Abstract
Using a mouse model engineered to measure estrogen receptor (ER) transcriptional activity in living organisms, we investigated the effect of long-term (21 d) hormone replacement on ER signaling by whole-body in vivo imaging. Estrogens and selective ER modulators were administered daily at doses equivalent to those used in humans as calculated by the allometric approach. As controls, ER activity was measured also in cycling and ovariectomized mice. The study demonstrated that ER-dependent transcriptional activity oscillated in time, and the frequency and amplitude of the transcription pulses was strictly associated with the target tissue and the estrogenic compound administered. Our results indicate that the spatiotemporal activity of selective ER modulators is predictive of their structure, demonstrating that the analysis of the effect of estrogenic compounds on a single surrogate marker of ER transcriptional activity is sufficient to classify families of compounds structurally and functionally related. For more than one century, the measure of drug structure-activity relationships has been based on mathematical equations describing the interaction of the drug with its biological receptor. The understanding of the multiplicity of biological responses induced by the drug-receptor interaction demonstrated the limits of current approach and the necessity to develop novel concepts for the quantitative analysis of drug action. Here, a systematic study of spatiotemporal effects is proposed as a measure of drug efficacy for the classification of pharmacologically active compounds. The application of this methodology is expected to simplify the identification of families of molecules functionally correlated and to speed up the process of drug discovery.
The use of reporter mouse facilitates the identification of SERMs able to mimic the estrous cycle by imaging estrogen receptor activity in living mice.
Estrogens are steroidal hormones produced primarily by the ovaries. Estrogens regulate reproductive functions and control target cell activities in the immune, nervous, cardiovascular, gastrointestinal, and muscle-skeletal systems by binding to specific receptors of which two, estrogen receptor (ER)α and ERβ, have been described. ERs are ligand-activated transcription factors (TFs), and there is strong evidence supporting their involvement in extranuclear signaling (1).
Given the wide range of activities of endogenous estrogens during the reproductive years and the significantly increased risk of cardiovascular, immune, and skeletal disorders after menopause (2,3,4,5,6), a major effort has been made to develop hormone replacement therapies aimed at providing aging women with the same biological advantages observed before cessation of ovarian functions (7,8,9). Having observed that the continuous administration of endogenous female sex hormones was associated with the risk of undesired hyperproliferation in the reproductive tissues and that synthetic estrogenic compounds displayed tissue-selective agonist/antagonist activity, an attempt was made to develop compounds agonists in nonreproductive tissues such as the skeleton and antagonists (or perhaps more appropriately, neutral compounds) in the reproductive organs [the so-called selective ER modulators (SERMs)] (10). Indeed, over two decades of concerted effort to develop SERMs has led to the generation of molecules with limitations in their clinical use despite the fact that they interact avidly with their intended target, the ER. The difficulty of identifying estrogenic compounds with the desired profile of activity and safety is still the object of a large debate in the scientific community (2,7,8,9). In the attempt to develop a truly specific SERM, complex comparative studies involving expression profiling (11,12), coregulator interactions (13), and molecular modeling (14) have been applied. These efforts provided a much deeper insight in our understanding of ER intracellular physiology and mechanism of action but minor advancement in the generation of a methodology able to consistently compare the effects of the synthetic compounds generated with the activity of endogenous estrogens in intact, cycling subjects.
A common trait of any methodology that has been applied to the systematic classification of new molecular entities is the lack of consideration of the time dimension. However, it is well known that in each target cell, the nature and the quality of the transcriptional response to estrogens is a function of the combinatorial interaction among at least four very dynamic populations: ligands (including their pharmacokinetic profile and their metabolites), ERs (including isoforms, splice variants, and hetero- vs. homodimers), ER-modifying enzymes (e.g. kinases, acetylases, and small-ubiquitin modifying enzymes), and coregulators (including a panoply of cis- and trans-acting factors) (1,15). These populations of factors may change significantly in response to uninterrupted ligand interaction with the receptor (16), and abundant data demonstrate that in the case of prolonged exposure to agonists, intracellular receptors may be subjected to down-regulation (17,18,19,20). Furthermore, increasing evidence shows that the nature of the stimulus responsible for the receptor transactivation activity may significantly change the dynamics of ER interaction with their responsive elements at the chromatin level (21). Thus, the time dimension in the analysis of ER activity is receiving increasing interest.
To test the power of temporal measurements in the assessment of drug efficacy, we studied the effect of ER synthetic ligands in a mouse model engineered to obtain whole-body expression of luciferase in response to ER activation. Thorough studies demonstrated that in this mouse, named ERE-Luc, luciferase activity is a faithful surrogate marker of ER transcriptional activity (22,23,24). The main advantage of the use of this model is that luciferase activity can be measured in living animals by quantitative analysis of photon emission from selected body areas (25); this facilitates the study of ER activity in the time dimension.
Here, the study of ER shows that structurally related compounds induce unique spatiotemporal profiles of transcription of the ERE-luciferase surrogate target. Based on these results, we propose a novel functional classification of estrogenic compounds that may speed the identification of more efficacious and safer therapies for the postmenopause and facilitate the comprehension of the overall effects of endocrine disruptors present in the environment and alimentary chain.
