Abstract
Animals eat different foods in proportions that yield a more favorable balance of nutrients. Despite known examples of these behaviors across different taxa, their ecological and physiological benefits remain unclear. We identified a surprising dietary shift that confers ecophysiological advantages in a dung beetle species. Thorectes lusitanicus, a Mediterranean ecosystem species adapted to eat semi-dry and dry dung (dung-fiber consumers) is also actively attracted to oak acorns, consuming and burying them. Acorn consumption appears to confer potential advantages over beetles that do not eat acorns: acorn-fed beetles showed important improvements in the fat body mass, hemolymph composition, and ovary development. During the reproductive period (October-December) beetles incorporating acorns into their diets should have greatly improved resistance to low-temperature conditions and improved ovarian development. In addition to enhancing the understanding of the relevance of dietary plasticity to the evolutionary biology of dung beetles, these results open the way to a more general understanding of the ecophysiological implications of differential dietary selection on the ecology and biogeography of these insects.
Introduction
The main reason animals eat is to acquire the mixture of nutrients required to fuel growth, development, and reproduction. A high-quality diet for insects permits the storage of different compounds (e.g., lipids, carbohydrates, or proteins) in the fat body, the most important organ for nutrient metabolism and storage in insects [1]. In fact, the fat body plays a crucial role not only in lipid storage and transport to the hemolymph, but also in lipid biosynthesis from carbohydrates, the biosynthesis of proteins such as antifreeze proteins (AFP) [2], and yolk deposition in oocytes or vitellogenesis [3]. Moreover, the fat body is the major organ for storing glycogen, the precursor of many cryoprotectants (mainly polyhydric alcohols such as glycerol and sorbitol and sugars such as trehalose) [4], [5], [6]. As a measure of cold thermal tolerance, the supercooling point (the temperature at which the insect freezes, SCP) is generally used to assess the cold acclimation and resistance. In cold conditions, freeze-avoiding insects may adopt one or both of these main mechanisms to decrease the SCP: (a) the production of AFPs that inhibit the growth of ice, modify the thermal hysteresis (the difference between the freezing point and the melting point of a solution) and stabilize supercooled hemolymph and other fluids [7], [8], [9], [10], and (b) the adjust concentration of multi-component cryoprotectant compounds that depress the SCP [11].
Nutritional changes can alter thermal tolerance by their influence on the production of antifreeze proteins [12], [13]. Although other dietary needs than the gathering of energy may be critical, the general predictions of the optimal foraging theory is that organisms forage to maximize their net rate of energy intake with the minimum loss of time; the animal increases its fitness by adopting feeding patterns that maximize the intake per unit time of some critical dietary currency, generally energy [14]. Some frequent predictions of this theory are “ that large foods should be preferred to small, patchy foods to spaced-out foods; calorie-rich foods to calorie-poor foods”. However, animals may eat different foods in proportions that yield a more favorable balance of nutrients than from any one of these foods alone, a behavioral strategy called dietary self-selection [14], [15], [16], [17]. Here we show for the first time how the diet diversification of a dung-beetle species to consume acorns allows the improvement of thermal tolerance and reproductive capacity under laboratory conditions. This, in turn may help to explain some ecological and distributional data of this species.
Thorectes lusitanicus (Scarabaeoidea, Geotrupinae) is an apterous dung beetle species endemic to the southern Iberian Peninsula. Recently, an astonishing and never before observed relationship between this dung beetle (Thorectes lusitanicus) and two oak species (Quercus suber and Q. canariensis) has been described for the Iberian Peninsula [18], [19]. Additionally, this behavior has been also recently described for Mycotrupes lethroides, a Geotrupinae dung beetle from Florida [20] as well as for the Iberian species Thorectes baraudi, the phylogenetically closest species to T. lusitanicus (unpublished data). Thorectes lusitanicus acts as a secondary disperser, capable of partially consuming (Fig. 1) dragging and burying a high number of viable acorns of both Quercus species during their reproductive period of activity (autumn-winter). Additionally, using olfactometry under laboratory conditions we have shown that specimens of T. lusitanicus are significantly attracted by acorns during their reproductive period, even preferring this food resource over the dung necessary to lay their eggs for breeding purposes [19]. During the autumn and winter, acorns are one of the most abundant and nutritious foods in temperate areas where oaks provide the principal forest structure. Nutritionally, acorns contain larger amounts of proteins (18.1%) and lipids (6.1%) [21] than cow dung (5% proteins and 0.4% lipids) [22]. Thus, although the tannins in acorns can cause serious damage to some mice [23], several wild animals, including mammals (such as red deer, wild boars and mice) and birds (such as Eurasian jays and common wood pigeons) consume acorns as a key part of their diet during the autumn and winter. In the case of T. lusitanicus the period of acorn production coincides with the reproductive period of this species [24].
Figure 1. Thorectes lusitanicus eating cork-oak acorns.
During their reproductive period, T. lusitanicus bury and feed on acorns of Quercus, with important ecological implications.
In addition to the possible ecological implications for forest regeneration, there is a great interest in understanding the advantages of this dietary shift in dung beetles from dung to acorns, as well as the possible physiological and reproductive implications of this diversification in the trophic niche. In this work, we present experimental evidence that helps to explain the possible benefits of this striking behavior [19]. Given the high content in polyunsaturated fatty acids, triglycerides, and sterols present in the acorns [25], we demonstrate that this diet expansion significantly affects fat body development, hemolymph composition, cold resistance and ovary development under laboratory conditions. Additionally we also include some field data and ecological and natural history evidences supporting the relevance of acorn consumption under natural conditions, thus suggesting that some insects probably have the capacity to enhance their environmental and reproductive capacity by changing their trophic behavior.
