) actually express endogenous ER-α36, which are consistent with the previous report that estrogen activates MAPK in native, nontransfected COS-7 cells (30).
ER-α36 transcript is initiated from a promoter located in the first intron of the ER-α66 gene (22), suggesting that ER-α36 expression is subjected to a transcription regulation different from ER-α66. The 5′-flanking region of ER-α36 harbors several AP-1 binding sites (22), suggesting that ER-α36 may be regulated by signaling pathways that lead to AP-1 activation. Our data here indicated that an AP-1 binding site located at the residues from −364 to −371 contributes to the activation of ER-α36 promoter activity by GPR30 signaling. Consistent with this, we found that the promoter activity activated by GPR30 signaling could be blocked by the MEK1/2 inhibitor U0126, and the Src inhibitor PP2, but not by the PI3K inhibitor LY294002. Our results thus indicated that GPR30 signals through the Src/MAPK/AP-1 pathway to activate the promoter activity and to induce ER-α36 expression. It is possible that other GPCR and growth factor-signaling pathways may also contribute to induction of ER-α36 expression via the Src/MAPK/AP-1 pathway, consistent with the finding that ER-α36 expression is frequently associated with HER2 expression in breast cancer specimens (29).
Using virtual and biomolecular screening methods, Bologa et al. (24) identified a molecule G1 and demonstrated that G1 is a selective GPR30 agonist in COS-7 cells transiently transfected with GPR30. Subsequently, several studies reported GPR activities in different systems using G1 as a selective GPR30 agonist (31,32,33,34). However, Otto et al. (18) reported that G1 did not show any estrogenic effect in two classical estrogen target organs, the uterus and the mammary gland, which suggested that GPR30-mediated nongenomic estrogen signaling is not involved in these classical estrogen activities. Here, we demonstrated that G1 failed to activate the transcription activities mediated by the original ER-α and -β and even to interfere with estrogen-stimulated transcription activities of ER-α66 and ER-β (data not shown), consistent with the previous report that G1 has a very low affinity for the agonist conformation of ER-α66 (24). Our results that an anti-ER-α36 antibody specifically against the last 27 amino acids located at the ligand-binding domain that are unique to ER-α36 was able to block G1 function, and G1 failed to induce the phosphorylation levels of the MAPK/ERK1/2 and intracellular Ca2+ mobilization in MDA-MB-231 and SK-BR-3 cells with knocked-down levels of ER-α36 expression strongly indicate that G1 is an agonist highly selective for ER-α36 but not for GPR30.
Antiestrogens such as tamoxifen, ICI 182,780 (Fulvestrant), and aromatase inhibitors (AIs) (anastrazole and letrozole) are widely used for the treatment of breast cancer, especially ER-positive breast cancer. Clinical evidence indicates that ER-negative breast cancer is less or not responsive to antiestrogen therapy, which would be at odds with our findings that ER-negative breast cancer cells that express endogenous ER-α36 retain nongenomic estrogen signaling. It is well established that tamoxifen acts as both agonist and antagonist. It is possible that the ineffectiveness of tamoxifen on ER-negative breast cancer was due to its agonist effects, because an earlier report indicated that enhanced Src activity that occurs often in ER-negative breast cancer can stimulate the agonist effects of tamoxifen in ER-negative cells (35). In our previous reports, we observed that tamoxifen acts as an agonist in ER-α36-expressing cells by activation of the MAPK/ERK signaling pathway (20) and patients with tumors highly expressing ER-α36 are resistant to tamoxifen treatment (29). ICI 182,780, a pure antiestrogen, works by a different mechanism; ICI 182,780 binds to ER-α66, impairs ER-α66 dimerization and nuclear localization, and accelerates degradation of ER-α66 protein (36). Recently, we reported that ICI 182,780 failed to induce degradation of ER-α36 (37), presumably because ER-α36 has a truncated ligand-binding domain that lacks the last four helices (helices 9–12) of ER-α66 (21). The helix-12 domain is critical in protein degradation induced by ICI 182,780, and different positioning of the helix 12 and the F domain of ER-α66 regulates functional differences between agonists and antagonists (38,39). This may provide a molecular explanation for the failure of these antiestrogens to block the nongenomic estrogen signaling mediated by ER-α36 in ER-negative breast cancer.