Results
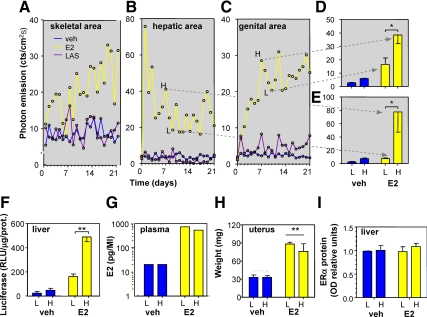
Longitudinal studies were carried out by administering the selected compounds for 21 consecutive days to groups of 5–10 adult female, heterozygous, ERE-Luc reporter mice with C57/BL6J background. Three weeks before initial SERM dosing, mice were ovariectomized (OVX) to eliminate circulating estrogens as a surrogate model of menopause. We also studied age-matched, cycling (intact) female mice as a positive control for the hormonal replacement studies. Mice were treated in the morning (0900 h) at the following daily concentrations: 17β-estradiol (E2), 6 μg/kg·d sc pellet (scp); conjugated estrogens (CE, Premarin), 3 mg/kg per os; bazedoxifene (BZA), 10 mg/kg per os; lasofoxifene (LAS), 50 μg/kg scp; ospemifene (OSP), 2 mg/kg scp; raloxifene (RAL), low 2 mg/kg and high 10 mg/kg per os and scp; and tamoxifen (TAM), 0.8 mg/kg scp. During the chronic study, photon emission was measured in selected body areas by means of a segmentation algorithm previously described (25) once a day (at 1500 h) (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). At the end of the study, we plotted the photon emission measured daily in each animal vs. time (Supplemental Figs. 2–6). In the body areas studied, each compound had a different profile of activity as better exemplified for the skeletal, hepatic and genital area after treatment with E2 and LAS in Fig. 1, A–C. In the skeletal and genital areas of mice treated with E2, luciferase activity was found to increase with time of exposure; in contrast, in the hepatic area, photon emission increased rapidly after E2 administration and decreased over time. LAS resulted in little to no change in the hepatic and skeletal areas, but in the genital area, photon emission became higher than in controls toward the end of the treatment interval. In the OVX mice, in all anatomical areas taken in consideration, ER activity did not change noticeably during the treatment. The analysis of the signals in each animal showed that photon emission fluctuated over consecutive days of exposure in all mice studied, including the OVX. Further in-depth analysis of the bioluminescence profile in time showed that with some of the drugs, this oscillatory trend appeared to have a fixed amplitude and frequency (e.g. LAS in the genital area) (Fig. 1C). By comparing the effects of the different treatments in each experimental group, we concluded that such an oscillation was a characteristic response to the specific ligand in the various tissues examined (Supplemental Figs. 2–6).
Figure 1.
Uninterrupted drug administration induces discontinuous reporter accumulation. Profile of photon emission in 21 d in skeletal (A), hepatic (B), and genital (C) areas of single, representative, OVX ERE-Luc mice after treatment with vehicle (veh), E2, or LAS. Drugs were administered via a dorsal implant of a continuous-release pellet delivering the compounds at a fixed concentration. Photon emission in discrete body regions was segmented in a Matlab environment using an algorithm previously described (25) and defined as the number of counts per second per centimeter square (cts/cm2s) corrected for instrument efficiency. In a parallel experiment, photon emission was measured daily in individual animals treated with vehicle (veh) or E2; groups of six mice were euthanized at the high (H) or low (L) photon emission (D and E), blood was collected, and uterus and liver tissues were dissected. F, Luciferase enzymatic activity in liver tissue extracts. G, E2 content was measured in samples of plasma pooled from two mice using gas chromatography mass spectrometry (see Materials and Methods). The analysis was done in duplicate on a total of three samples for each experimental condition. H, Weight of uterus frozen tissue. Bars represent mean ± sem (n = 6). *, P = 0.043 (D); *, P = 0.043 (E); **, P = 0.002 (F); **, P = 0.003 (H) (ANOVA followed by Bonferroni). I, ERα protein content as measured by Western blot in liver tissue extracts (n = 6).
To establish that the oscillatory pathway was not due to imaging artifacts, we measured photon emission from the hepatic and genital areas in a group of vehicle- and E2-treated OVX ERE-Luc mice for several days. Mice were then euthanized when bioluminescence was highest or lowest (Fig. 1, D and E). Enzymatic quantitative analysis of luciferase activity in tissue extracts proved that the changes in bioluminescence reflected a differential accumulation of the enzyme in time in liver (Fig. 1F) and in vagina (not shown). To investigate whether the pulses of photon emission were induced by changes of circulating ER ligands, we measured E2 in plasma. We found serum levels of E2 to be identical in animals euthanized at the phases of high or low photon emission (Fig. 1G). In line with this observation, the uterus weight did not change in relation to the state of ER transcriptional activity but was clearly responsive to OVX and E2 treatment (Fig. 1H). Thus, the changes in bioluminescence clearly reflected changes in luciferase content in the tissues and were unlikely to be caused by fluctuations of ligands in the bloodstream. Next we tested whether the oscillations of photon emission were produced by ligand-dependent changes in the expression of ERs (4,5,6). Figure 1I shows that in liver, ER content was stable in time and not regulated by OVX or prolonged treatment with E2.