Results
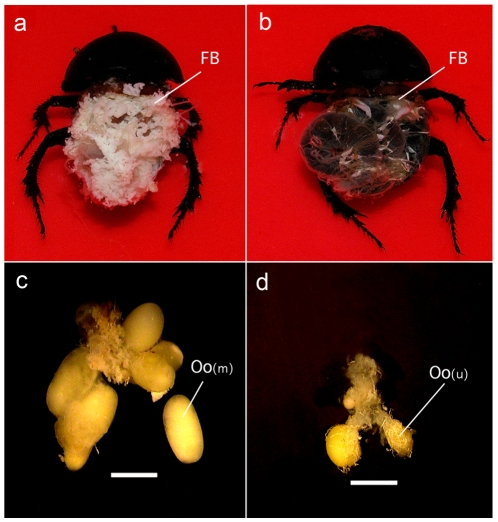
Beetles fed with acorns developed a fat body 4.6-fold heavier than those fed with cow dung (Table 1). The impressive size reached by this tissue in acorn-fed beetles was even evident to the naked eye (Fig. 2A).
Table 1. The effect of food on the fat body mass, hemolymph composition, thermal tolerance and activity of Thorectes lusitanicus.
| Food source | |||
| Acorns | Cow dung | Samples/diet | |
| Fat body (% body mass) | 11.26±2.85 | 2.46±0.85 | n = 10 |
| Glycerol (g/L) | 0.027±0.009 | 0.017±0.0002 | n = 10 |
| Protein (mg/ml) | 129.29±16.36 | 28.34±4.13 | n = 10 |
| SCP (°C) | −13.48±1.15 | −8.50±2.17 | n = 10 |
| THA (°C) | 0.64±0.04 | 0.41±0.02 | n = 5 |
| Palmitic acid (mg/ml) | 1.25±0.25 | 0.39±0.06 | n = 3 |
| Palmitoleic acid (mg/ml) | 0.12±0.01 | 0.04±0.04 | n = 3 |
| Stearic acid (mg/ml) | 0.15±0.02 | u.c. | n = 3 |
| Oleic acid (mg/ml) | 3.75±0.45 | 1.21±0.27 | n = 3 |
| Linoleic acid (mg/ml) | 0.81±0.13 | 0.40±0.12 | n = 3 |
| Linolenic acid (mg/ml) | 0.02±0.03 | u.c | n = 3 |
| Activity at 20°C (%) | 91.3±2.4 | 43.6±7.9 | n = 6 |
| Activity at 10°C (%) | 85.5±4.3 | 17.2±4.9 | n = 6 |
(Mean ± sd). All measurements are statistically significant (P≤0.01) according to a Mann-Whitney non-parametric test. SCP: Supercooling point; THA: Thermal hysteresis; u.c.: undetectable concentrations (≈0).
Figure 2. The effect of diet on the fat body and ovary development in T. lusitanicus.
Diets based on acorns (A and C) and cow dung (B and D). FB: fat body; Oo(m): mature oocyte; Oo(u): undeveloped oocyte.
The hemolymph composition was also extensively altered by the diet, particularly in the fatty acid type and content and in the protein content. All fatty acids found in the hemolymph of cow dung-fed beetles (palmitic, 16:0; palmitoleic, 9-cis 16:1; oleic, 9-cis 18:1; and linoleic acid, 9,12-cis 18:2) increased two to three fold in the acorn-fed beetle hemolymph (Table 1). The acorn diet also determined the presence in hemolymph of stearic (18:0) and linolenic acid (9,12,15-cis 18:3), which were both undetectable under the cow dung diet (Table 1).
Probably, the most outstanding feature of the hemolymph of the acorn-fed beetles was related to the protein content, which was 4.6 times higher than that found in the hemolymph of cow dung-fed beetles (Table 1). Although the type and identity of these proteins are now currently being investigated in our laboratory, a first consequence of this great difference in the protein content derived from the diet was the variation in some biophysical features of the hemolymph (Table 1). We found a reduction in the supercooling point (SCP) from −8.50±2.17°C (mean ± sd) in the hemolymph of cow dung-fed beetles to −13.48±1.15°C of that of the acorn-fed beetles. Besides the modification of the hemolymph SCP, the diet also brought about a change in the thermal hysteresis activity (THA) detected in the hemolymph, which increased from 0.41±0.02°C in the cow dung-fed beetles to 0.64±0.04°C in the acorn-fed beetles. Glycerol was found at similar concentrations in the hemolymph irrespective to the diet (Table 1).
Behavioral data on the activity of T. lusitanicus under laboratory conditions showed that those individuals feeding on acorns were significantly more active at the two examined environmental temperatures. Ninety-one percent and 85.5% of individuals feeding on acorns remain active both at 20°C and 10°C, while 43.6% and 17.2% of individuals feeding on cow dung were active at both temperatures, respectively (Table 1).
Finally, beetles fed with acorns also showed a higher development of their ovaries, a higher number of mature oocytes per ovary, and a higher mean weight of oocytes that beetles fed with cow dung. Furthermore, the number of undeveloped ovaries was higher in beetles fed with cow dung (Table 2) than in beetles fed acorns.
Table 2. The effect of food on correlates of reproductive success in Thorectes lusitanicus.
| Food source | ||
| Acorns | Cow dung | |
| Ovary weight (% body mass) | 3.66±1.03 | 0.87±0.28 |
| Number of oocytes/ovary | 2.10±0.55 | 0.40±0.22 |
| Mean weight of oocytes (mg) | 0.011±0.002 | 0.003±0.002 |
| Number of undeveloped ovaries | 1 | 7 |
All measurements are statistically significant (P≤0.01) according to a Mann-Whitney non-parametric test (n = 10 for each diet) (Mean ± sd).