Previously, Razandi et al. (6) reported that membrane and nuclear ER-α and -β originate from a single transcript and their activities could be blocked by ICI 182,780. Accumulating evidence indicates that more than one membrane-initiated signaling pathway is associated with estrogen action. Data from many laboratories using the membrane-impermeable compound estradiol-BSA (E2β-BSA) suggest the existence of two functionally distinct membrane-associated pathways: one sensitive to antiestrogens and one resistant (5). For example, the earlier version of ER-α66/knockout mice retained rapid estrogen-stimulated membrane effects in neurons that were not blocked by ICI 182,780 (9). It is worth noting that the earlier version of ER-α66/knockout mice that was generated by insertion of a Neo cassette into the first coding exon of the mouse ER-α gene (40) (the exon that is skipped in the generation of the transcript encoding ER-α36) retains ER-α36 expression (Sharon, E., Petach Tikva and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel personal communication). This version of ER-α66-deficient mice also retained antiestrogen ICI 182,780-insensitive nongenomic estrogen signaling in different systems (9,10,11,12). Our current study suggested that ER-α36 may be involved in the nongenomic estrogen signaling resistant to antiestrogens.
The AIs, on the other hand, inhibit the action of the enzyme aromatase, which converts testosterone to E2 (estradiol) and androstenedione to estrone. The third-generation AIs, exemplified by anastrazole and letrozole, provide a second-line therapeutic strategy in advanced ER-positive patients (41). It was reported that anastrozole suppressed the plasma level of E2 to a mean of 2.6 pmol/liter and letrozole to a mean of 2.1 pmol/liter (42). Here, we found that these ER-negative breast cancer cells responded to a very low concentration of estrogen: activation of the MAPK/ERK signaling at the picomolar range, suggesting that cells expressing high levels of ER-α36 are hypersensitive to estrogen. Our findings here are reminiscent of the adaptive hypersensitivity observed in ER-positive breast cancer MCF7 cells after long-term deprivation of estrogen (LTED) (43). It was reported that LTED cells exhibited hypersensitivity to estrogen (respond to estrogen at the picomolar range) and marked enhancement of nongenomic estrogen signaling such as the activation of the MAPK/ERK (44). We recently found that ER-positive breast cancer MCF7 cells expressing high levels of recombinant ER-α36 exhibited estrogen hypersensitivity like LTED cells (Zhang, X., unpublished observations), suggesting that high levels of ER-α36 expression may be one of the mechanisms underlying estrogen hypersensitivity. Our finding here that ER-negative breast cancer cells that express endogenous ER-α36 are hypersensitive to estrogen may provide an explanation for the failure of ER-negative breast cancer that retains nongenomic estrogen signaling to respond to AIs.
Here, we also demonstrated that ER-α36 is also involved in estrogen-induced Ca2+ mobilization. In COS-7 cells transiently transfected with the GPR30 expression vector, both E2 and G1 elicited a strong Ca2+ mobilization, which was abrogated by cotransfection of an ER-α36 shRNA expression vector. ER-negative breast cancer cells, however, exhibited a modest but consistent Ca2+ mobilization, which is similar to a pattern reported before by others (26). Recently, Pedram et al. (17) also found that E2 stimulated a strong increase of intracellular Ca2+ mobilization in ER-positive breast cancer MCF7 cells and a modest increase in ER-negative breast cancer SK-BR-3 cells. The biological significance of this modest increase of Ca2+ mobilization in ER-negative breast cancer requires further investigation.
The exact mechanism by which ER-a36 contributes to Ca2+ is currently unclear. Coimmunoprecipitation assays revealed that GPR30 physically interacted with ER-α36 (data not shown). It is possible that GPR30 not only induces ER-α36 expression but also acts as a partner for ER-α36 to mediate some activities of nongenomic estrogen signaling. However, our results in GPR30-negative but ER-α36-positive MDA-MB-231 cells strongly indicated that ER-α36 is able to function independently of GPR30 to mediate nongenomic estrogen signaling presumably through interaction with other signaling proteins.
In summary, we conclude that ER-α36, not GPR30, is involved in nongenomic estrogen signaling, and GPR30, as an inducer of ER-α36 expression and a possible partner of ER-α36 function, contributes as an indirect factor to some activities mediated by ER-α36 in some cell contexts.