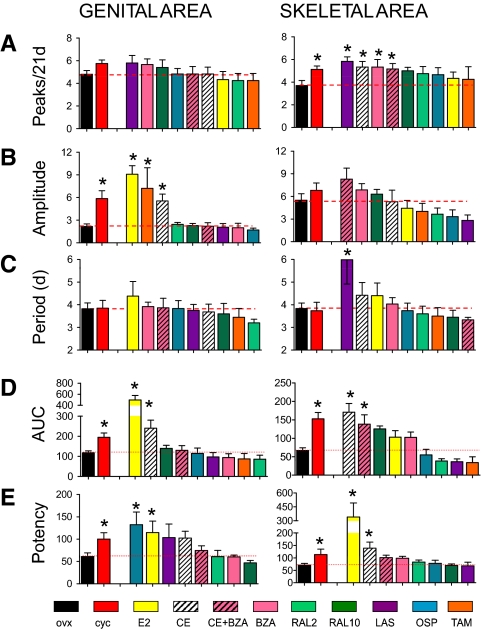
Having established that the fluctuations in photon emission were likely to reflect changes in the ability of the ligand-ER complex to interact with the transcriptional apparatus, we concluded that the quantitative analysis of such oscillatory behavior might have provided an insight on the pharmacological efficacy of each drug. Thus, we further studied the curves relative to luciferase-induced activity in the 21 d of observation. Using differential calculus (local maxima analysis), we counted the number of peaks of luciferase activity induced by each treatment in the 21-d timeframe. The amplitude and frequency of each cycle was measured by spectral analysis. Figure 2 shows exemplificative results obtained in two of the body areas where photon emission had been studied: genital and skeletal tissues. In intact cycling females, the number and amplitude of the peaks displayed was higher than in OVX females, confirming that ER transcriptional activity is reduced with estrogen deficiency (e.g. postmenopause). Interestingly, several compounds, including LAS, CE, BZA, and CE plus BZA increased significantly the numbers of peaks in the skeletal but not in the genital area (Fig. 2A). Conversely, quite sensitive to the treatments was the amplitude of the cycles in the genital areas, as shown by the significant enhancement induced by E2, TAM, and CE (Fig. 2B). The 4-d periodicity of the cycles observed in the cycling animals was conserved in most treatments (surprisingly also in OVX mice). The only exception found was in the skeletal area where LAS decreased the frequency of the pulses to about 6 d (Fig. 2C).
Figure 2.
Comparative analysis of the effect of treatment with selected SERMs on luciferase accumulation in skeletal and genital areas of ERE-Luc OVX mice. A–D, OVX females ERE-Luc mice were treated daily with 6 μg/kg sc E2, 3 mg/kg per os CE, 10 mg/kg per os BZA, 50 μg/kg sc LAS, 2 mg/kg sc OSP, 2 or 10 mg/kg per os RAL, or 0.8 mg/kg per os TAM. Photon emission measured in individual ERE-Luc mice was plotted against time (21 d). Bars represent the mean ± sem of a minimum of five animals per group. A, Peak number. Data were scored from the second-derivative plot using GraphPad Prism. On the basis of the variability of photon counting (coefficient of variation 12%), peaks less than the 15% of the distance between the minimum and the maximum are ignored [*, cycling (cyc) P = 0.023; LAS P = 0.004; CE P = 0.021; BZA P = 0.036; BZA+CE P = 0.028]. B, Peak amplitude. Photon emission was centered on the y-axis by the first derivative, and photon emission rates were then processed by fast Fourier transformation into its component sine waves with a 64 zero padding. Analyzed spectra were windowed at bins 3 and 10. The amplitude, estimating the degree of displacement from the resting state, was calculated as the square root of the 95th percentile of the power spectra (*, cyc P = 0.002; E2 P = 0.001; TAM P = 0.011; CE P = 0.003). C, Peak period. Periodicity is estimated by the inverse of the frequencies under the amplitude previously calculated (*, LAS P = 0.019). D, AUC. The AUC of the plot of photon emission in the 21 d treatment was calculated using GraphPad Prism by a trapezoidal approximation (*, genital area cyc P = 0.012, E2 P = 0.001, and CE P = 0.004; skeletal area cyc P = 0.001, E2 P = 0.001, and BZA P = 0.002). E, Groups of six ERE-Luc OVX mice were treated as in A, and photon emission in individual areas was measured 6 h after drug administration (*, genital area cyc P = 0.021, OSP P = 0.038, and E2 P = 0.017; skeletal area cyc P = 0.020, E2 P = 0.008, and CE P = 0.013). Bars represent mean ± sem (n = 6). Statistical analysis was done with ANOVA followed by Bonferroni comparing the effect of each experimental group with OVX and cycling mice.
Next, to measure the overall effect of the treatment over time in each animal, we calculated the area under the curve (AUC) relative to the 21 d treatment (Fig. 2D). In cycling mice, the AUC was significantly higher than in the OVX animals in both the genital and skeletal tissue compartments. As expected, E2 and CE significantly increased the AUC in the genital area. Interestingly, E2 failed to affect the AUC in the skeletal area where CE and CE plus BZA where active. In this site, RAL was able to increase photon emission significantly, however, only at the higher dose, which is known to protect against resorptive bone loss (26). Finally, to evaluate the potency of the effect of each drug at the dosage selected for the 21-d study, the ER ligands were administered to groups of six OVX females at time 0, and photon emission was measured at 0, 3, 6, 16, and 24 h after treatment (data not shown). In line with previous observations made in our laboratory (22,23), the highest photon emission was observed at the 6-h time point in all body areas. The amount of luciferase produced at 6 h was therefore selected as a measurement of the potency of each drug in acute treatment. Figure 2E shows photon emission in the genital and skeletal areas at 6 h. Photon emission in cycling mice was measured at the highest point of its oscillation in each organ; thus, bioluminescence was found to be significantly higher than in OVX mice in both areas. With regard to the different treatments, at the dosage used, only OSP and E2 significantly increased ER activity in the genital area, whereas in the skeleton, increased bioluminescence was seen only with E2 and CE.