Discussion
In insects, the main indicator of the nutritional reserve level is the fat body, which appears as a spongy, aggregated mass of cells (trophocytes), whitish in color, and irregularly distributed in the perivisceral space of the thorax and the abdomen. It is known that the fat body is responsible for the synthesis of the fatty acids and proteins found in the hemolymph [2], [3]. In our case, the impressive size reached by this tissue as a consequence of the acorn ingestion coincided with significant variations in the measured ecophysiological parameters. Thus, the hemolymph composition was altered by the diet, particularly in the fatty-acid type and content. All the fatty acids detected in the hemolymph of the cow dung-fed beetles (palmitic, palmitoleic, oleic and linoleic) were significantly increased two to three-fold in the hemolymph of acorn-fed beetles. In addition, the acorn diet caused the appearance of stearic and linolenic acids in the hemolymph. Both stearic and linolenic acids were undetectable in the cow dung diet. Insects utilize lipids efficiently for development, reproduction and flight, and the amount and composition of lipids in an insect vary considerably among the developmental stages and tissues and are influenced by several factors, including nutrition [26]. In insect physiology, polyunsaturated fatty acids have diverse roles in extreme temperature tolerance, reproduction and development [27], [28]. For example, in Sarcophaga crassipalpis, oleic acid promotes cell membrane fluidity at low temperature and also allows the cell membrane to maintain a liquid crystalline state if temperatures increase in the cell membrane [27].
Diet has not only an important effect on the fat body mass, but also on the cryoprotectant content of the hemolymph. In our study, the changes in the concentration of hemolymph fatty acids related with the acorn consumption seem to be associated to the temperature tolerance of T. lusitanicus. We found a significant reduction in the supercooling point (SCP) in the hemolymph from acorn fed dung-beetles. Besides the modification of the hemolymph SCP, the diet change also brought a change in the thermal hysteresis activity (THA) detected in the hemolymph. Interestingly, although the type and identity of these proteins is now being investigated in our laboratory, we suspect that this great difference in the protein content derived from the dietary shift is related to the changes in the biophysical characteristics of the hemolymph and the observed higher thermal tolerance and activity rates at lower temperatures. The effect of diet on thermal tolerance was also observed in other arthropods. In the Antarctic midge Belgica antartica, antifreeze and cryoprotectant modulation is dependent on the dietary carbon source [29], but this process does not seem to be universal [30]. In the case of T. lusitanicus, the consumption of acorns and the accompanying metabolic changes significantly increased the tolerance to lower temperatures. In fact, for an overwintering species such as T. lusitanicus, the capacity to increase the cold tolerance could be advantageous by enabling the beetle to survive hard frosts during winter, but also by extending its activity during the cold conditions in which other species remain inactive. In the locality at which this behavior was first observed [18], [19], Thorectes lusitanicus is the only dung beetle species that remains active in the field during winter according to our field experience. Our results on the activity test support this observation; those individuals feeding on acorns were more active at both temperatures considered but especially during cold conditions (10°C) maintaining 85.5% activity in contrast to individuals with a dung-exclusive diet which only 17.2% of individuals maintained activity (Mann-Whitney U test: U = 36, P = 0.001; for both temperature conditions) being also able to have a higher rate of survival to winter conditions. Preliminary laboratory observations suggest that a higher survival of T. lusitanicus individuals was obtained under a diet based on acorns compared to beetles under a dung-based diet (unpublished results).
In freeze-intolerant and overwintering species such as T. lusitanicus, the SCP and THA are two characteristics that increase during autumn to promote survival during the winter. In the case of T. lusitanicus, the modulation of the SCP and THA levels during the winter may be an important mechanism in preventing the formation of inoculative freezing (by contact with ice) during periods of frosts. In the distribution area of T. lusitanicus, snow and frost periods are scarce, although in some occasions the environmental temperature registered during winter can reach −5°C. However, a moderately chill-tolerant species such as T. lusitanicus can take advantage under natural situations when the SCP diminishes because the survival rate at low temperatures may drastically increase. In moderately chill-tolerant and chill susceptible species, changes in survival rates are related to SCP values [31] being the increase of SCP a frequent strategy to survive during coldest periods [32]. For example, in the case of the curculionid beetle Rhynchaenus fagi, although this species has an SCP of −25°C, less than 30% of individuals survive after 50 days at −15°C [33].
Acorn consumption seems to also affect the reproductive capacity of this species. Ovary weights, the number of oocytes per ovary and the mean weight of oocytes were significantly higher in the acorn-fed individuals (see Fig. 2C and Table 2) than in the beetles fed with cow dung, which also had a higher number of undeveloped ovaries. This higher ovarian development may be enhanced by the high content of fatty acids. Palmitic, oleic, and linoleic acids are the most abundant during the reproductive cycle of many insects [34]. Most insects have a nutritional requirement for polyunsaturated fatty acids, and many studies have pointed out that either linoleic or linolenic acids satisfy this nutritional need. The absence or deficiency of these compounds results in failures in metamorphosis, nymph growth, and the emergence of well-formed adults [35]. In the case of T. lusitanicus, a diet based on acorns not only provides high concentrations of fatty acids, but is also the unique source of the essential linolenic acid.
Is there any relationship between acorn density and population size or body size of T. lusitanicus under natural conditions? A review of the available data on the composition of dung beetle assemblages within the distribution area of T. lusitanicus, was shown in six published studies that collected this species [36], [37], [38], [39], [40], [41]. The total number of individuals collected in them oscillates between 3 and 241 (mean = 49 individuals) despite being annual studies. However, in the locality in which this surprising behavior has been discovered [18], the mean number of T. lusitanicus per trap in a 48-hours study was higher than the yearly mean of other sites. (51.4±5.1; n = 30; unpublished results). Moreover, variation in abundance between sampling plots was highly related to variation in acorn density (65% of the total variability; 2.5±0.8 (mean± se) individuals per m2 were collected directly in the soil [19], and 1,542 total individuals were collected). Preliminary field observations were carried out during November 2009 in four locations 20 km apart, and the locations characterized by their different tree cover values of Quercus species (approximately 90% of Quercus suber, 10% of Q. suber, 10% of Q. rotundifolia and 5% of Q. rotundifolia, respectively). The four locations had T. lusitanicus nest densities of 8.6±2.1, 3.2±0.8, 2.0±0.7, 1.2±0.6 (mean± se) nests/m2 (Kruskal-Wallis test: T = 11.89; P<0.01; n = 3 sites/location), respectively, preliminarly supporting the influence of the consumption acorns on the population size of T. lusitanicus. Furthermore, the mean body size of T. lusitanicus individuals also differed (Kruskal-Wallis test: T = 30.59; P<0.0001) between the surveyed localities. Individuals (n = 14, 10, 11 and 13, for each tree cover category) inhabiting the most dense forest of Quercus had a mean body size of 6.35±0.12 mm (mean± se), while those individuals belonging to the other tree cover categories had mean body sizes of 5.26±0.15, 5.18±0.09, and 4.79±0.12 mm, respectively. It is well established that both the nutritional quality and quantity of food determines the body size of emerged adult dung beetles [42]. Although more field studies are necessary to corroborate the former detected tendencies, the current evidence clearly suggest the existence of a positive association between the abundance of Quercus trees and the population and/or body sizes of T. lusitanicus.
