Abstract
The mean resonance frequency of the human middle ear under air conduction (AC) excitation is known to be around 0.8–1.2 kHz. However, studies suggest that the mean resonance frequency under bone conduction (BC) excitation is at a higher frequency around 1.5–2 kHz. To identify the cause for this difference, middle-ear responses to both AC and BC excitations were measured at the umbo and lateral process of the malleus using five human cadaver temporal bones. The resonance modes identified from these measurements, along with finite element analysis results, indicate the presence of two ossicular modes below 2 kHz. The dominant mode under AC excitation is the first mode, which typically occurs around 1.2 kHz and is characterized by a “hinging” ossicular motion, whereas the dominant mode under BC excitation is the second mode, which typically occurs around 1.7 kHz and is characterized by a “pivoting” ossicular motion. The results indicate that this second mode is responsible for the translational component in the malleus handle motion. The finding is also consistent with the hypothesis that a middle-ear structural resonance is responsible for the prominent peak seen at 1.5–2 kHz in BC limit data.
INTRODUCTION
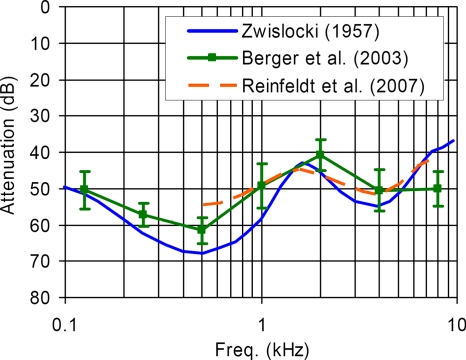
There is an ongoing need to provide more effective and reliable hearing protection devices for people who work in extremely noisy environments, such as on the flight deck of an aircraft carrier. Typical hearing protectors, such as earmuffs and earplugs, reduce the risk of hearing damage by suppressing the sound that reaches the inner ear through the air conduction (AC) pathway. However, the maximum amount of hearing protection provided by a conventional hearing protector is limited by the presence of bone conduction (BC) pathways, such as acoustically induced skull vibrations, through which residual acoustic energy is transmitted to the cochlea. The hearing protection performance limit due to BC is commonly called the “BC limit” (or the “BC threshold”) in the literature. Figure 1 shows several estimates of the BC limit (Zwislocki, 1957; Berger et al., 2003; Reinfeldt et al., 2007).
Figure 1.
Estimates of the mean BC limit (i.e., the BC threshold): The data by Zwislocki (1957) and Reinfeldt et al. (2007) were obtained with a finer frequency resolutions than those by Berger et al. (2003) (error bars=±1S.D.). Note the prominent peak feature between 1.5 and 2 kHz, indicating a significant amount of BC sound transmission in this frequency range.
The most notable feature of the BC limit estimates shown in Fig. 1 is the presence of a prominent peak between 1.5 and 2 kHz. The maximum overall attenuation obtainable by a typical hearing protection device is significantly restricted by the presence of this peak since it limits the mean attenuation level to about 40 dB in the critical mid-frequency range. It has been hypothesized that this peak may be associated with a middle-ear structural resonance. This hypothesis stems from the current understanding of the primary mechanisms of BC sound transmission, which are typically classified into the following three types (Silman and Silverman, 1991; Stenfelt et al., 2002): (a) compressional, (b) inertial-ossicular, and (c) external-canal. The compressional BC mechanism refers to the case where the skull vibration is transmitted directly to the cochlea via vibrational distortions of the bone enclosing the cochlea fluid. The inertial-ossicular mechanism refers to the case where the BC excitation is transmitted to the cochlea through vibrations of the middle-ear ossicles. This is called the “inertial-ossicular” mode since it is the inertia of the middle-ear ossicles that leads to relative motion between the ossicles and the vibrations of the surrounding bone, which in turn causes cochlear excitation through vibrations of the stapes footplate. The external-canal mechanism is the case where BC-induced vibrations of the cartilaginous ear canal and ear plug produce acoustic pressure in the ear canal that excites the tympanic membrane (TM).
There have been a number of studies that point to the inertial-ossicular mechanism as the dominant BC hearing mechanism in the mid-frequency range around 2 kHz. A study by Carhart (1971) has shown that BC hearing sensitivity especially decreases at 2 kHz on average for patients with a condition of stapes fixation (“otosclerosis”), a phenomenon known as the “Carhart notch.” Tonndorf (1972) attributed this effect to a loss of the middle-ear BC resonance contribution by observing correlation between the middle-ear resonance frequency and the frequency of BC hearing sensitivity loss for various animals. Linstrom et al. (2001) showed that BC hearing improved most significantly at 2 kHz after performing middle-ear reconstruction surgery on patients with middle-ear impairments.