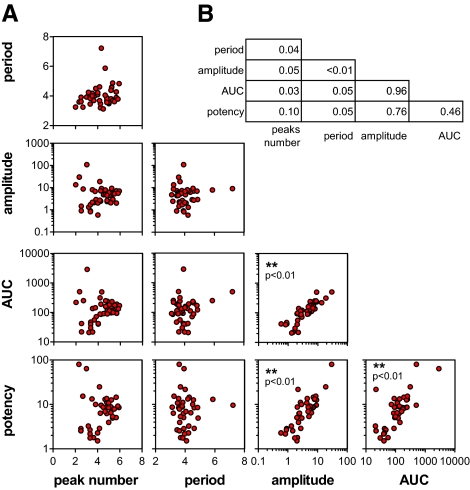
As clearly indicated in Fig. 2, the parameters, namely descriptors, used to measure ER transcriptional activity spatiotemporally were modulated differently by each of the ER ligands. Thus, we asked whether the set of descriptors identified were necessary and sufficient to provide a quantitative assessment of the pharmacological efficacy of the drug tested. First, we verified that the drug property described by each parameter was independent from the others; this was achieved by direct comparison of the parameters in pairs and measuring their coefficient of correlation (Pearson’s R2). Eighty percent of the R2 values indicated a significant lack of correlation among the descriptors selected. The low coefficient of correlation found for all pairs (Fig. 3) indicated that each of the parameters selected described a unique feature of the drug effect and therefore was suitable to be used in a clustering algorithm aimed at verifying the extent to which drugs behave differently or similarly with each other. More importantly, by comparing these sets of parameters of drug-treated mice with those found in OVX or cycling animals, we were able to have a measure of how much each drug was effective in replacing the endogenous hormones and thus constituted the best replacement therapy.
Figure 3.
Extent of functional correlation among each descriptor of drug activity. A, Correlation analysis was done for each pair of descriptors to verify the degree of redundancy of the parameters selected for the clustering analysis; B, degree of correlation in each pair of drug activity descriptors as measured by Pearson’s R2.
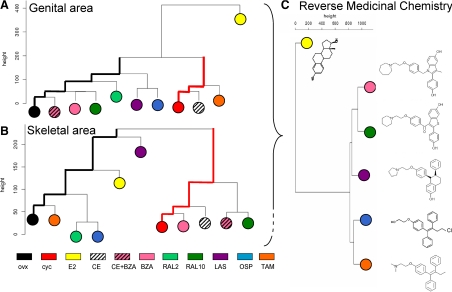
Drug classification was obtained using clustering analysis (27). For each anatomical area, a dissimilarity matrix based on Manhattan distances between descriptors was analyzed. The resulting dendrograms grouped SERMs according to their relative similarities in biological activity. Most interestingly, Fig. 4 shows the large Manhattan distance of both genital (Fig. 4A) and skeletal (Fig. 4B) areas in OVX and cycling females. This was also observed in the other body areas such as tail, abdomen, and thymus (data not shown) and indicates that these two biological conditions represent two extremes for ER activity on the ERE-Luc reporter gene. As expected, the compounds studied grouped differentially in the two body areas. In the genital area RAL and BZA, two well-described antagonists of ER in uterus (28,29), clustered with OVX mice, whereas CE was found very close to cycling animals. However, when CE was administered in association with BZA, the combination therapy moved into the OVX cluster. OSP and LAS, two SERMs known to have an activity on vaginal atrophy (30,31), emerged as basal branches in the OVX cluster. Unlike CE, E2 did not group with cycling mice, possibly indicating that the mixture of estrogenic compounds in CE are able to better mimic the state of ER activation during the estrous cycle (32). A different picture was obtained by the cluster analysis in the areas representative of the skeletal tissue where CE, BZA, and RAL (only at the higher dosage) were found to group with cycling animals (9). E2 and LAS emerged closer to (but still rather distant from) OVX animals (33), although the closest neighbors of the OVX mice were TAM (34) and a subcluster containing OSP and low-dose RAL. These data led us to conclude that the descriptors used to define the efficacy of drug action in the different tissues provided a view of the activity of the compounds used in this study that were in line with our current knowledge of their biological action.
Figure 4.
Phenetics of drug action. Each descriptor (peak number, amplitude, period, AUC, and drug potency) was normalized on the average calculated on intact cycling (cyc) females (considered equal to 100). A matrix was built for each anatomical area: each column contains one descriptor, and each cell contains the descriptor averages for each experimental group. Agglomerative hierarchical clustering (27) was computed with a Manhattan metric and a complete linkage method with an R code available online (Agglomerative Nesting version 1.0.2, Office for Research Development and Education; http://www.wessa.net/). In the dendrogram, distances between branch lengths represents the distance of the menopause model (OVX) vs. the physiology model (cyc); hormone replacement efficacy is measured by its ability to mimic ER activity in the cycling mice. A and B, SERM classification (hierarchical clustering) in the genital area (A) and in skeletal area (B); C, multidimensional imaging descriptors from all anatomical areas measured (genital, skeletal, hepatic, abdominal, and thymic) are clustered as above; dendrogram branches group families of structurally related compounds.