Biochemical reactions and metabolic rates are highly dependent on temperature. Hence, fully or partly endothermic animals profit from the ability to heat themselves, because it enables them to adjust their temperature to enhance metabolic rates across a broad range of environmental conditions [43]. Although most animal groups (except birds and mammals) cannot completely regulate their body temperature, many have physiological, behavioral, and morphological strategies to adjust their body temperature, in response to thermal environmental changes [44]. The main physiological mechanism to elevate body temperature in dung beetles is the shivering of flight muscles [45]. However, in Thorectes wing muscles are atrophied because these species are apterous, having lost their capacity to fly. The apterism could have facilitated the evolutionary emergence of alternative strategies to elevate body metabolism through trophically mediated mechanisms. Alternatively, the acquisition of a new and energy-rich food source, that implies the increase of fat body, may increase the caloric energy by circulating metabolic substrates (lipids, proteins, polyalcohols, etc.), which are replenished by the fat body, and subsequently the tolerance to cold conditions, thus diminishing the selective pressure of the use of flight muscles to elevate body temperatures. A recent phylogenetically-based analysis showed that apterism evolved recurrently throughout Geotrupidae evolution (Cunha, Verdú, Lobo and Zardoya, in preparation). According to this phylogenetic hypothesis, Thorectes baraudi is the closest species to T. lusitanicus. However, Mycotrupes lethroides belongs to a relatively unrelated American lineage. The absence of phylogenetic propinquity in this diet-shift behavior suggest that apterism, a common condition of three species with this acorn diet-shift, could be the facilitation mechanism behind the evolutionary emergence of alternative diet-shift behavior in these dung beetles.
Most Geotrupinae species can be considered cool-temperature adapted species whose ancestral tribes are not strictly specialized in the consumption of herbivore dung. The ancestral shift in feeding habits from saprophagy to coprophagy in the Scarabaeoidea probably coincided with the appearance of large terrestrial herbivores, particularly grazing Mesozoic reptiles and subsequently mammals [46]. In general, an evolution towards coprophagy is evident in several Scarabaeoidea lineages. The diet of the most primitive geotrupids [47] is likely based on humus and fungi, whereas many Geotrupinae are basically coprophagous but possess modifications in the adult mouthparts that facilitate the consumption of more hard resources [48]. Thorectes species are characterized by the presence of mandibles with strongly developed scissorial areas and well-developed molar areas capable of triturating dry-pellet, an adaptation that suggest that these species are dung-fiber consumers and not dung-juice consumers [48]. Thus, although these species inevitably seem to require the presence of dung in order to build their nests and feed their larvae, their mouthparts are able to exploit trophic resources such as dry dung pellets and vegetable compounds (such as acorns). The wide range of trophic resources consumed by Geotrupidae dung beetles and the generally accepted saprophagous habits of the Scarabaeoidea ancestors [49], [50] suggest that the consumption of acorns constitutes a faculty that takes advantage of previous morphological adaptations.
This flightless Geotrupinae lineage would have evolved to reduce the loss of body water under arid conditions, as is common in other Coleoptera groups colonizing dry environments [51]. Subsequently, the loss of the flight capacity would have diminished the possibility of using an ephemeral resource such as dung, promoting the morphological adaptations directed to consume hard organic substances such as acorns, an evolutionary decision to maximize the net energy intake [14]. At the same time, this apterism would also have caused the isolation and diversification of this group. Thorectes (sensu lato) constitutes the most diversified Geotrupinae lineage in the Western Mediterranean region [52], with 36 endemic or narrowly distributed species (74% of the total, approximately) present in the Iberian Peninsula and North Africa, where the distribution seem to be highly conditioned by river-basin configurations [53]. In our opinion, both the capacity to consume hard resources and the apterism of Thorectes lusitanicus, T. baraudi and Mycotrupes lethroides are key characteristics that may explain the observed dietary shift and the astonishing relevance to thermal tolerance observed for T. lusitanicus. Mouthparts capable of crushing hard vegetable products would have facilitated the dietary shift towards a more energetically rich trophic resource such as acorns, which are able to provide the proteins and lipids necessary for enhancing cold resistance in the absence of the flight-muscle shiver mechanism.
In summary, the consumption of acorns by T. lusitanicus and the accompanying metabolic changes significantly increased the thermal tolerance of the species, and probably also the reproductive success of the species. Dietary acorn-selection by T. lusitanicus represents an unexpected behavior, which demonstrates that an apparently simple change in the diet can potentially drastically enhance the ecological capacity of an insect species, creating new life history possibilities from existing plasticity. Considering the large size of T. lusitanicus populations [18], more research is needed to examine the relevance of this dietary shift to the long term dynamic between the forest regeneration processes and the action of the large herbivore communities with which these Thorectes dung consumer species are associated. Furthermore, long-term additional evidences under natural conditions are necessary to establish a direct causal link between the acorn consumption and fitness or survival variables.
