Abstract
Previous cross-sectional study of ventral prefrontal cortex (VPFC) implicated progressive volume abnormalities during adolescence in bipolar disorder (BD). In the present study, a within-subject, longitudinal design was implemented to examine brain volume changes during adolescence/young adulthood. We hypothesized that VPFC volume decreases over time would be greater in adolescents/young adults with BD than in healthy comparison adolescents/young adults (HC). Eighteen adolescents/young adults (10 with BD I and 8 HC participants) underwent two high resolution magnetic resonance imaging scans over approximately two-years. Regional volume changes over time were measured. Adolescents/young adults with BD displayed significantly greater volume loss over time, compared to HC participants, in a region encompassing ventral and rostral PFC and extending to rostral anterior cingulate cortex (p<0.05). Additional areas where volume change differed between groups were observed. While data should be interpreted cautiously due to modest sample size, this study provides preliminary evidence to support the presence of accelerated loss in ventral and rostral PFC volume in adolescents/young adults with BD.
Keywords: adolescents, bipolar disorder, magnetic resonance imaging, prefrontal cortex, development, longitudinal
Ventral prefrontal cortex (VPFC), a key regulatory component of the cortico-limbic neural system involved in emotional and motivated behavior, is implicated in bipolar disorder (BD) (Blumberg et al., 2002). Neuroimaging studies of adults with BD provide evidence for abnormal VPFC morphology and functioning. The VPFC undergoes structural and functional maturation during adolescence and early adulthood (Giedd et al., 1999; Gogtay et al., 2004; Sowell et al., 1999), coinciding with a peak for acute BD episodes. Abnormal VPFC morphology and function in BD, combined with the intersection of VPFC developmental maturation and the onset of BD, indicate that abnormalities in VPFC neurodevelopment may play a role in the behavioral expression of BD in adolescence and young adulthood. VPFC disturbances may not be expressed fully until the region passes through its course of programmed development in late adolescence/early adulthood, when characteristics of the adult phenotype emerge (Blumberg et al., 2004).
The progressive course of PFC neurodevelopment may contribute to differing results obtained in morphometric study of the region in diverse age groups of those with BD. Gray matter volume decreases in multiple VPFC subregions, including subgenual anterior cingulate cortex (ACC), orbitofrontal cortex, and inferior frontal cortex, are consistently reported in adults with BD (Blumberg et al., 2006; Drevets et al., 1997; Lopez-Larson et al., 2002; Lyoo et al., 2004). In contrast, studies of adolescents with BD have yielded inconsistent findings with reports of volume decreases in sub- and pre-genual ACC and orbitofrontal cortex (Kaur et al., 2005; Wilke et al., 2004) or failures to detect differences (Adler et al., 2007; Chang et al., 2005; Dickstein et al., 2005; Frazier et al., 2005; Sanches et al., 2005). VPFC volume decreases reported by Blumberg et al.(Blumberg et al., 2006) were statistically significant in young adults, but not adolescents, with BD. Based on these cross-sectional results, the authors suggested that VPFC volume abnormalities may progress over the course of adolescence and therefore be less likely to emerge as significantly divergent until late adolescence or early adulthood. We conducted a prospective, longitudinal study of brain volume in adolescents and young adults with BD in order to address this issue empirically. We hypothesized that adolescents/young adults with BD would show greater volume loss in VPFC over time relative to healthy comparison participants (HC).
Method
Participants
Participants consisted of 10 adolescent/young adult outpatients with BD I (5 females) and 8 adolescent/young adult HCs (6 females), as described previously (Blumberg et al., 2005). The HCs did not meet criteria for DSM-IV Axis I diagnoses (American Psychiatric Association, 2000) and had no family history of DSM-IV Axis I diagnoses in their first-degree relatives. Participants with BD were referred from the Yale School of Medicine Medical Center, the Veterans Affairs Connecticut Healthcare System, and the greater New Haven, Connecticut community, and the HCs were recruited from the community. At the time of initial study participation, individuals with BD were between 10 and 21 years of age and HCs were between 11 and 19 years of age. Follow-up study participation occurred on average 2.28 ± 0.55 SD years after baseline participation. Participants were without a history of other neurological disorders, loss of consciousness for longer than five minutes, or significant medical illness, with the exception of one BD participant with treated hypothyroidism.