To further challenge the ability of the method to classify drugs on the basis of their spatiotemporal activity, we used all the descriptors identifying the effect of SERMs on ER in the different ERE-Luc anatomical areas (genital, skeletal, hepatic, abdominal, and thymic) to create a single phenogram. This analysis, by containing the features of drug action in time in different organs, may be considered a multivariate fingerprint of drug efficacy. Figure 4C shows that the analysis of the biological data describing the activity of E2, BAZ, RAL, LAS, OSP, and TAM led to clusters of compounds very related from the structural point of view, thus demonstrating that the detailed analysis of the effects of the different treatments in space and time carried out by us on a single surrogate marker was sufficient to group compounds belonging to the same chemical family, thus proving the unique strength of the methodology applied here.
Discussion
The present study provides the first demonstration that in living organisms subjected to long-term stimulation with natural and synthetic ligands, ER-mediated transcriptional activity oscillates with pulses of a frequency and amplitude of the transcription pulses that is strictly associated with the type of estrogenic compound administered, its dosage, and the organ investigated. The accurate spatiotemporal measurement of such oscillations also in comparison with OVX and cycling mice provides a unique and novel methodology to measure drug efficacy.
It is now well accepted that transcriptional regulation by nuclear receptors is a dynamic and cyclical process (35). Biochemical analysis (chromatin immunoprecipitation and nuclear run-on) as well as molecular imaging in living cells demonstrated significant oscillations in transcription mediated by inducible TFs such as ER (36), androgen receptor (37), glucocorticoid receptor (35), or nuclear factor-κB (38). Depending on the mechanism involved in the phenomenon, TF-dependent transcription oscillates in a timeframe of seconds, minutes, or hours (39). Initial chromatin immunoprecipitation studies showed that upon E2 stimulation, the levels of ER and its coregulator at the promoter of target genes can cycle with 20- to 40-min intervals (36); these observations were supported by nuclear run-on assay on selected endogenous target genes that expanded the phase of the pulse to up to 2 h. In vivo imaging studies based on fluorescence recovery after photobleaching enabled to visualize ER dynamic activity at the chromatin level and demonstrate dissimilar pulses upon ER stimulation with natural or synthetic ligands (40). Specific mechanisms have been recognized to explain the dynamics of oscillation described by studies at the cellular level (35,39). These include 1) shuttling of the TF between nucleus and cytoplasm; 2) complex interactions between multiple activating regulatory proteins, OVX, and the chromatin template at the promoter of target genes; and 3) fluctuation of the stimulating effector.
Here we report that in response to a continuous stimulus induced by natural and synthetic ER ligands, the oscillations of ER-induced transcriptional activity have a timeframe of days. We tested several hypotheses made to explain the mechanism of daily pulsatility. First, we ruled out the hypothesis of a discontinuous presence of the stimulating ligands because E2 plasma concentration and uterine weight (the classical estrogenic bioassay) were both found unchanged at a low or high level of luciferase transcription. Indeed, the same conclusion had been suggested by the observation that in each animal, not all organs cycled synchronously and with the same frequency. Furthermore, the periodicity of oscillation was independent of the half-life of each ligand (e.g. BAZ and RAL have a half-life in mouse of 2–4 h, yet the period of oscillation was about 4 d in uterus like LAS (Komm, B., personal communication), which has a half-life in mouse of about 7 d (Komm, B., personal communication).
Next, we considered the possibility of changes in ER protein content. Previous studies have shown that ER protein is decreased after ligand stimulation (17,18,19,20,21), although ER turnover is quite rapid (3–5 h); thus, the levels of ER may be quickly replenished. This notion played against cycles of pulses in a timeframe of days. Furthermore, a protective mechanism against loss of hormone responsiveness was described during continuous stimulation whereby ERα protein is stabilized due first to a decreased rate of proteolysis and second to the accumulation of proteasome-resistant, phosphorylated form of receptor (41,42). In keeping with this observation, we found the same content of ERs at both phases of high or low transcriptional activity when we measured ERα content in liver stimulated with E2. Finally, we discarded the hypothesis of transcriptional pulses generated by changes in the ratio or interaction between the two ER receptors, ERα and ERβ, because the transcription pulses were observed also in liver, an organ that expresses only ERα (43 and data not shown).
Thus, the cause and the mechanism responsible for the oscillations here described remains to be elucidated in molecular terms, but we believe that the dynamics of ER transcriptional activity are very relevant from the physiological point of view and need to be reproduced faithfully in hormone replacement therapy. It has been long known that pulsatility represents a common mechanism for most hormones acting through intracellular as well as membrane receptors. Hormones such as gonadotropins or GH fail to exert their physiological effects if not administered with the correct rhythmicity, and ultradian hormone stimulation is essential to induce a truthful transcriptional response to GR stimulation (44). Endogenous estrogens are released as a result of activity of highly regulated and dynamic connections in the hypothalamic-pituitary-gonadal axis. The temporal activity of the hypothalamic-pituitary-gonadal axis is set to ensure a well- controlled gene expression, necessary to maintain all the physiological functions of estrogens in reproductive and nonreproductive organs. Thus, to maximize the efficacy of hormone replacement therapies, we should identify ligands that can closely mimic the temporal effects of endogenous hormones. Clustering compounds on the bases of their effects in space and time on ER transcriptional activity and assessing the extent to which the pharmacological treatment overcomes the effect of ovariectomy and mimics the activity of ER in cycling mice may open the way to the identification of an efficacious and safe treatment for the postmenopause. Furthermore, classifying alimentary and environmental endocrine disrupters and comparing their activity to well-studied compounds may facilitate the understanding of their real toxicity.