Based on the evidence suggesting the dominance of the middle-ear BC mechanism around 2 kHz, it is logical to hypothesize that the prominent peaks occurring in the 1.5–2 kHz range in the BC limit data of Fig. 1 would most likely be due to the middle-ear BC mechanism, provided that BC is dominating over AC (which is considered to be the case as the terms BC limit and BC threshold suggest by definition). It is pointed out that the higher frequency-resolution data by Zwislocki (1957) and Reinfeldt et al. (2007) in Fig. 1 indicate the prominent BC limit peak to be at around 1.6–1.7 kHz, rather than exactly at 2 kHz as indicated by the BC limit data by Berger et al. (2003) with limited frequency resolution (which is typical of many hearing-related studies including the aforementioned studies indicating the main frequency of the middle-ear BC contribution to be exactly at 2 kHz.)
However, this appears to be inconsistent with knowledge that the mean middle-ear resonance frequency for AC is around 0.8–1.2 kHz (Silman and Silverman, 1991; Wada et al. 1998). Measurements carried out by Wada et al. (1998) on 275 ears from live subjects show the mean resonance frequency (± 1 S.D.) for AC to be 1.17(±0.27) kHz. This raises the following question: Why does the observed middle-ear resonance frequency appear in the 1.5–2 kHz range for BC rather than the 0.8–1.2 kHz range which is the commonly acknowledged middle-ear resonance frequency for AC? The reasons for this apparent difference in the middle-ear resonance frequencies between BC and AC have not been clarified in the past.
In order to identify the mechanisms responsible for this difference, measurements of both BC and AC middle-ear responses were conducted using five human temporal bone specimens. Furthermore, a finite element (FE) model of a middle-ear structural system was developed to gain insight into the modal characteristics observed in the temporal bone measurement data. Understanding the fundamental modal characteristics, such as the natural frequencies and normal modes, is of critical importance in understanding middle-ear dynamics at low-to-mid frequencies. This study is a first step toward the goal of identifying the mechanisms behind the mid-frequency BC limit peak at 1.5–2 kHz, which could ultimately lead to further improvements in hearing protection devices used for extreme noise environments.
METHODS
Temporal bone measurements
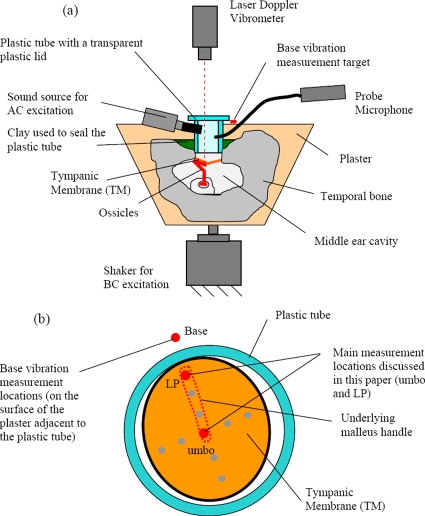
Human temporal bone measurements were conducted to investigate the dynamic characteristics of the middle ear for both AC and BC excitations. The experimental setup is shown in Fig. 2.
Figure 2.
Temporal bone experiment setup for measuring the AC and BC middle-ear responses: (a) cross-sectional view; (b) laser measurement locations on the TM surface from the perspective of the otoscope integrated with the laser Doppler vibrometer. The measurement locations reported in this paper, which indicate the underlying malleus handle motion, are the LP and the umbo.
Temporal bone preparations
Temporal bones were extracted from human cadavers within 48 h of death, at the time of autopsy. A total of five temporal bones (TBs) were used in this study (labeled as TB1, TB2, TB3, TB4, and TB5), which were extracted from an 84 year old male (left ear), a 61 year old male (right ear and left ear for TB2 and TB3), an 81 year old male (right ear), and an 83 year old female (right ear), respectively. For all preparations, the attached extraneous tissues were removed and the bony wall of the external ear canal was drilled down to 2 mm from the tympanic annulus. A 10-mm-long plastic tube with an 8.5 mm internal diameter was placed against the bony ear canal remnant such that the axis of the tube was approximately perpendicular to the plane defined by the tympanic annulus around the edge of the tympanic membrane (TM). This plane is referred to as the “TM plane” in this study. The plastic tube was held in place with clay, and the majority of the temporal bone was encased in plaster. A transparent plastic disk was attached to the tube opening using beeswax in order to acoustically isolate the tube canal, and also to increase the efficiency of introducing sound for AC excitation. The effects of the resulting closed air volume were checked by measuring and comparing the BC responses with and without the plastic disk, and no significant differences were observed. The AC responses were not affected since they are normalized by the acoustic pressure in the air volume.