Materials and Methods
Study site and animals
Individuals of Thorectes lusitanicus were captured from the Los Alcornocales Natural Park in the Aljibe Mountains in southern Spain (36°31′54″N, 5°34′29″W) during the autumn (November-December 2006, 2007, 2008 and 2009), to coincide with the production of acorns and with the reproduction period of T. lusitanicus. Each year, in 48-h periods, we used pitfall traps baited with cow dung to capture live beetles. All of the individuals were maintained in plastic containers (60×40×40 cm) at 20°C until their arrival at the laboratory, where they were maintained at 10°C in a climate chamber (a temperature similar to the average one experienced in the field during autumn). The substrate was fallen leaves of Quercus to eliminate stress for the beetles.
We considered several methodological requirements to assure a common physiological state for all of the individuals, thus allowing the comparison of physiological measures between the diets: (a) we randomly selected only mature specimens according to cuticular deterioration of tibial in conjunction with the hardness of the cuticle employed in age determination of dung beetles [54], which permit the identification of individuals of approximately the same age; (b) a sex ratio of 1∶1 was maintained in each experiment; (c) to avoid the effect of diet and gut content on physiological measures, after a captive feeding period, all beetles were starved for a ten-day acclimation period prior to the SCP determination and extraction of hemolymph. The individuals submitted to different diets were separated in different rooms in order to avoid the presence of olfactory information on the two resources at the same time.
Types of diet and acclimation temperatures
Two plastic containers (60×40×40 cm) with 100 individuals of T. lusitanicus (50 males and 50 females) were installed with a substrate of sterile dry vermiculite. These individuals were exclusively fed with randomly selected acorns. Two additional plastic containers with 100 individuals were fed cow dung exclusively. The four containers were placed in a climate chamber at 20°C, with 60% relative humidity (RH) with a photoperiod of 15∶9 (light∶dark). Individuals were supplied with fresh dung or acorns every four days to ensure that the food supply was not a limiting factor in the analysis. After 20 days, 50 animals from each container were randomly selected and placed into two new containers at 10°C, 60% RH, with a photoperiod of 15∶9 (light∶dark) without further feeding. After ten days in these conditions, the hemolymph was extracted and analyzed.
Fat body mass and ovary development
Prior to the dissection, 20 females (n = 10 per treatment) were numbered and weighed. The fat body tissue and ovaries were dissected from ice-cold beetles into ice-cold insect Ringer's solution. For each individual, all the fat body tissue was removed and immediately placed on a dry paper to eliminate excess water prior to weighing. The same procedure was used for the ovary weight measurement. Also, the number of oocytes per ovary and their developmental status were noted. Finally, the fat body tissues and ovaries were stored at −85°C for later analyses.
Extraction of hemolymph
For each treatment, samples of hemolymph were obtained in autumn-winter coinciding with the reproductive period of the species. Hemolymph collection was done by piercing the dorsal surface of the thorax with a pin and collecting the hemolymph with capillary tubes. Each individual extraction was transferred to a 1.5-ml Eppendorf vial, with a total of 10 individual extractions aggregated to form a single sample per vial. After the extraction of the hemolymph, samples and beetles were frozen and stored at –85°C.
Protein analysis
The total protein content in the hemolymph was estimated using the Bradford method [55]. Briefly, the extracted hemolymph was centrifuged at 8000×g for 5 min to separate the particulate material. Then 20 µl-aliquots of the supernatant were diluted with water to a final volume of 100 or 1000 µl, depending on whether the samples came from excrement- or acorn-fed beetles, respectively. Aliquots of 100 µl each of the diluted samples were then mixed with 3 ml of Bradford reagent and left to stand at room temperature for 10 min. The absorbance at 595 nm of the mixture was measured in the context of the proper blank in a Shimadzu UV-VIS spectrophotometer UV-6001. The protein concentration was calculated from a calibration plot constructed under the same conditions as described above using bovine serum albumin (BSA) as the standard and taking into account the different dilution made with both samples.
Glycerol analysis
The glycerol present in the hemolymph of the beetles was analyzed with the Glycerol Assay Kit from Megazyme®. This procedure is based in the phosphorylation of the glycerol present in the samples to L-glycerol-3-phosphate by a reaction catalyzed by glycerokinase, which yields adenosine-5′-diphosphate (ADP) as a by-product. The ADP formation is then coupled to the cleavage of pyruvate from phosphoenolpyruvate by pyruvate kinase. Finally, the pyruvate formed in this reaction is reduced to L-lactate by L-lactate-dehydrogenase with the concomitant formation of NAD+, which is photometrically assessed at 340 nm. The amount of NAD+ formed is stoichiometric with the amount of glycerol originally present in the samples. The concentration of glycerol in the samples was inferred from a Mega-Calc™ spreadsheet.
Fatty acid analysis
To extract and analyze the fatty acids from the hemolymph, 30-µl aliquots of hemolymph were mixed with 440 µl chloroform: methanol (1∶2) and 100 µl of MethPrepII© methylating reagent. The mixture was maintained for 30 min in an ultrasound bath and then centrifuged to settle any particulate material. The fatty acid composition in the supernatant was analyzed by GC-MS.