The results of the clustering analysis in the genital area are in line with data presented in clinical and preclinical literature and underline the known activity of TAM (9), OSP (30), and LAS (31) in the vagina. More puzzling are the results in bone where TAM was found to group with OVX mice. Indeed TAM has never been prescribed for the prevention of osteoporosis in women due to the observation that it caused loss of bone mineral density in premenopausal women (34); however, additional studies in postmenopausal women showed protective activity of TAM in bone (9). These findings indicate a complexity of action of SERMs in bone that awaits further experimental explanation.
We believe that the relevance of the present study goes far beyond the field of estrogen action. The methodology here developed that enables the classification of molecular entities on the basis of their actions in the four dimensions (thus including the time dimension) may represent the highly needed, novel, paradigm to measure drug efficacy. Drug classification is currently based on principles set at the beginning of the last century. In fact, it dates at that time the introduction of the drug-receptor interaction theory that enabled the measurement of the ability of a given molecule to interact with its receptor (namely affinity) and to induce biological effects (efficacy) (45,46,47). Drug efficacy, originally evaluated in organ cultures, is now generally characterized in cell cultures. However, the present understanding of the plasticity and promiscuity of intracellular signaling pathways highlighted the current limitations to provide an unambiguous classification of drug efficacy (48,49,50) and the necessity to find novel, more efficacious ways to compare families of molecular entities. The best example of the difficulties in categorizing biologically active compounds was provided by ER ligands (51,52). Compounds such as TAM, RAL, or BZA were described to either block or induce ER transcriptional activity depending on the target tissue or gene under consideration (53,54), and a novel terminology was introduced (SERMs) in an attempt to differentiate these molecules from other estrogens. Yet, when studying SERM effects in a single target cell, each of these molecules may be redefined as an ER agonist or antagonist dependent upon the signaling pathway considered. This lack of a definite method to classify drug actions is common to all classes of drugs and is considered as a major obstacle in drug development (55).
A well-known feature in drug action is the transformation of its pharmacological effect with time due to the mechanisms of defense that each organism has against xenobiotics. As a consequence, drug efficacy should be evaluated in different organs and at different times. The use of reporter animals offers for the first time the opportunity to measure drug effects spatiotemporally, opening the way to the generation of novel methods to classify drugs. In addition, the study of drug effects on a well- defined target facilitates the assessment the efficacy of the treatment in models of disease and the direct comparison of the effect of the drug with the physiological, healthy status. In our view, the present study provides compelling evidence for the power of longitudinal imaging and anticipates the possibility of a reverse approach in medicinal chemistry where the spatiotemporal measurement of an intended target drives the classification of chemically related compounds.
Materials and Methods
Experimental animals
All animal experimentation was carried out in accordance with European guidelines for animal care and use of experimental animals, approved by the Italian Ministry of Research and University, and controlled by the panel of experts of the Department of Pharmacological Sciences, University of Milan.
Compounds tested
E2, RAL, and TAM were purchased from Sigma-Aldrich (Pomezia, Italy); EC and BAZ were from Wyeth (Collegville, PA); and LAS and OSP were from Hormos Medical Ltd. (Turku, Finland). Compounds were administered at doses equivalent to those used in humans as calculated by the allometric approach and further harmonized with the companies that developed the drugs to be closer to their previous preclinical data. All control groups (OVX) received vehicles.
In vivo imaging
Raw bioluminescence was measured with tiff images of 512 × 512 pixels at 16 bits. Each pixel contained the number of counts detected over the exposure period of 5 min at the resolution of about 0.3 pixels/mm. Background was estimated on the average of 10 background acquisitions and arithmetically subtracted from the raw images. Before each imaging session, instrumental efficiency was measured with an external source of photons (Glowell, Lux Biotechnology, Edinburgh, UK). Anatomical areas (limb and tail/skeletal, genital, hepatic, abdominal, and thymic) were segmented in a Matlab environment using an algorithm previously described. In each anatomical area, photon emission was defined as the number of counts per second per centimeter squared corrected for instrument efficiency. All the measurements were in the linearity range of the detector (IVIS Lumina; Caliper Life Sciences, Hopkinton, MA).
Quantitative analysis of plasma E2
Steroids were extracted according to Caruso et al. (56) with minor modification. Briefly, the deuterated internal standard 2,4,16,16-D4-17β-estradiol (D4-17β-E; CDN Isotope, Pointe-Claire, Quebec, Canada) was added to 100–200 μl plasma. After addition of acetic acid (1% in methanol), samples were loaded onto C18 cartridges (Discovery DSC-18, 500 mg; Supelco, Milano, Italy). The steroid fraction was eluted with methanol (5 ml), and the organic residue was reconstituted with methanol/water (1:1) before the injection in a RP-C18 analytical column (Hypersil GOLD; Thermo Fisher Scientific Inc., Rodano, Italy; 3 μm, 100 mm × 3 mm inner diameter). The HPLC (Surveyor LC Pump Plus; Thermo Fisher Scientific Co., Waltham, MA) was coupled to a linear ion trap mass spectrometer (LTQ; Fisher Scientific) equipped with an atmospheric pressure chemical ionization source operating in the positive ion mode. E2 was identified on the basis of both the retention time and the tandem mass spectrometry spectrum of reference compounds. The quantitative analyses were done monitoring specific ions (selected ion chromatogram mode) in the tandem mass spectrometry spectrum obtained by collision of precursor ion in the mass spectrometry spectrum (56) using calibration curves generated with deuterium-labeled internal standards.