The middle-ear air cavity (tympanic cavity plus mastoid cavity) also formed a closed air volume since it was not vented to the outside. However, the contribution of the middle-ear air cavity to the overall middle-ear dynamics is considered to be insignificant (Zwislocki, 1962). It is not thought to have a recognizable effect unless the middle-ear air volume is reduced to the extreme, such that the whole mastoid cavity is sacrificed (McElveen et al., 1982; Voss et al., 2000). Based on qualitative inspections of the temporal bones used in this study, it is likely that a significant portion of the mastoid air volume was retained in each specimen. Therefore, it is considered unlikely that the middle-ear air cavities provided a sufficient acoustic stiffness to significantly affect the dynamics. This was also supported by the observation that the mean value of the measured AC resonance frequency among the temporal bones, which is proportional to the square root of the middle-ear stiffness, was largely consistent with the normal expected value as shown later in Sec. 4.
The state of the cochlear fluid was not inspected during the temporal bone preparations, on the assumption that the fluid would be mostly intact for fresh temporal bones. Also, it has been shown that the cochlear input impedance is predominantly resistive (Aibara et al., 2001), which implies that leakage of cochlear fluid mostly has the effect of reducing the amount of damping in the system, and this is not of critical importance in this study.
AC∕BC excitation methods
The plaster-encased temporal bone specimens were attached to a shaker (B&K type 4810, B&K, Nærum, Denmark) to provide BC stimulation. The temporal bone preparations were rigidly attached to the shaker using a screw and cement in such a way that the direction of vibration was approximately perpendicular to the TM plane. AC excitations were provided by a small acoustic transducer, which introduced acoustic pressure into the plastic tube positioned above the TM. The peak sound pressure level for the AC stimulation was 90–93 dB, which is well below the range where nonlinear distortions are expected to occur (120–130 dB) (Voss et al., 2000). The middle-ear vibrations produced by the BC excitation were comparable to or slightly smaller than those produced by the AC excitation: the BC-induced umbo displacement magnitudes ranged from 1 to 40 μm, while the AC-induced magnitudes ranged from 2 to 80 μm. Therefore, the middle-ear structures were not overdriven during the measurements.
Response measurements
A probe tube microphone (ER-7C, Etymotic Research, Elk Grove Village, IL) was also installed to measure the acoustic pressure within the plastic tube. The tip of the probe tube was positioned at a distance of about 2 mm from the TM. A laser Doppler vibrometer sensor head (HLV-1000, Polytec, Tustin, CA) with a joystick-controlled mirror was mounted on an operating microscope to enable the laser beam to be aimed at the desired measurement points. Measurements were obtained at multiple locations on the surface of the TM, as shown in Fig. 2b. Retroreflective microbeads were positioned at the measurement locations to increase the reflected signal. These microbeads were negligibly small in size (about 5–10 μm in diameter and 0.001 mg in mass), and thus provided no potential for mass loading. Although measurements were obtained at a number of TM locations during the experiments, the discussion in this paper is limited to the lateral process (LP) and umbo locations. Since the TM is firmly attached to the malleus bone at these two locations, they are suitable targets for measuring the malleus handle motion. As it will become clear in subsequent discussions, the rigid-body motions of the underlying malleus handle, observed from the two-point measurements, offer essential clues regarding the overall three-dimensional (3D) motion characteristics of the middle ear.
Response data format
AC responses were calculated by normalizing the umbo and LP velocities, vumbo and vlp, by the pressure in the plastic “ear-canal” tube, pec. BC responses were measured at the same locations along the malleus handle, but with the BC excitation introduced by the shaker. Since the surrounding bone structure encasing the middle ear was also vibrating in this case, the ossicular responses are expressed in terms of the differential velocity (Stenfelt et al., 2002), Δv,
| (1) |
where vbase is the base vibration velocity introduced by the BC excitation. The base velocity was obtained at a point near the tube on the plastered surface of the temporal bone, as shown in Fig. 2. The differential velocity, Δv, in the BC response is equivalent to the absolute velocity, v, in the AC response, with the only difference being that the base velocity is zero for AC.