Materials and Methods
Chemicals and antibodies
17β-Estradiol (E2) was purchased from Sigma Chemical Co. (St. Louis, MO), and G1 was obtained from Cayman Chemicals (Ann Arbor, MI). The MEK1/2 inhibitor U0126, the Src inhibitor PP2, and the PI3K inhibitor LY294002 were purchased from Tocris Bioscience (Ellisville, MO). Antiphospho-p44/42 ERK (Thr202/Tyr204) (197G2) mouse monoclonal antibody and anti-p44/42 ERK (137F5) rabbit monoclonal antibodies were purchased from Cell Signaling Technology (Boston, MA). Anti-GPR30 antibody was purchased from LifeSpan BioSciences (MBL International Corp., Woburn, MA). Polyclonal anti-ER-α36 antibody was generated and characterized as described before (20).
Cell culture and treatment
Breast cancer cell lines MCF7, SK-BR-3, MDA-MB-231, African green monkey kidney cells COS-7 and HEK293 cells were obtained from American Type Culture Collection (Manassas, VA). MCF7 cells were maintained in Improved MEM with 10% heat-inactivated fetal bovine serum (FBS), 1% nonessential amino acids, 1% HEPES buffer, 1% antibiotic-antimycotic, and 2 μg/ml bovine insulin at 37 C and 5% CO2 in a humidified incubator. COS-7, SK-BR-3, MDA-MB-231, and HEK293 cells were maintained in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic at 37 C and 5% CO2 in a humidified incubator.
For E2 and G1 treatment, cells were maintained in phenol red-free media with 2.5% charcoal-stripped fetal calf serum for 2 d, and then in serum-free media for 24 h before experimentation. For ERK activation assays, cells were treated with vehicle (dimethylsulfoxide) and indicated concentrations of E2 or G1 for 10 min.
Transient transfection and establishment of stable cell lines
HEK293 cells were plated at a density of 1×105 cells/60 mm dish and transfected 24 h later with an HA-tagged ER-α36 expression vector driven by the CMV promoter, an empty expression vector, or ER-α66 expression vector using the FuGene6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN). The ER-α36 expression vector was constructed as described before (20). The ER-α66 expression vector was provided by Dr. Nawaz Zafar at the University of Miami. Forty eight hours after transfection, transfected cells were replated and selected in medium containing 500 μg/ml G418 (Invitrogen Corp., Carlsbad, CA). More than 20 individual clones from transfected cells were pooled, named as HEK293/36 and HEK293/66, respectively, and used for the experiments. The empty expression vector-transfected HEK293 cells (HEK293/V) were used as controls.
For the MCF7 stable cell lines, MCF7 cells were transfected with an ER-α36 expression vector or an empty expression vector and then selected in medium containing 400 μg/ml G418 as described above. These two cell lines were named MCF7/V and MCF7/36, respectively.
To establish cell lines with ER-α36 expression knocked down by the shRNA method in MDA-MB-231 and SK-BR-3 cells, we constructed an ER-α36-specific shRNA expression vector by cloning the DNA oligonucleotides 5′-TTAACCGTACCACTCTGCTGATTGATATCCGTCAGCAGAGTGGTACGGTTA-3′ targeting the 3′-untranslated region of ER-α36 cDNA into the pRNAT-U6.1/Neo expression vector from GenScript Corp. (Piscataway, NJ). Stable cell lines were established as described previously. Briefly, cells transfected with the empty expression vector or the ER-α36-specific shRNA expression vector were selected in medium containing 400 μg/ml G418 for 3 wk, and more than 20 individual clones of selected cells were pooled and were named as SK-BR-3/V and SK-BR-3/36Sh, or 231/V and 231/36Sh, respectively. The knocked down levels of ER-α36 expression were confirmed by Western blot and RT-PCR analysis.
For transient HA-GPR30 transfection, HEK293 and COS-7 cells were plated at a density of 2 × 106 cells per 100-mm dish for 24 h and transfected with the HA-GPR30 expression vector or an empty vector using the FuGene6 transfection reagent (Roche Molecular Biochemicals) as instructed by the manufacturer. Expression of both GPR30 and ER-α36 in these transfected cells was examined with Western blot and RT-PCR analysis.
For transient transfection of GPR30 siRNA, MCF7 and SK-BR-3 cells were plated at a density of 2 × 106 cells per 100-mm dish for 24 h and transfected with GPR30 siRNA or control siRNA purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) using Lipofectamine RNAiMAX transfection reagent from Invitrogen Corp. as instructed by the manufacturer. Expression levels of both GPR30 and ER-α36 in these transfected cells were assessed with Western blot and RT-PCR analysis.