The revised Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSAD-PL) (Kaufman et al., 1997) was administered to the participants and a parent/guardian for participants 18 years of age and under by a board-certified child psychiatrist or a psychologist expert in childhood mood disorders. For those participants over 18 years of age, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Version 2.0) (First et al., 1995) was administered by a board-certified adult-psychiatrist. These interviews were administered for each participant at both time points. Final DSM-IV diagnoses were established by the consensus of clinical and structured interviews. At the time of the baseline neuroimaging scan, five participants with BD were unmedicated, six met criteria for rapid cycling, nine were in the midst of a mood episode and one was euthymic. At the time of the second neuroimaging session, seven participants with BD were unmedicated, seven met criteria for rapid cycling, and three were euthymic. Psychotropic medications at the first scan included mood stabilizers (n=2), anticonvulsants (n=3), antidepressants (n=2) (both tricyclics and selective serotonergic reuptake inhibitors), atypical antipsychotics (n=2), stimulants (n=1), levothyroxine (n=1) and clonidine (n=1). Psychotropic medications at the second time point included mood stabilizers (n=1), anticonvulsants (n=2), antidepressants (n=2) (both tricyclics and selective serotonergic reuptake inhibitors), atypical antipsychotics (n=2), stimulants (n=2), and atomoxetine (n=1). All five participants who were not taking psychotropic medication at the time of both scans had been exposed to either lithium or valproate or other psychotropic medications. Co-morbidities included attention-deficit/hyperactivity disorder (n=1), oppositional defiant disorder (n=2), social phobia (n=1), posttraumatic stress disorder (n=1), learning disorder not otherwise specified (n=2) and developmental coordination disorder (n=1). One participant had a history of alcohol dependence, and hallucinogen and marijuana abuse, in remission for more than one-year prior to baseline scan. All BD participants reported positive family history, within their first or second -degree relatives, of a mood spectrum disorder, including BD, major depressive disorder, or alcohol abuse. For further detail on sample characterization see (Blumberg et al., 2005).
Following a complete description of the research, written informed consent was obtained from parents/guardians and participants older than 18 years. Written informed assent was obtained from minors. This research was approved by the human investigation committees of the Yale School of Medicine and the Department of Veterans Affairs.
Magnetic Resonance Image (MRI) Acquisition and Processing
MRI scans at baseline and follow-up timepoints were obtained using the same 1.5-T scanner (GE Signa; General Electric, Milwaukee, Wis.). Head positioning was standardized using canthomeatal landmarks. Images were obtained using a 3-dimensional sagittal spoiled gradient echo sequence (repetition time, 24 milliseconds; echo time, 5 milliseconds; flip angle, 450; frequency encoding superior/inferior; no wrap; 256 × 192 matrix; field of view, 30 × 30 cm2; 2 excitations; slice thickness, 1.2 mm; and 124 contiguous slices).
Morphometric measures were computed using the Yale Bioimage Suite software package (http://www.bioimagesuite.org/) running on Linux workstations. We employed a non-rigid registration method that relied on the normalized mutual information metric. The transformation was parameterized using a Free-Form Deformation Model (FFD) (Papademetris et al., 2004; Rueckert et al., 1999). Using this non-rigid registration technique, the follow-up scans for each participant were pairwise registered to the baseline scans. The resulting transformations were then used to produce Jacobian maps of local expansion/contraction that were generated based on the determinant of the Jacobian of the displacement field generated by each transformation (Staib et al., 2006). The Jacobian maps had a resolution of 1.2 × 1.2 × 1.2 mm3 where each voxel has a value representing the local volume change required to map an individual participant's baseline time-point to his/her follow-up time point (i.e. 1=no volume change, >1=follow-up time-point was larger than baseline time-point and <1=baseline time-point was larger than follow-up time-point). The Jacobian maps were checked to ensure that the transformations were all free of singularities (i.e. |J|<0). Next, the baseline images for each participant were normalized to the Colin Brain template which is in Montreal Neurological Institute (MNI) space using the same non-linear registration algorithm and the individual Jacobian maps were then warped to the space of the MNI template using the second registration. Jacobians were computed at each voxel throughout the brain enabling exploration of differences in volume change over time in regions not hypothesized a priori.