Identification of the primary resonance frequency
Primary resonance frequencies in AC and BC, which are designated as and , respectively, were identified for each individual temporal bone from the middle-ear response data. A system identification procedure, based on the invfreqs function in MATLAB (Mathworks, Natick, MA), was performed for this task. The invfreqs function accepts complex frequency response data and uses a curve-fitting algorithm to find an approximate representation of these data as a rational transfer function, H(s), that consists of the ratio of a numerator polynomial, B(s), (of order m) and a denominator polynomial, A(s), (of order n):
| (2) |
This transfer function expresses an input-output relationship of a dynamic system in the frequency domain (i.e., the s-domain). The polynomial orders, m and n, are selected iteratively by the user until a transfer function is found that produces the best fit to the frequency response data in the frequency range of interest. [That is, all the polynomial coefficients, bm and an, in Eq. 2 are determined.] The natural frequencies, ωn, and damping ratios, ξ, associated with the resonance peaks in the frequency response are then extracted from the complex-valued roots of the denominator polynomial, A(s) [i.e. the roots of the characteristic equation, A(s)=0], as
| (3) |
which are also called “system poles” and represent the complex-valued eigenvalues of the dynamic system. In the present case, the purpose of this system identification procedure was to identify the characteristic parameters, ωn and ξ, associated with the primary resonance peak, which is the first major resonance peak visible in the mid-frequency range. Once a good fit was achieved, and the natural frequency, ωn, associated with the primary resonance peak was determined, the primary resonance frequency in hertz was given as f=ωn∕2π. In practice, there was some variability in the identified values of the natural frequency and damping ratio due to the dependence on user judgment for choosing the appropriate polynomial orders, m and n, and also due to the relatively high level of damping that existed in the middle-ear frequency responses, which also tended to increase the variability. In an effort to minimize this variability, the system identification procedure was performed on both umbo and LP responses, and then the mean of the two values was used. This is a valid technique since natural frequencies and damping ratios are properties inherent to the structural system, and therefore the same modal information should be contained in both frequency responses, which are taken from different locations of the same system.
It should be noted that, in this study, the “resonance frequency” is synonymous with the natural frequency associated with the resonance. Strictly speaking, the center frequency of a resonance peak can be shifted slightly from the natural frequency depending on the damping level. However, for the damping ratio, ξ, presently observed for the middle ear, which is about 0.2 on average, the resonance peak center frequency is approximately equal to the natural frequency, with the potential theoretical difference being only up to about 4% (James et al., 1994). It should also be noted that the natural frequency is a property determined only by the mass and stiffness of the structure, and thus is independent of the damping.
Frequency normalization
Further processing of the measured data was performed, in which the frequency axis of each individual frequency response was normalized by its primary resonance frequency, (or ). This was done in order to compensate for the variability in resonance frequencies among the temporal bones, whose basic modal characteristics were otherwise highly similar aside from being shifted in frequency according to the resonance frequency values. In previous middle-ear dynamics studies, the frequency responses obtained from a number of temporal bone specimens were typically averaged to obtain mean response data, based on which middle-ear models were developed. However, this approach is not suitable in this case, since the averaging process tends to eliminate or “smear” the detailed modal characteristics that were otherwise present in individual responses. Since the current focus is on the middle-ear modal characteristics, it was necessary to observe the individual middle-ear responses without averaging.
Finite element analysis
A FE middle-ear model was developed in order to help reveal the 3D dynamic characteristics of the overall middle-ear structure, which underlies the malleus handle motions observed in the measured data.
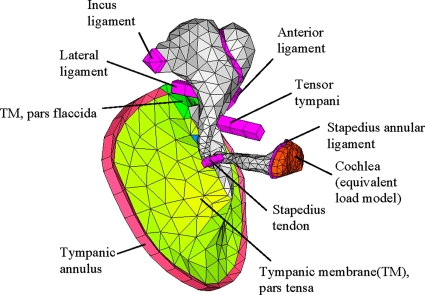
Human middle-ear FE model
Figure 3 shows the FE model of a left middle ear. The middle-ear FE model consisted of the TM, ossicles (malleus, incus, and stapes), ligaments, and tendons. The cochlea was modeled as an equivalent mechanical load based on the cochlear input resistance value reported by Aibara et al. (2001). The geometries of the ossicles and the TM were based on micro-CT imaging data from an actual human middle-ear sample (Sim et al., 2007). Then, a corresponding FE mesh model was created using the FE pre∕post processing software HYPERMESH (Altair Engineering, Tory, MI). Tendons and ligaments were not directly based on the micro-CT imaging data, but approximated using columns with constant cross sections. Solid elements were used for most of the components except for the TM, which instead consisted of “solid-shell” elements (based on the shell equations, but with 3D solid element topologies).
Figure 3.
A FE model of a human middle-ear structure (left ear). The model consists of the TM, ossicles, ligaments, tendons, and a simplified model of the cochlea. The air volumes of the ear canal and the middle-ear cavity were not included in the model.