Luciferase assay
HEK293 cells were transfected using FuGene 6 transfection reagent (Roche Applied Science, Indianapolis, IN) with the pER36- 736-Luc, pER36-584-Luc, pER36-513-Luc, or pER36-296-Luc reporter plasmids as described previously (22) and an empty expression vector or the expression vector for HA-GPR30 (a kind gift from Dr. Edward Filardo at Rhode Island Hospital and Brown University, Providence, RI). Cells were cotransfected with a cytomegalovirus-driven Renilla luciferase plasmid, pRL-CMV (Promega Corp., Madison, WI) to establish transfection efficiency. Cells were treated, 24 h after transfection, with vehicle, 10 μm of U0126, PP2, or LY294002 for 24 h. Cell extracts were prepared 48 h after transfection, and luciferase activity was determined and normalized using the Dual-Luciferase Assay System (Promega) and a TD 20/20 Luminometer (Turner BioSystems, Inc., Sunnyvale, CA) as instructed by the manufacturer.
To test G1 activity, HEK293 cells were cotransfected with vectors to express a VP16-ER-α36 fusion protein, ER-α66, or ER-β with a plasmid encoding the firefly luciferase gene driven by a promoter bearing two-tandem estrogen response elements, p2×ERE-Luc reporter plasmid (a kind gift from Dr. Katarine Pettersson at Karolinska Institute, Stockholm, Sweden) and pRL-CMV expression vector. Cells were treated with vehicle (ethanol) and different concentrations of E2 or G1 for 12 h. Cell extracts were normalized 48 h after transfection and analyzed for luciferase activity.
Western blot analysis
Western blot analysis was performed exactly as described elsewhere (45).
RNA purification and RT-PCR
Total RNA was prepared with the TRIzol RNA purification reagent. Total RNA (1 μg) was reversely transcribed using the ProtoScript II RT-PCR kit (New England Biolabs, Ipswich, MA) with random primers at 42 C for 1 h. RT-PCR analysis of ER-α36, GPR30, and β-actin was performed using gene-specific primers as the following. ER-α36: forward primer, 5′-CAAGTGGTTTCCTCGTGTCTA AAG-3′; reverse primer, 5′-TGTTGAGTGTTGGTTGCCAGG-3′; GPR30: forward primer, 5′-GGCTTT GTGGGCAACATC-3′; reverse primer, 5′-CCTGCAAGCAGTCTTTCCG -3′; β-actin: forward primer, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′; reverse primer, 5′-CTAGAAGCATTTGCGGTG GACGATGGAGGG-3′.
PCR procedure was carried out as described previously (22). PCR products were analyzed by electrophoresis in a 1.5% agarose gel and visualized by ethidium bromide staining under UV illumination.
DNA mutagenesis
To mutate the AP-1 consensus binding site from 5′-TGAGTCAC-3′ to 5′-TGAGTtgC-3′, the mutated primers ER-α36P-AP-1m forward, 5′-CACGGGGCGCTTTGAGTtgCTTGGGAAGGTCTCG-3′, and reverse, 5′-CGAGACCTTCCCAAGcaACTCAAAGCGCCCCGTG-3′, were synthesized by the Integrated DNA Technologies (Coralville, IA). The mutagenesis was performed with the QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. The mutation was verified by DNA sequencing, and the plasmid was used for the transfection assay.
Intracellular calcium mobilization
To perform transient transfection of COS-7 cells, 1 × 106 cells were transfected with 1 μg of GPR30 expression vector together with 9 μg of the empty expression vector or the ER-α36 shRNA expression vector using Microporator (MP-100, Digital Bio., Korea) for 48 h. Changes in Ca2+ mobilization were measured using the Ca2+-sensitive fluorescent probe Fluo-4/AM (Invitrogen, Carlsbad, CA). All cells were maintained in phenol red-free medium with 2.5% charcoal-stripped fetal calf serum for 2 d and seeded at 1×105 on a cover glass slip in 35-mm dish in serum-free medium 24 h before experiments. The medium was removed to eliminate sources of residue fluorescence. Cells were washed three times with HBSS buffer (0.137 m NaCl, 5.4 mm KCl, 0.25 mm Na2HPO4, 0.44 mm KH2PO4, 1.3 mm CaCl2, 1.0 mm MgSO4, 4.2 mm NaHCO3), and incubated in 1 ml of 4 μm Fluo4 with F127 solution (20%, 1:1; Invitrogen) in the dark for 1 h at 37 C. Cells were then washed twice and stored in HBSS buffer without Fluo-4/AM for another 30 min at 37 C. Measurements of Fluo4 fluorescence were performed at 37 C and determined by excitation at λex 494 nm and emission at λem 516 nm using the Wallac 1420 Victor2 multilabel counter. The fluorescent values were recorded by the Victor System (PerkinElmer, Waltham, MA) and transferred to Microsoft Excel for analysis.