Data Analysis
Two-tailed two-sample t-tests (BD vs. HC) were performed using BioImage Suite with Jacobian values as the dependent variables. Findings of volume changes in the brain over time were considered significant at p<0.05 uncorrected, and clusters>50 contiguous voxels. Post-hoc exploratory analyses were performed to determine the potential effects of any demographic factors with large effect sizes, as well as presence or absence of psychotropic medication at time of scanning, on the dependent measures. These exploratory analyses (including analyses covarying for any demographic factors with large effect sizes and an analysis of variance comparing those BD participants who were medicated at either time-point (n=5) to those participants with BD who were unmedicated at both time-points (n=5)) were conducted using signal extracted from each participant's normalized Jacobian maps from the maximum point of the prefrontal regions where volume change over time differed between the two groups.
Results
The groups did not differ significantly in age at the baseline or follow-up scans, time between scan, gender, or SES (see Table 1). Volume decreases from baseline to follow-up were greater in the BD group, relative to the HCs, in bilateral PFC as displayed in orthogonal views in Figure 1. While HCs showed approximately a 0.66% relative volume decrease in this region over time, the BD group showed 3.59% volume decrease in this region. The left PFC cluster included superior and medial frontal gyri [Brodmann's area (BA) 10] and extended to rostral ACC (BA 32) and ventrally to BA 11. The right PFC cluster was in the medial frontal gyrus (BA 10). Differences were also observed in other areas not hypothesized a priori and description for all significant clusters is provided in Table 2. Post-hoc analyses revealed that, amongst the BD participants, no significant effects were detected with regard to the presence or absence of psychotropic medication. While inter-scan interval had a large effect size, on average the interval-scan interval differed by ½-year between groups and analysis using this factor as a covariate resulted in the group difference in volume change over time in the prefrontal regions maintaining statistical significance.
Table 1.
Demographic characteristics of the samples
| Variable | BD (n=10) | HC (n=8) | Test for significance | p | Effect size |
|---|---|---|---|---|---|
| Age at time 1 (yrs) | 15.03 (3.95) | 15.34 (2.82) | t = −0.19 | 0.86 | d = 0.09 |
| Age at time 2 (yrs) | 17.53 (3.88) | 17.36 (2.77) | t = 0.10 | 0.92 | d = 0.05 |
| Inter-scan interval (yrs) | 2.49 (0.43) | 2.03 (0.61) | t = 1.90 | 0.08 | d = 0.88 |
| SES | 2.56 (0.88)* | 2.38 (1.06) | Mann-Whitney U = 32.50 | 0.72 | r = 0.09 |
| Female | 5 (50) | 6 (75) | X2 = 1.17 | 0.28 | w = 0.26 |
| Age at symptom onset (yrs) | 9.60 (4.01) | ----- | ----- | ----- | ----- |
| Disease duration (yrs) | 5.43 (3.58) | ----- | ----- | ----- | ----- |
n=9
Values given as mean (standard deviation), excluding gender given as whole number (percentage). BD=bipolar disorder, HC=healthy comparison participants, SES=socioeconomic status.
Figure 1. Regions of volume loss in prefrontal cortex over time.
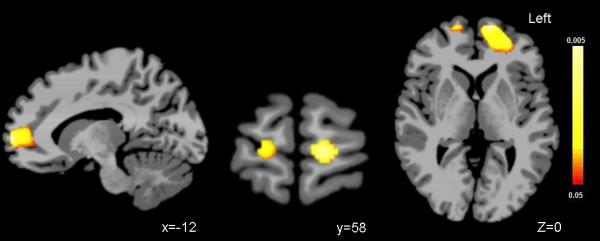
Sagittal (Left), coronal (middle) and axial (right) images show regions of statistically significant volume loss over two years in the ventral and rostral prefrontal cortex in adolescents/young adults with bipolar disorder, compared to healthy comparison adolescents (p<.05). The color bar represents that range of p values from red p<.05 to yellow p<.005.
Table 2.