The material properties of various components were initially obtained from the existing literature (Fay et al., 2006; Sim et al., 2007), including past studies of FE middle-ear modeling (Koike et al., 2002; Gan et al., 2004). In the previous FE middle-ear modeling studies, material property values for the ligaments and tendons were typically adjusted by matching the simulated responses to the experimental dynamic responses. The material property values in the present model were also tuned by comparing against the temporal bone data. However, the approach in this study differed from the previous approaches in some aspects. The elastic modulus values of the ligaments and tendons in the present FE model were calibrated by performing the normal mode analysis first, so that natural frequencies were in agreement with the average values observed in the temporal bone measurements. This is in contrast to previous approaches where the FE model was calibrated against the mean AC response data, whose detailed modal characteristics were no longer identifiable due to the averaging process across a number of temporal bones. Having determined the elastic modulus values of the components, the damping in the model was then adjusted so that the simulated responses (for both AC and BC) exhibited the level of damping that was consistent with that observed in corresponding temporal bone responses. The damping was adjusted through varying the material loss factors associated with the components. Table 1 shows the final material property values of the middle-ear components as a result of this model tuning process, along with the initial reference values obtained from the literature.
Table 1.
Material properties of various components in the middle-ear FE model. The initial material values obtained from the literature are shown in curly brackets.
| Component | Elastic modulusE1 (N∕m2) | Density ρ (kg∕m3) | Loss factor η |
|---|---|---|---|
| Incus ossicle | 1.41×1010a | 2.15×103c | 0.01 (constant) |
| Malleus ossicle | 1.41×1010a | 2.39×103c | 0.01 (constant) |
| Stapes ossicle | 1.41×1010a | 2.20×103a | 0.01 (constant) |
| Malleus∕incus joint | 1.41×1010a | 2.39×103 | 0.01 (constant) |
| Incus∕stapes joint | 4.4×105 {6×105a} | 1.2×103a | 0.15 at 1 kHz |
| Tensor tympani | 1.9×107 {7.0×107a} | 1.2×103 | 0.15 at 1 kHz |
| Anterior ligament | 1.5×107 {2.1×107a} | 1.2×103 | 0.15 at 1 kHz |
| Lateral ligament | 5.0×105 {6.7×106a} | 1.2×103 | 0.15 at 1 kHz |
| Stapes tendon | 3.8×105 {5.2×107a} | 1.2×103 | 0.15 at 1 kHz |
| Incus ligament | 4.8×106 {6.5×106a} | 1.2×103 | 0.15 at 1 kHz |
| Tympanic membrane, Pars tensa | 3×107b | 1.2×103d | 0.15 at 1 kHz |
| Tympanic membrane, Pars flaccida | 0.7×107 {1∕3Epars tensa} | 1.2×103d | 0.15 at 1 kHz |
| Tympanic annulus | 6×105d | 1.2×103 | 0.15 at 1 kHz |
| Stapes annular ligament | 4.12×105e | 1.2×103 | 0.25 at 1 kHze |
| Cochlear load | Effective resistance: 30 GΩf (mass and stiffness are assumed to be insignificant) | ||
Aibara et al. (2001) [with 1.41 correction factor applied; see O’Connor and Puria (2008)].
The current FE model did not include air volumes associated with the ear canal and the middle-ear cavities. This is because the impedance magnitudes associated with these air cavities were expected to be significantly smaller than those associated with the structural part of the middle ear and consequently the effects of the air cavities on the dynamics of the middle ear were considered to be insignificant (Zwislocki, 1962). Therefore, the current FE model excluded the air cavities, which allowed concentration on mechanically the most significant part of the middle-ear structures.
Forced response analysis
Simulations were performed using the FE simulation software ACTRAN (Free Field Technologies, Belgium), which was developed specifically for vibroacoustic problems. Two kinds of FE analysis were performed: one was the forced response analysis, and the other was the normal mode analysis. In the forced response analysis, the middle-ear responses to external excitations (either AC or BC) are simulated by solving the following matrix equation of motion (EOM):
| (4) |
where ω is the radian frequency and x is the displacement vector to be solved as a response to the forcing vector, f. The matrices, K and M, are the stiffness and mass matrices. The stiffness and damping properties associated with the structural components are represented by the frequency-dependent complex-valued material modulus:
| (5) |
where E1, is called the “storage” modulus which represents the stiffness, and the imaginary part, E2, is called the “loss” modulus which defines the damping. As a result, the stiffness matrix, K, in the EOM of Eq. 4 is complex valued and frequency dependent:
| (6) |
where K1 and K2 represent the overall stiffness and damping of the system, respectively.