Receptor binding assay
Cells were maintained in phenol red-free media with 2.5% charcoal-stripped fetal calf serum until confluent. Confluent cells were harvested and washed with cold lysis buffer containing 10 mm Tris, 1.5 mm disodium EDTA, 10% (vol/vol) glycerol, 12 mm monothioglycerol, 25 mm sodium molybdate (pH 7.4). All subsequent procedures were conducted at 4 C. Cells were resuspended in 1.5 ml lysis buffer, homogenized using a Teflon pestle, and centrifuged at 104,000 × g for 45 min. The supernatant was diluted to 1.0 mg protein/ml in the lysis buffer immediately before binding assays. Aliquots (100 μl) of protein preparation were used for each assay. For saturation analysis, total binding curves were generated by incubating one set of wells containing a range (0.1 to 10 nm) of [3H]-E2 alone. Nonspecific binding was determined from a parallel set containing 100-fold excess (10 nm to 1 μm) unlabeled E2, as a competitor. For competitive binding assays, wells containing 5 nm [3H]E2 and the steroid competitors (concentration range 0.1 nm to 1 μm) were incubated with 100 μl of protein preparation. After incubation for 2 h, the binding reactions were terminated by adding 10% trichloroacetic acid and filtering the precipitated mixture over presoaked GF/B glass-fiber microfilter plates (PerkinElmer) to separate bound steroid from free steroid. The filter was then washed twice with wash buffer [25 mm HEPES, 10 mm NaCl, and 1 mm EDTA (pH 7.6)]. Microscint-20 (30 μl, PerkinElmer) was added to each well, and radioactivity was counted with a Multi-purpose Scintillation Counter (Beckman Coulter, Fullerton, CA). Disintegrations per minute were calculated by count per minute/efficiency (∼20–30%). The displacement of [3H]E2 binding by steroid competitors was expressed as a percentage of the maximum specific binding of E2 (cpm = ∼2000–3000). Each assay point was run in quadruplicates, and the assays were repeated using at least two different batches of cultured cells for each test chemical. Linear and nonlinear regression analyses for all receptor-binding assays and calculations of dissociation constant (KD) and binding capacity were performed using GraphPad Prism for Windows (version 4.01; GraphPad Software, La Jolla, CA).
Homology modeling and docking analysis
The structure of the ligand-binding domain of ER-α36 was built with the Build Homology Models in Discovery Studio 2.1 (Accelrys, Inc., San Diego, CA) with the template structure being the agonist structure (Protein Data Bank accession code: 1ERE) of ER-α66. The molecular docking studies were performed on the homology model of ER-α36, and the GROMACS MD simulation package was used to further optimize the structure. A snapshot of the optimized structure was taken after 5 nsec of simulation and was later used in docking experiments. Both enantiomers of G1 were docked into ER-α36 model with Autodock-4 (46).
Statistical analysis
Data were summarized as the mean ± se using the GraphPad InStat software program. Tukey-Kramer multiple comparisons test was also used, and the significance was accepted for P < 0.05.
Acknowledgments
We thank Dr. Edward Filardo (Division of Biology and Medicine, Brown University, Providence, RI) for the generous gift of the HA-GPR30 expression vector and Dr. Katarine Pettersson (Karolinska Institute, Stockholm, Sweden) for the ER-β expression vector and the 2XERE luciferase reporter plasmid.
Footnotes
This work was supported by a National Institutes of Health Grant DK070016 (to Z.Y.W.) and Nebraska Tobacco Settlement Biomedical Research Program Awards LB-595 and LB692 (to Z.Y.W. and Y.T.)
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 2, 2010
Abbreviations: AI, Aromatase inhibitor; AP-1, activator protein 1; CMV, cytomegalovirus; E2, 17β-estradiol; EGF, epidermal growth factor; ER, estrogen receptor; ERE, estrogen-response element; GPR, G protein-coupled receptor; HA, hemagglutinin; HEK, human embryonic kidney; LTED, long-term deprivation of estrogen; MEK, MAPK kinase; PI3K, phosphoinositide 3-kinase; shRNA, small hairpin RNA; siRNA, small interfering RNA.