Western analysis
Was carried out Western analysis as previously described. Briefly, after quantification of the proteins in whole-cell extracts, 28 μg cell protein was loaded onto discontinuous gradient SDS-PAGE (10–5%) gels. After electrophoresis, proteins were transferred to nitrocellulose membrane and incubated first with the primary antibodies (all 1:1000) overnight and then with the peroxidase-conjugated secondary antibody (Bio-Rad, Hercules, CA) for 1 h. Proteins were detected by chemiluminescence (Amersham Biosciences, Arlington Heights, IL).
Statistical analysis
P values were calculated with one-way ANOVA followed by Bonferroni post hoc test with GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank G. E. Rovati and V. Capra for advice on assessment of drug efficacy, E. Casiraghi and C. Lenardi for discussions on the use of bioinformatic and mathematic tools, M. Unkila for insights on SERM activity, P. Chambon for an overall view on the study, Monica Rebecchi, Clara Meda, and Renata Janczara for technical assistance, and Paolo Sparaciari for veterinary assistance.
Footnotes
Support grants were received from the European Union (STREP Estrogen in Women Ageing (EWA) LSHM-CT-2005-518245; NoE Diagnostic Molecular Imaging (DiMI) LSHB-CT-2005-512146; IP CRESCENDO LSHM-CT-2005-018652), National Institutes of Health (RO1AG027713), and Wyeth Pharmaceutical Co.
Disclosure Summary: The authors declare to not have any conflict of interest with regard to the content of the present manuscript.
First Published Online March 2, 2010
Abbreviations: AUC, Area under the curve; BZA, bazedoxifene; CE, conjugated estrogens; E2, 17β-estradiol; ER, estrogen receptor; LAS, lasofoxifene; OSP, ospemifene; OVX, ovariectomized; RAL, raloxifene; scp, sc pellet; SERM, selective ER modulator; TAM, tamoxifen; TF, transcription factor.
References
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA 2006 International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781 [DOI] [PubMed] [Google Scholar]
- Bolego C, Vegeto E, Pinna C, Maggi A, Cignarella A Selective agonists of estrogen receptor isoforms: new perspectives for cardiovascular disease. 2006 Arterioscler Thromb Vasc Biol 26:2192–2199 [DOI] [PubMed] [Google Scholar]
- Imai Y, Kondoh S, Kouzmenko A, Kato S 2009 Regulation of bone metabolism by nuclear receptors. Mol Cell Endocrinol 310:3–10 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB 2003 Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 290:1729–1738 [DOI] [PubMed] [Google Scholar]
- Straub RH The complex role of estrogens in inflammation. 2007 Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S 2004 Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- Johnson KA 2006 The SERM of my dreams. J Clin Endocrinol Metab 91:3754–3756 [DOI] [PubMed] [Google Scholar]
- Tannen RL, Weiner MG, Xie D, Barnhart K 2007 Estrogen affects post-menopausal women differently than estrogen plus progestin replacement therapy. Hum Reprod 22:1769–1777 [DOI] [PubMed] [Google Scholar]
- Komm BS 2008 A new approach to menopausal therapy: the tissue selective estrogen complex. Reprod Sci 15:984–992 [DOI] [PubMed] [Google Scholar]
- Jordan VC 2004 Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5:207–213 [DOI] [PubMed] [Google Scholar]
- Levenson AS, Kliakhandler IL, Svoboda KM, Pease KM, Kaiser SA, Ward 3rd JE, Jordan VC 2002 Molecular classification of selective oestrogen receptor modulators on the basis of gene expression profiles of breast cancer cells expressing oestrogen receptor α. Br J Cancer 87:449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafoglio C, Ambrosino C, Cicatiello L, Altucci L, Ardovino M, Bontempo P, Medici N, Molinari AM, Nebbioso A, Facchiano A, Calogero RA, Elkon R, Menini N, Ponzone R, Biglia N, Sismondi P, De Bortoli M, Weisz A 2006 Comparative gene expression profiling reveals partially overlapping but distinct genomic actions of different antiestrogens in human breast cancer cells. J Cell Biochem 98:1163–1184 [DOI] [PubMed] [Google Scholar]
- Kremoser C, Albers M, Burris TP, Deuschle U, Koegl M 2007 Panning for SNuRMs: using cofactor profiling for the rational discovery of selective nuclear receptor modulators. Drug Discov Today 12:860–869 [DOI] [PubMed] [Google Scholar]
- Wu YL, Yang X, Ren Z, McDonnell DP, Norris JD, Willson TM, Greene GL 2005 Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell 18:413–424 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen JA, O'Malley BW, Katzenellenbogen BS 1996 Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol 10:119–131 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Jensen EV, Suzuki T, Numata M, Smith S, DeSombre ER 1969 Estrogen-binding substances of target tissues. Steroids 13:417–427 [DOI] [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, Martin MB 1988 Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol 2:1157–1162 [DOI] [PubMed] [Google Scholar]
- Nardulli AM, Katzenellenbogen BS 1986 Dynamics of estrogen receptor turnover in uterine cells in vitro and in uteri in vivo. Endocrinology 119:2038–2046 [DOI] [PubMed] [Google Scholar]
- Wu RC, Feng Q, Lonard DM, O'Malley BW 2007 SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129:1125–1140 [DOI] [PubMed] [Google Scholar]