The complex material modulus of Eq. 5 above can be alternatively written as
| (7) |
where the loss factor, η, specifies the material damping. In the current middle-ear FE model, the storage modulus, E1, is assumed to be constant, which means that the matrix K1 in Eq. 6 is also constant. The loss factor for the middle-ear ligaments and tendons are assumed to be proportional to frequency, that is,
| (8) |
where c is a constant. In Table 1, the proportionality constants, c, for components are specified indirectly by a reference loss factor value at 1 kHz. This material damping model is essentially equivalent to the Rayleigh damping model (with only the stiffness-proportional constant β being used, and the mass-proportional constant α being set to 0), which has been used in previous middle-ear FE model studies. It can be shown that the constant β of the Rayleigh damping model is equivalent to the constant c in the loss factor model of Eq. 8 (James et al., 1994). It should be noted that the loss factor, η, which describes the material damping in individual components, should be distinguished from the damping ratio, ξ, of Eq. 3, which describes the total damping in the system resulting from the cumulative effects of the component-level damping.
AC excitations were simulated by assigning a uniformly distributed dynamic pressure over the TM surface on the ear-canal side. This is considered reasonable for the current study since the acoustic wavelength is still fairly large compared to the TM dimensions for the frequencies of interest (up to 4–5 kHz). The BC excitations were simulated by assigning uniform displacement vectors (both magnitude and phase) at the boundaries of the structure, such as at the ends of the ligaments and tendons, and the edge of the tympanic annulus. This essentially simulated the rigid-body vibration of the base temporal bone structure. The direction of the BC excitation was perpendicular to the TM plane (i.e., the plane of the tympanic annulus), which is consistent with the temporal bone measurements.
Normal mode analysis
The other type of FE analysis performed in this study was the normal mode analysis, in which the following equation is solved:
| (9) |
This equation is obtained from the EOM of Eq. 4 above with the excitation vector, f, set to zero. It should be noted that the stiffness matrix is, K1, which is only the real part of the original stiffness matrix, K, and does not include the damping part, K2. This renders Eq. 9 a real-valued eigenproblem, which is solved to obtain the natural frequencies (eigenfrequencies), ωn, and the mode shapes (eigenvectors), x. The removal of the system damping was achieved in the present model by eliminating the imaginary parts of the elastic modulus values for all the components, including the cochlear fluid load. In a normal mode analysis, it is typical to solve this real-valued eigenproblem without damping, since the natural frequencies and mode shapes are properties that depend solely on the mass and stiffness characteristics of a dynamic structure, and are independent of the damping and excitation.
RESULTS
This section presents the results obtained from the temporal bone measurements, followed by the simulation results obtained by the FE analyses.
Temporal bone measurements
Measured middle-ear responses
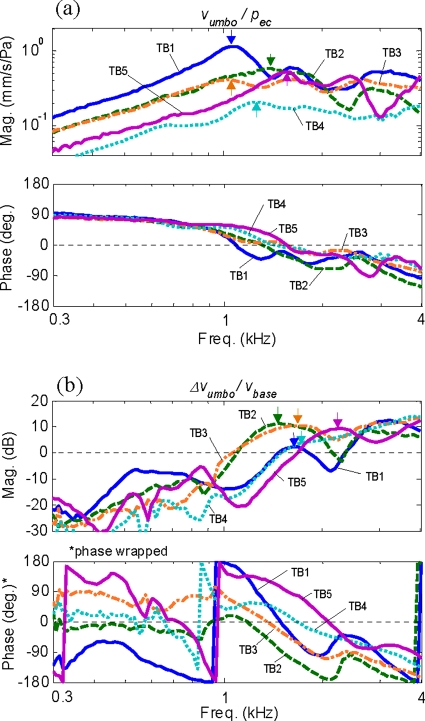
Figure 4 shows the middle-ear responses measured at the umbo for five temporal bones, with the AC responses, vumbo∕pec, shown in Fig. 4a, and the BC responses, Δvumbo∕vbase, shown in Fig. 4b. The arrows indicate the primary resonance peaks. It can be observed from the two plots that the primary resonance peaks for BC occur at higher frequencies than for AC.
Figure 4.
Measured umbo responses from the five temporal bones: (a) AC responses—ratio of the umbo velocity (mm/s), vumbo, to the ear-canal acoustic pressure (Pa), pec; (b) BC responses—ratio of the differential umbo velocity, Δvumbo (=vumbo−vbase), to the base excitation velocity, vbase. The arrows indicate the primary resonance peaks.