References
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER 2007 Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD 2002 Connections and regulation of the human estrogen receptor. Science 296:1642–1644 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276: 36869–36872 [DOI] [PubMed] [Google Scholar]
- Segars JH, Driggers PH 2002 Estrogen action and cytoplasmic signaling cascades. I. Membrane-associated signaling complexes. Trends Endocrinol Metab 13:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Levin ER 2002 Cellular functions of plasma membrane estrogen receptors. Steroids 67:471–475 [DOI] [PubMed] [Google Scholar]
- Fu XD, Simoncini T 2008 Extra-nuclear signaling of estrogen receptors. IUBMB Life 60:502–510 [DOI] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL 1999 Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 140:660–666 [DOI] [PubMed] [Google Scholar]
- Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB 1997 Estrogenic responses in estrogen receptor-α deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA 94:12786–12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD 2001 Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology 142:5145–5148 [DOI] [PubMed] [Google Scholar]
- Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF 2002 The AF-1 activation-function of ERα may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA 99:2205–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Ahola TM, Alkio N, Manninen T, Ylikomi T 2002 Progestin and G protein-coupled receptor 30 inhibit mitogen-activated protein kinase activity in MCF-7 breast cancer cells. Endocrinology 143:4620–4626 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH 2008 G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856 [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH 2009 GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF 2006 A variant of estrogen receptor-α, hER-α36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103:9063–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF 2005 Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem Biophys Res Commun 336:1023–1027 [DOI] [PubMed] [Google Scholar]
- Zou Y, Ding L, Coleman M, Wang Z 2009 Estrogen receptor-alpha (ER-α) suppresses expression of its variant ER-α 36. FEBS Lett 583:1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY 2008 ER-α36, a novel variant of ER-α, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res 28:479–483 [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M 2007 G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Walsh DE, Dockery P, Doolan CM 2005 Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [Ca2+]i in human breast cancer cells. Mol Cell Endocrinol 230:23–30 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ 2008 Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E 2006 Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366 [DOI] [PubMed] [Google Scholar]
- Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Wang Z, Xie Y 2009 Expression of ER-α36, a novel variant of estrogen receptor α, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol 27:3423–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethrapalli IS, Tinnikov AA, Krishnan V, Lei CD, Toran-Allerand CD 2005 Estrogen activates mitogen-activated protein kinase in native, nontransfected CHO-K1, COS-7, and RAT2 fibroblast cell lines. Endocrinology 146:56–63 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Noel SD, Keen KL 2009 Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol 21:316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E 2009 Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanga-Sollo E, White ME, Chung KY, Johnson BJ, Dayton WR 2008 Potential role of G-protein-coupled receptor 30 (GPR30) in estradiol-17β-stimulated IGF-I mRNA expression in bovine satellite cell cultures. Domest Anim Endocrinol 35:254–262 [DOI] [PubMed] [Google Scholar]
- Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Andò S, Maggiolini M, Pezzi V 2008 The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17β-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 149:5043–5051 [DOI] [PubMed] [Google Scholar]
- Shah YM, Rowan BG 2005 The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor α promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol 19:732–748 [DOI] [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C, Wakeling AE 2000 ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer 89:817–825 [DOI] [PubMed] [Google Scholar]
- Kang L, Wang ZY 2009 October 15 Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with downregulation of estrogen receptor-α36. J Cell Mol Med 10.1111/j.1582-4934.2009.00877.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoudi A, Roulet E, Dauvois S, Parker MG, Wahli W 1995 Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc Natl Acad Sci USA 92:4206–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce ST, Liu H, Jordan VC 2003 Modulation of estrogen receptor α function and stability by tamoxifen and a critical amino acid (Asp-538) in helix 12. J Biol Chem 278:7630–7638 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ 2003 Inhibition of aromatase: insights from recent studies. Steroids 68:559–567 [DOI] [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE 2002 Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20:751–757 [DOI] [PubMed] [Google Scholar]
- Masamura S, Santner SJ, Heitjan DF, Santen RJ 1995 Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab 80:2918–2925 [DOI] [PubMed] [Google Scholar]
- Shim WS, Conaway M, Masamura S, Yue W, Wang JP, Kmar R, Santen RJ 2000 Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology 141:396–405 [DOI] [PubMed] [Google Scholar]
- Kang L, Ding L, Wang ZY 2009 Isothiocyanates repress estrogen receptor α expression in breast cancer cells. Oncol Rep 21:185–192 [PMC free article] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ 1998 Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662 [Google Scholar]