- O'Malley BW 2009 The “fourth dimension” of gene transcription. Mol Endocrinol 23:587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A 2001 Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol 15:1104–1113 [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A 2003 In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- Rando G, Biserni A, Ciana P, Maggi A 2010 Profiling of drug action using reporter mice and molecular imaging. Methods Mol Biol 602:79–92 [DOI] [PubMed] [Google Scholar]
- Rando G, Casiraghi E, Arca S, Campadelli P, Maggi A, 2009 Automatic segmentation of mouse images. Proc 10th European Congress of International Society for Stereology, Bologna, Italy, 2009, p 60 [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR, Frolik CA, Termine JD, Bryant HU 1994 Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ 1990 Finding groups in data: an introduction to cluster analysis. New York: Wiley [Google Scholar]
- Ashby J, Odum J, Foster JR 1997 Activity of raloxifene in immature and ovariectomized rat uterotrophic assays. Regul Toxicol Pharmacol 25:226–231 [DOI] [PubMed] [Google Scholar]
- Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR 2005 Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology 146:3999–4008 [DOI] [PubMed] [Google Scholar]
- Voipio SK, Komi J, Kangas L, Halonen K, DeGregorio MW, Erkkola RU 2002 Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas 43:207–214 [DOI] [PubMed] [Google Scholar]
- Ke HZ, Paralkar VM, Grasser WA, Crawford DT, Qi H, Simmons HA, Pirie CM, Chidsey-Frink KL, Owen TA, Smock SL, Chen HK, Jee WS, Cameron KO, Rosati RL, Brown TA, Dasilva-Jardine P, Thompson DD 1998 Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology 139:2068–2076 [DOI] [PubMed] [Google Scholar]
- Yasui T, Uemura H, Takikawa M, Irahara M 2003 Hormone replacement therapy in postmenopausal women. J Med Invest 50:136–145 [PubMed] [Google Scholar]
- McClung MR, Siris E, Cummings S, Bolognese M, Ettinger M, Moffett A, Emkey R, Day W, Somayaji V, Lee A 2006 Prevention of bone loss in post-menopausal women treated with lasofoxifene compared with raloxifene. Menopause 13:377–386 [DOI] [PubMed] [Google Scholar]
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S 1996 Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14:78–84 [DOI] [PubMed] [Google Scholar]
- Hager GL, McNally JG, Misteli T 2009 Transcription dynamics. Mol Cell 35:741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Kang Z, Pirskanen A, Jänne OA, Palvimo JJ 2002 Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 277:48366–48371 [DOI] [PubMed] [Google Scholar]
- Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G 2006 A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-κB-dependent gene activity. EMBO J 25:798–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MH, Salvatore L, De Lorenzi R, Indrawan A, Pasparakis M, Hager GL, Bianchi ME, Agresti A 2009 Sustained oscillations of NF-κB produce distinct genome scanning and gene expression profiles. PLoS One 4:e7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O'Malley BW, Mancini MA 2001 FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nat Cell Biol 3:15–23 [DOI] [PubMed] [Google Scholar]
- Valley CC, Métivier R, Solodin NM, Fowler AM, Mashek MT, Hill L, Alarid ET 2005 Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor α N terminus. Mol Cell Biol 25:5417–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET 2008 Temporal variation in estrogen receptor-α protein turnover in the presence of estrogen. J Mol Endocrinol 40:23–34 [DOI] [PubMed] [Google Scholar]
- Gao H, Fält S, Sandelin A, Gustafsson JA, Dahlman-Wright K 2008 Genome-wide identification of estrogen receptor α-binding sites in mouse liver. Mol Endocrinol 22:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL 2009 Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ 1937 General pharmacology. In: Handbuch der experimentallen Parmakologie. Berlin: Springer Verlag [Google Scholar]
- Stephenson RP 1956 A modification of receptor theory. Br J Pharmacol Chemother 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP 2006 The receptor concept: pharmacology’s big idea. Br J Pharmacol 147(Suppl 1):S9–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB 2007 Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Kenakin TP 2008 Pharmacological onomastics: what’s in a name? Br J Pharmacol 153:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M 2007 The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, McDonnell DP, Martin KA, Wise PM 2004 Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304:1269–1273 [DOI] [PubMed] [Google Scholar]
- Pink JJ, Jordan VC 1996 Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res 56:2321–2330 [PubMed] [Google Scholar]
- McDonnell DP 1999 The molecular pharmacology of SERMs. Trends Endocrinol Metab 10:301–311 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2000 An issue of tissues: divining the split personalities of selective estrogen receptor modulators. Nat Med 6:960–962 [DOI] [PubMed] [Google Scholar]
- Kenakin T 2005 New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov 4:919–927 [DOI] [PubMed] [Google Scholar]
- Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC 2008 Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int 52:560–568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.