Identified primary resonance frequencies
Table 2 summarizes the values of the primary resonance frequencies, and , and associated damping ratios, ξ, identified for the five temporal bones. As shown in the table, the mean AC resonance frequency, , is 1.26(±0.20) kHz and the mean BC resonance frequency, , is 1.72(±0.34) kHz, which is clearly higher than the mean AC resonance frequency, . The BC resonance frequency is observed to be higher than the AC resonance frequency in every temporal bone, typically by a factor of around 1.4–1.5. The mean damping ratios, ξ, identified for the AC and BC resonances are 0.19(±0.03) and 0.20(±0.06), respectively.
Table 2.
Primary AC and BC resonance frequencies, and , along with the associated damping ratios identified from the measured temporal bone responses.
| Temporal bone | Primary AC resonance | Primary BC resonance | ||
|---|---|---|---|---|
| Frequency (kHz) | Damping ratio, ξ | Frequency (kHz) | Damping ratio, ξ | |
| TB1 | 1.06 | 0.18 | 1.66 | 0.18 |
| TB2 | 1.30 | 0.21 | 1.44 | 0.18 |
| TB3 | 1.13 | 0.23 | 1.62 | 0.29 |
| TB4 | 1.23 | 0.15 | 1.59 | 0.21 |
| TB5 | 1.57 | 0.17 | 2.31 | 0.14 |
| Mean(±S.D.) | 1.26(±0.20) | 0.19(±0.03) | 1.72(±0.34) | 0.20(±0.06) |
Frequency-normalized middle-ear responses
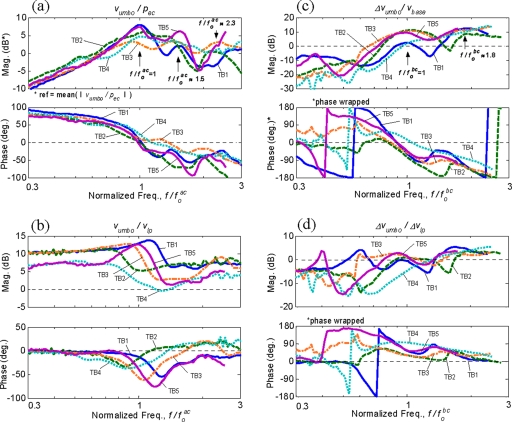
Figure 5 shows the frequency-normalized middle-ear responses. Figures 5a, 5c show the frequency-normalized umbo responses for AC, vumbo∕pec, and for BC, Δvumbo∕vbase, respectively. The magnitude of each umbo AC response has also been normalized by its mean magnitude calculated over the frequency range shown. Figures 5b, 5d show the velocity ratios between the umbo and LP, which indicate the two-dimensional motion of the malleus handle for AC, vumbo∕vlp, and for BC, Δvumbo∕Δvlp.
Figure 5.
Frequency-normalized middle-ear responses of the five temporal bones: (a) umbo AC response, vumbo∕pec; (b) umbo/LP AC velocity ratio, vumbo∕vlp; (c) umbo BC response, Δvumbo∕vbase; (d) umbo/LP BC velocity ratio, Δvumbo∕Δvlp. The frequency axes are normalized by their respective resonance frequencies, and . Each umbo AC response in (a) are also normalized by its mean magnitude calculated over the frequency range shown.
As shown in Fig. 5, the normalization clearly reveals common characteristics, which are otherwise difficult to distinguish, among the temporal bone data. First, in the umbo AC responses shown in Fig. 5a, the primary resonances occur at by definition, as a result of the normalization. The phase responses tend to cross or approach 0° around . There also tend to be two additional resonance peaks at higher frequencies at and 2.3. The umbo∕LP velocity ratio plot in Fig. 5b also reveals common characteristics among the temporal bones. At low frequencies, the umbo and LP motions are in phase, but the magnitudes are significantly higher for the umbo than the LP. Then, around the primary AC resonance frequency, , there is a transitional region where the magnitude of the umbo∕LP response ratio shifts to a smaller value. This is accompanied by a dip in the phase around this frequency.
The frequency-normalized umbo BC responses in Fig. 5c show the primary BC resonance at , by definition, and the phase responses are mostly around −90° at this frequency. An additional resonance peak is also recognizable at around , and the magnitudes become significantly smaller for frequencies below . Figure 5d shows the umbo∕LP velocity ratio for BC, Δvumbo∕Δvlp, which exhibits significant similarity among the temporal bones at frequencies above . The figure shows that the magnitude and the phase of the umbo∕LP response ratios tend to approach zero at the primary BC resonance frequency, . At frequencies above , there tends to be a slight dip in magnitude, but then it recovers to about 2–5 dB at higher frequencies.