Regions where volume change over time differed between groups
| Brain Region | x | y | z | Cluster volume (mm3) | Maximum t | p value | d |
|---|---|---|---|---|---|---|---|
| Regions of greater volume decrease over time in BD | |||||||
| Left BA 10, BA 11, BA 32 | −12 | 59 | 2 | 350 | 3.14 | 0.006 | 1.57 |
| Right BA 10 | 15 | 59 | 2 | 84 | 2.64 | 0.018 | 1.32 |
| Left BA 44 | −50 | 20 | 11 | 147 | 2.85 | 0.012 | 1.42 |
| Right BA 44, BA 6 | 49 | −3 | 18 | 250 | 3.08 | 0.007 | 1.54 |
| Left BA 6 | −60 | −19 | 26 | 92 | 2.87 | 0.011 | 1.43 |
| BA 7/31 | −1 | −29 | 46 | 201 | 2.57 | 0.021 | 1.28 |
| Right BA 21 | 45 | −30 | −11 | 193 | 4.16 | 0.001 | 2.08 |
| Regions of greater volume increase over time in BD | |||||||
| Left BA 17 | −24 | −92 | −9 | 97 | 3.53 | 0.003 | 1.77 |
| Left posterior cerebellum | −32 | −79 | −27 | 284 | 3.38 | 0.004 | 1.69 |
| Left anterior cerebellum | −44 | −57 | −32 | 138 | 3.65 | 0.002 | 1.83 |
| Right posterior cerebellum | 47 | −55 | −30 | 452 | 5.34 | <0.001 | 2.67 |
| Left frontal periventricular WM | −21 | −23 | 30 | 1504 | 4.39 | 0.001 | 2.19 |
| Right frontal periventricular WM | 16 | −19 | 29 | 419 | 2.69 | 0.016 | 1.34 |
| Right parietal WM | 21 | −40 | 51 | 180 | 3.36 | 0.004 | 1.68 |
x, y, z coordinates of the maximum point within Talairach space; t statistic for maximum point; BD bipolar disorder; WM white matter; BA Brodmann's area
Discussion
We observed greater volume reductions over time in ventral and rostral PFC bilaterally in adolescents/young adults with BD relative to HCs, extending our previous cross-sectional findings in providing evidence for progressive PFC abnormalities during adolescence in the disorder (Blumberg et al., 2006). To the best of our knowledge, this work, together with our report of persistent amygdala abnormalities over the adolescent developmental epoch (Blumberg et al., 2005), represent the first comparison of volume change over time in adolescents with BD, relative to HCs. While amygdala volume reductions are present early in the disorder and remain stable over this two-year interval, changes in PFC volume progress over this time period suggesting that subcortical and cortical disturbances in BD may emerge at different points in development. Other longitudinal neuroimaging work in BD includes the study of an adolescent sample before and after disease onset by Gogtay et al. (2007) and longitudinal study of an adolescent/young adult BD sample by Farrow et al. that did not incorporate repeat neuroimaging of their HC sample (Farrow et al., 2005). ACC volume loss is consistently reported in these three longitudinal imaging efforts and suggests that the region may be particularly important for the emergence of BD symptomatology in adolescence. Disparities in regions of relative change reported herein and by Gogtay et al. may be related to sampling or methodological differences, such as whether baseline scanning occurred before or after illness onset and the degree of psychosis present in the two samples. While we employed a whole brain analysis, Gogtay et al. studied the cortical surface. Volume changes reported by Gogtay et al. may relate more to onset of mania, while the present findings may convey an interaction between disease progression and neurodevelopmental changes of adolescence. As it is possible that the volume changes detected in our BD group may relate to progressive changes in the disease over time, future longitudinal neuroimaging studies that quantify changes in degree of symptomatology would be of interest.
The progressive course of PFC neurodevelopment may also contribute to divergent results obtained in functional study of the VPFC in diverse age groups of those with BD. Deficits in VPFC activation during Stroop performance have been observed repeatedly in adults with BD (Blumberg, Leung et al., 2003; Kronhaus et al., 2006; Malhi et al., 2005), but these deficits were not detected during a study of Stroop performance in adolescents with BD (Blumberg, Martin et al., 2003). In the latter study, age-related increases in VPFC activation were observed in healthy adolescents but not in adolescents with BD, supporting the presence of abnormalities in VPFC functional maturation over the course of adolescence in BD. Some recent studies have reported VPFC dysfunction in adolescents with BD, including VPFC deficits during commission errors on a stop signal task (Leibenluft et al., 2007), as well as both increases and decreases in VPFC activation relative to controls during the performance of emotional processing tasks (Pavuluri et al., 2007; Rich et al., 2006). These recent study designs may have been especially sensitive to VPFC dysfunction that emerges earlier in adolescence while other aspects of VPFC functional maturation may progress during adolescence and be difficult to detect prior to late adolescence/early adulthood.