Supercooling point
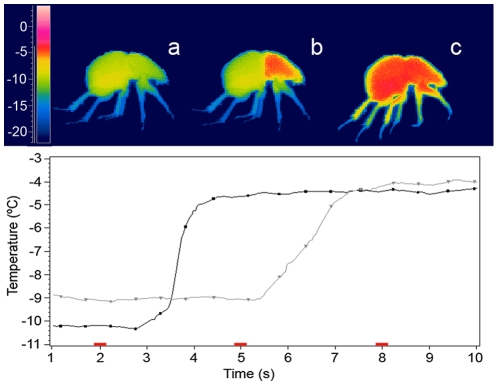
The temperatures of cooling of the hemolymph below its freezing point without it becoming solid were measured with a FLIR Thermacam P620 thermal infrared camera with a resolution of 640×480 pixels and a microbolometer Focal Plane Array detector with a spectral range of 7.5–13 µm and a thermal sensitivity of ±0.06°C at 30°C. We used the ThermaCAM™ Researcher v 2.9 software [56] to record and analyse the thermal images obtained. The supercooling points were measured by determining the temperature at which the exothermic reaction of crystallization (latent heat of crystallization) occurs at the onset of freezing [57] (Fig. 3). In order to standardize all measures, we have selected the supercooling point obtained in the thorax (Fig. 3). We fixed the cooling rate at 0.5°C/min. The cooling rates were maintained using a MIR-153 programmable heated and cooled incubator (SANYO Electric Co., Ltd) with an accuracy of ±0.2°C. The supercooling point experiments were carried out with a hemolymph sample of at least ten individuals for each diet.
Figure 3. The supercooling process in Thorectes lusitanicus.
(A) The prefreezing state when the supercooling point was measured; (B) the exothermic crystallization of the hemolymph contained in the thorax (see black curve below); and (C) the crystallization of the hemolymph contained in the abdomen (see grey curve below) and the rest of the body. The red marks indicate the instant that correspond with each thermography and temperature curves (the thoracic temperature in the black curve, and the abdominal temperature in the grey line).
Thermal hysteresis
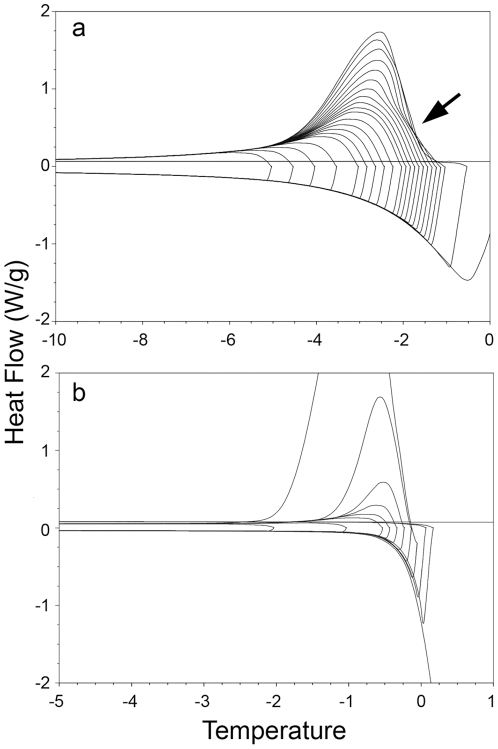
These measurements were made using a differential scanning calorimeter (DSC; Q-100, TA Instruments). Each sample of hemolymph (1 µl) was sealed in an aluminum pan using an empty pan as control. The samples were weighed before the DSC run. Bovine serum albumin was used as a standard AFP-free solution to compare with samples containing AFP [58]. Following Hansen and Baust [59], the samples were cooled and warmed at 1°C/min, and both the crystallization and melting points were recorded. We used hemolymph without oil and varied some experimental parameters: the samples were then cooled and equilibrated, in this case, to −30°C (rate of 1°C/min), maintained for 5 min (isothermal time), warmed to successive partial melt temperatures between –10 and 0°C in 0.1°C increments, and held for 5 min at each increment to allow for the ice–protein interaction and system stabilization. Finally, the samples were then slowly cooled to −30°C (1°C/min), and the hold and onset of the freeze exothermal curve were recorded for each DSC cycle (Fig. 4). The onset was calculated as the start of the crystallization exothermal curve and was analyzed using the Universal Analysis 2000 v. 4.1D program (TA Instruments).
Figure 4. The thermal hysteresis activity (THA) of Thorectes lusitanicus.
The differential scanning calorimetry curves of refreezing (rate of 0.1°C/min) of (A) partially melted hemolymph of Thorectes lusitanicus, and (B) partially melted bovine serum albumin (BSA; as control). No thermal hysteresis effect was observed in the curves with a lower heat flow (temperature ranges from –5 to –2°C), whereas THA was observed in temperature ranges oscillating from −2 to −1°C (see curves marked by an arrow). Frozen BSA was heated to partially melt at the different hold temperatures and then cooled to recrystallize. No thermal hysteresis effect was observed in this case.
Effect of diet on activity
In order to explore the diet effect on the activity of T. lusitanicus, twelve plastic containers (20×20×15 cm) with 20 individuals of T. lusitanicus (ten males and ten females) were installed with a substrate of vermiculite. Previously, six containers were supplied with acorns and six with cow dung during 20 days in 2008 and 2009. Three containers of each diet treatment were placed in separated climate chambers at 20°C or 10°C, 60% of relative humidity and a photoperiod of 15∶9 (light∶dark). Individuals were checked after four days, and the number of active individuals was counted. Individuals were only recorded as “active” if they exhibited normal behaviour, such as coordinated walking and extension of antennae. Null responses (inactive) correspond to immobile individuals in that as much the legs as the antennae remained folded to body during the experiment. This experiment was made during 2008 and 2009.
Statistical analyses
Mann–Whitney non-parametric tests were used to examine the differences among the treatments using StatsDirect 2.5.7 [60].
Acknowledgments
The authors wish to thank S. L. Chown, B. A. Hawkins, A.T. Peterson, J. Hortal and E. Galante for their constructive criticisms and comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Ministerio de Ciencia e Innovacion-Spain (CGL2008-03878). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van der Horst DJ, Ryan RO. 2005. pp. 225–246. in Comprehensive Molecular Insect Science, eds Gilbert L, Latrou IK, Gill SS (Vol. 4, Elsevier, Amsterdam)
- 2.Xu L, Duman JG. Involvement of juvenile hormone in the induction of antifreeze protein production by the fat body of larvae of the beetle Dendroides canadensis. . J exp Zool. 1991;258:288–293. [Google Scholar]
- 4.Storey KB, Storey JM. Biochemical adaptations for winter survival in insects. Adv Low-Temp Biol. 1992;1:101–140. [Google Scholar]
- 5.Wolfe GR, Hendrix DL, Salvucci ME. A thermoprotective role for sorbitol in the silverleaf whitefly, Bemisia argentifolii. . J Insect Physiol. 1998;44:597–603. doi: 10.1016/s0022-1910(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Fernandes JR, Arrese EL, Wells MA. Allosteric effectors and trehalose protect larval Manduca sexta fat body glycogen phosphorylase B against thermal denaturation. Insect Biochem Mol Biol. 2000;30:473–478. doi: 10.1016/s0965-1748(00)00016-3. [DOI] [PubMed] [Google Scholar]
- 7.Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- 8.Duman JG, Bennett V, Sformo T, Hochstrasser R, Barnes BM. Antifreeze proteins in Alaskan insects and spiders. J Insect Physiol. 2004;50:259–266. doi: 10.1016/j.jinsphys.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Davies PL, Baardsnes J, Kuiper MJ, Walker VK. Structure and function of antifreeze proteins. Philos Trans R Soc Lond B Biol Sci. 2002;357:927–935. doi: 10.1098/rstb.2002.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair BJ, Chown, SL Hemolymph osmolality and thermal hysteresis activity in 17 species of arthropods from sub-Antarctic Marion Island. Polar Biol. 2002;25:928–933. [Google Scholar]
- 11.Storey KB, Storey JM. Natural freezing survival in animals. Ann Rev Ecol Syst. 1996;27:365–386. [Google Scholar]
- 12.Sømme L, Block W. Cold hardiness of Collembola at Signy Island, maritime Antarctic. Oikos. 1982;38:168–176. [Google Scholar]
- 13.Denlinger DL, Lee RE., Jr 1998. pp. 55–96. in Insects and Application in Integrated Pest Management, eds Hallman GJ, Denlinger DL (Westview Press, Boulder)
- 14.Waldbauer GP, Friedman S. Self-selection of optimal diets by insects. Annu Rev Entomol. 1991;36:43–63. [Google Scholar]
- 15.Cangussu JA, Zucoloto FS. Self-selection and perception threshold in adult females of Ceratitis capitata (Diptera, Tephritidae). J Ins Physiol. 1995;41:223–227. [Google Scholar]
- 16.Friedman S, Waldbauer GP, Eertmoed JE, Naeem M, Ghent AW. Blood trehalose levels have a role in the control of dietary self-selection by Heliothis zea larvae. J Ins Physiol. 1991;37:919–928. [Google Scholar]
- 17.Fielding DJ, Defoliart LS. Discriminating tastes: self-selection of macronutrients in two populations of grasshoppers. Physiol Entomol. 2008;33:264–273. [Google Scholar]
- 18.Pérez-Ramos IM, Marañon T, Lobo JM, Verdú JR. Acorn removal and dispersal by the dung beetle Thorectes lusitanicus: ecological implications. Ecol Entomol. 2007;32:349–356. [Google Scholar]
- 19.Verdú JR, Lobo JM, Numa C, Pérez-Ramos IM, Marañón T, et al. Acorn preference by the dung beetle, Thorectes lusitanicus, under laboratory and field conditions. Anim Behav. 2007;74:1697–1704. [Google Scholar]
- 20.Beucke K, Choate P. Notes on the feeding behavior of Mycotrupes lethroides (Westwood) (Coleoptera: Geotrupidae), a flightless North American beetle. Col Bull. 2009;63:228–229. [Google Scholar]
- 21.Bouderoua K, Selselet-Attou G. Fatty acid composition of abdominal adipose tissue in broilers fed green-oak (Quercus ilex), cork oak acorn (Quercus suber L.) based diets. Anim Res. 2003;52:377–382. [Google Scholar]
- 22.Negro JJ, Grande JM, Tella JL, Garrido J, Hornero D, et al. Coprophagy: An unusual source of essential carotenoids. Nature. 2002;416:807–808. doi: 10.1038/416807a. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Saitoh T. Negative effects of acorns on the wood mouse Apodemus speciosus. Popul Ecol. 2003;45:7–17. [Google Scholar]
- 24.Martín-Piera F, López-Colón JI. Coleoptera, Scarabaeoidea I. Fauna Ibérica, 14, ed. 2000. Ramos MA, Nieves-Aldrey JL, Museo Nacional de Ciencias Naturales, CSIC, Madrid.
- 25.Lopes IMG, Bernardo-Gil MG. Characterisation of acorn oils extracted by hexane and by supercritical carbon dioxide. Eur J Lipid Sci Technol. 2005;107:12–19. [Google Scholar]
- 26.Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Michaud MR, Denlinger DL. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. J Insect Physiol. 2006;52:1073–1082. doi: 10.1016/j.jinsphys.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Arrese EL, Soulages JL. Insect Fat Body: Energy, Metabolism, and Regulation. Ann Rev Entomol. 2010;55:207–255. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baust JG. Environmental triggers to cold hardening. Comp Biochem Physiol A. 1982;73:563–570. [Google Scholar]
- 30.Lavy D, Verhoef HA. Cold hardiness in the collembolan Orchesella cincta under different food conditions. Comp Biochem Physiol. 1997;118:699–704. [Google Scholar]
- 31.Chown SL, Nicholson SW. Insect Physiological Ecology: Mechanisms and Patterns. In: Chown SL, Nicholson SW, editors. Oxford: Oxford University Press; 2004. 115 [Google Scholar]
- 32.Atapur M, Moharramipour S. Changes of cold hardiness, supercooling capacity, and major cryoprotectants in overwintering larvae of Chilo suppressalis (Lepidoptera: Pyralidae) 2009. [DOI] [PubMed]
- 33.Bale JS. Classes of insect cold hardiness. Funct Ecol. 1993;7:751–753. [Google Scholar]
- 34.Sayah F. Changes in the lipid and fatty acid composition of hemolymph and ovaries during the reproductive cycle of Labidura riparia. Entomol Sci. 2008;11:55–63. [Google Scholar]
- 35.Dadd RH. 1985. pp. 313–390. in Comprehensive Insect Physiology Biochemistry and Pharmacology, eds Kerkut GA, Gilbert LI (Pergamon, Oxford)
- 36.Ávila JM, Sandoval P, Schmidt J, Sánchez-Piñero Contribución al conocimiento de los Scarabaeoidea (Coleoptera) coprófagos de los excrementos de conejo de la provincia de Granada (España). Elytron. 1988;2:41–50. [Google Scholar]
- 37.Ávila JM, Sánchez-Piñero F. Contribución al conocimiento de las comunidades de escarabeidos de Chiclana de la frontera (Cádiz) (Coleoptera, Scarabaeoidea). 1988. pp. 707–714. Autoecología de las especies de las familias Geotrupidae y Hybosoridae. Actas III Congr Ibérico Entomol.