The PFC region of greater volume reduction in adolescents/young adults with BD reported herein extended to include a rostral/frontopolar region, consistent with functional abnormalities reported in rostral PFC in adults with BD performing both emotional and cognitive tasks (Blumberg, Leung et al., 2003; Blumberg et al., 1999; Rubinsztein et al., 2001) and the involvement of rostral PFC in reward processing (Rogers et al., 1999) and emotional regulation (Bermpohl et al., 2006), functions impaired in adults with BD. The loss of PFC volume over time in adolescents with BD we observed is similar to the phenomena reported in adolescents with schizophrenia. However, the patterns of localized volume loss differed between the disorders with significant decreases in dorsolateral PFC regions found in schizophrenia (Thompson et al., 2001), suggesting that the regional localization of volume loss over time may contribute to the differing phenotypes. While the pathophysiological mechanisms that may contribute to the progressive prefrontal abnormalities we observed cannot be determined based on this work, it is possible that similar to what is postulated in schizophrenia this volume loss represents an acceleration of normal regressive processes in gray matter during this developmental stage (Weinberger, 1987).
The VPFC is a key component of a larger corticolimbic neural system that is implicated in BD. Consistent with the involvement of other brain regions in BD, we report herein other regions not hypothesized a priori where volume change over time differed between individuals with BD and HCs. The observed greater volume increases over time in posterior and cerebellar regions in the adolescents/young adults with BD relative to the HCs is consistent with a report of volume increases in these regions in first episode patients with BD (Adler et al., 2007). Furthermore, our report of greater volume increases over time in periventricular white matter is supported by findings of increased white matter in BD (Nugent et al., 2006). The specificity to BD of the regional developmental abnormalities reported herein should be evaluated in future studies via direct comparison with other diagnostic groups.
These findings are preliminary and further investigation is required due to limitations including the modest sample size, a wide age range, an inter-scan interval that varied between groups and possible confounding factors such as medication exposure. We did not detect significant effects of presence of medication or inter-scan interval. However, our ability to detect effects could have been limited by inadequate power and future within subject, longitudinal imaging investigations in adolescents with BD with larger sample sizes and increased power to address associations with clinical factors would be important.
Acknowledgements
This article is dedicated to Ms. Kathleen Colonese whose devotion towards advancing bipolar disorder research and aiding those suffering from psychiatric illnesses helped make this longitudinal study possible. The authors thank Karen Martin, R.T.R.M.R., Terry Hickey, R.T.R.M.R. and Hedy Sarofin, R.T.R.M.R. for their technical expertise and the research subjects for their participation.
Funding Sources: This work was supported by grants from the NIMH R01MH69747 (HPB), R01MH070902 (HPB), T32MH14276 (JHK), NIH/NINDS R01NS35193 (JSD, LHS, RTC, XP), the Department of Veterans Affairs Research Enhancement Award Program (HPB), the National Alliance for Research in Schizophrenia and Depression (HPB, JHK), the Attias Family Foundation (HPB), Marcia Simon Kaplan (JHK), the Ethel F. Donaghue Women's Investigator Program at Yale (HPB) and the Klingenstein Foundation (JHK). BioImage Suite was supported in part by a grant from the NIH/NIBIB R01EB006494.
Footnotes
Dr. Blumberg has consulted to Pfizer, Inc. and has received honoraria from Abbott and Lilly.
The authors report no other potential conflicts of interest.
References
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61(6):776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, D.C.: 2000. Text Revision ed. [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Attentional modulation of emotional stimulus processing: an fMRI study using emotional expectancy. Hum Brain Mapp. 2006;27(8):662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7(4):243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7(6):570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, Martin L, Gerard E, Charney DS, Peterson BS. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59(7):611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60(6):601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160(7):1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156(12):1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, Ketter T, Reiss AL. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol Psychiatry. 2005;58(3):197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, Biederman J, Hodge SM, Dieterich ME, Gerstein ED, Kennedy DN, Rauch SL, Cohen BM, Caviness VS. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7(6):555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, 3rd, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48(9):852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, Hatch JP, Keshavan MS, Ryan N, Birmaher B, Soares JC. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162(9):1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, Andrew CM, Phillips ML. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8(1):28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55(6):648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7(Suppl 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Integrated intenstiy and point-feature nonrigid registration. MICCAI. 2004;1:763–770. doi: 10.1901/jaba.2001.3216-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor M, M., Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19(20):9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ. Decision-making in mania: a PET study. Brain. 2001;124(Pt 12):2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging. 1999 doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sanches M, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Hatch JP, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138(1):43–49. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Staib L, Jackowski M, Papademetris X. Paper presented at the ISBI. 2006. Brain shape characterization from deformation. [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]