- 38.Hidalgo JN, Cardenas NM. Escarabeidos coprófagos de las Sierras Subbéticas (provincia de Córdoba, España) (Coleoptera, Scarabaeoidea). Nouv Rev Ent. 1994;11:217–231. [Google Scholar]
- 39.Hidalgo JM, Bach C, Cárdenas AM. II. Los Scarabaeoidea (Coleoptera) Coprófagos de las comarcas naturales de la provincia de Córdoba: II. Trogidae, Geotrupidae y Scarabaeidae. Boln Asoc Esp Ent. 1998;22:203–230. [Google Scholar]
- 40.Sánchez-Piñero F, Ávila JM. Análisis comparativo de los Scarabaeoidea (Coleoptera) coprófagos de las deyecciones de conejo y de otros mamíferos. Estudio preliminar. Eos. 1991;67:23–34. [Google Scholar]
- 41.Romero-Alcaraz E, Ávila JM, Sánchez-Piñero F. Inventario faunístico y corología de los escarabeidos coprófagos (Coleoptera) de la Sierra de Baza (Granda, sureste de la Península Ibérica. Zool Baetica. 1998;9:47–67. [Google Scholar]
- 42.Emlen DJ, Lavine LC, Ewen-Campen B. On the origin and evolutionary diversification of beetle horns. Proc Natl Acad Sci. 2007;104:8661–8668. doi: 10.1073/pnas.0701209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grigg GC, Beard LA, Augee ML. The evolution of endothermy and its diversity in mammals and birds. Physiol Biochem Zool. 2004;77:982–987. doi: 10.1086/425188. [DOI] [PubMed] [Google Scholar]
- 44.Chown SL, Terblanche JS. Physiological diversity in insects: ecological and evolutionary contexts. Adv Insect Physiol. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartholomew GA, Heinrich B. Endothermy in African dung beetles during flight, ball making, and ball rolling. J Exp Biol. 1978;73:65–83. [Google Scholar]
- 46.Halffter G, Edmonds WD. Evolución de la nidificación y de la cooperación bisexual en Scarabaeinae (Ins.: Col.). An escuela nac cien biol. 1982;25:117–144. [Google Scholar]
- 47.Verdú JR, Galante E, Lumaret JP, Cabrero-Sañudo F. Phylogenetic analysis of Geotrupidae (Coleoptera, Scarabaeoidea) based on larvae. Syst Entomol. 2004;29:509–523. [Google Scholar]
- 48.Verdú JR, Galante E. Behavioural and morphological adaptations for a low-quality resource in semi-arid environments: dung beetles (Coleoptera, Scarabaeoidea) associated with the European rabbit (Oryctolagus cuniculus L.). J Nat Hist. 2004;38:705–715. [Google Scholar]
- 49.Halffter G, Matthews EG. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae), Fol Entomol Mex. 1966;12–14:1–312. [Google Scholar]
- 50.Cambefort Y. 1991. pp. 22–35. in Dung beetle ecology, ed Hanski I, Cambefort Y (Princeton University Press, Oxford)
- 51.Chown SL, Pistorius P, Scholtz CH. Morphological correlates of flightlessness in southern African Scarabaeinae (Coleoptera: Scarabaeidae): testing a condition of the water conservation hypothesis. Can J Zool. 1998;76:1123–1133. [Google Scholar]
- 52.Löbl I, Smetana A. 2006. 690. Catalogue of Palaearctic Coleoptera,Vol. 3: Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea, Byrrhoidea. Appollo Books, Stenstrup, Denmark.
- 53.Lobo JM, Verdú JR, Numa C. Environmental and geographical factors affecting the Iberian distribution of flightless Jekelius species (Coleoptera: Geotrupidae). Divers Distrib. 2006;12:179–188. [Google Scholar]
- 54.Tyndale-Biscoe M. Age-grading methods in adult insects: a review. Bull Entomol Res. 1984;74:341–377. [Google Scholar]
- 55.Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 56.Flir Systems Ltd. ThermaCAM™ Researcher v 2.9 software. 2007. User's manual.
- 57.Lee RE. 1991. pp. 17–46. in Insects at Low Temperature, eds Lee RE, Denlinger DL (Chapman & Hall, New York, 1991)
- 58.Lu M, Wang B, Li Zh, Fei Y, Wei L, et al. Differential scanning calorimetric and circular dichroistic studies on plant antifreeze proteins. J Thermal Anal Calorim. 2002;67:689–698. [Google Scholar]
- 59.Hansen N, Baust JG. Differential scanning calorimetric analysis of antifreeze protein activity in the common mealworm, Tenebrio molitor. Biochim Biophys Acta. 1988;957:217–221. doi: 10.1016/0167-4838(88)90275-0. [DOI] [PubMed] [Google Scholar]
- 60.StatsDirect Ltd. StatsDirect Statistical Software, StatsDirect, U.K 2005.