It is observed in Figs. 5c, 5d that the BC responses show significant variability, especially in phase responses, at low frequencies below . The likely reason for this variability is that the BC response is obtained by taking the difference between two velocity measurements (e.g., vumbo=vumbo−vbase), such that when the two velocities become more similar at low frequencies, the phase of their difference becomes more sensitive to small variations between the two velocities. Furthermore, some temporal bone data are seen to contain low-frequency peaks, for example, at in TB1 and in TB5 in Fig. 5c, which are not found in the other temporal bones. These extra peaks may be the result of minor low-frequency rocking motions of the temporal bone∕shaker assembly, caused by an imperfect alignment of the temporal bone’s center of gravity with the shaker’s axis of vibration. However, the inconsistency in the BC response data at low frequencies does not affect the current discussion since the main frequency range of interest for BC is , where the BC response magnitudes become significant due to the middle-ear BC resonance at .
Finite element analysis
Normal mode analysis
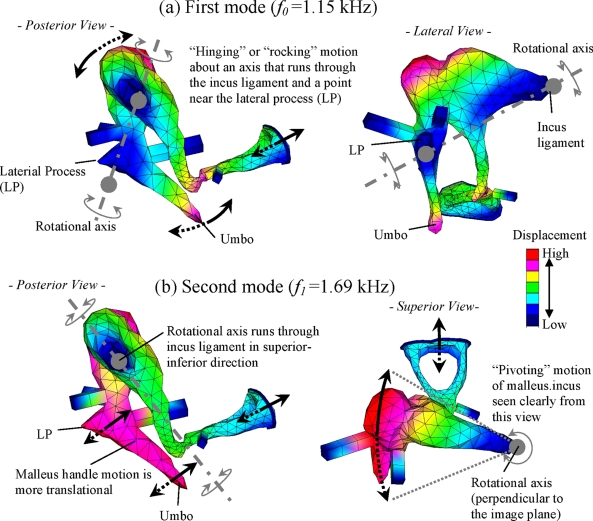
The normal mode analysis identified a total of three modes in the frequency range below 3 kHz whose natural frequencies, designated as f0, f1, and f2, are 1.15, 1.69, and 2.76 kHz, respectively. Although the analysis identified additional modes at frequencies above 3 kHz, these higher-order modes are not discussed in this study since the focus is on the characteristics of the low-to-mid frequencies. The first two modes, found at 1.15 and 1.69 kHz, are attributed to rigid-body motions of the ossicles, which are supported by flexible ligaments and tendons, and are therefore referred to as “ossicular modes” in this study. Figure 6 shows qualitative illustrations of the characteristic middle-ear motions (i.e., mode shapes) associated with these two ossicular modes. Figure 6a shows the first mode, at f0=1.15 kHz, which is characterized by a hinging motion of the malleus-incus complex about an axis that connects the incus ligament and a point near the LP. On the other hand, the second mode, at f1=1.69 kHz, shown in Fig. 6b involves an ossicular motion that is significantly different from that of the first mode at f0. The characteristic motion of the second mode can be described as the malleus-incus assembly “pivoting” about an axis running through the incus ligament in the superior-inferior direction, which is clearly seen by the superior view in Fig. 6b. The posterior view of this mode in Fig. 6b shows a translational movement of the malleus handle where the umbo and the LP are moving in phase and in parallel. The stapes motions associated with both modes are observed to be rather “piston-like,” but with some amount of rocking component also observable. The third mode, at f2=2.76 kHz, is observed to be mainly associated with the TM, and is characterized by a prominent displacement in the posterior region of the TM consistent with the measurements by Tonndorf and Khanna (1972). This TM resonance mode was not of primary interest in this study, therefore and was not illustrated in Fig. 6.
Figure 6.
Characteristic motions (mode shapes) associated with the two middle-ear modes identified by the FE normal mode analysis below 2 kHz: (a) The first mode (“hinging mode”) at f0=1.15 kHz; (b) The second mode (“pivoting mode”) at f1=1.69 kHz. The color map indicates relative displacement amplitude and the arrows suggest general motional directions.
Forced response analysis
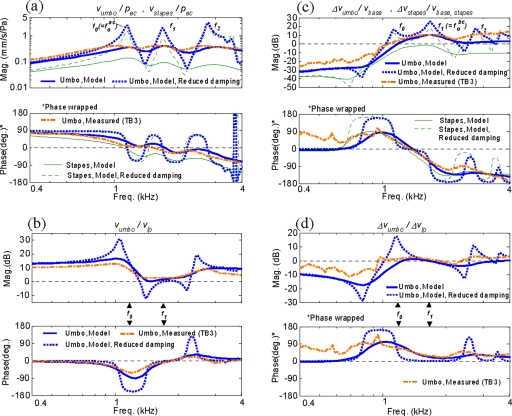
Figure 7 shows the simulated AC and BC middle-ear responses in comparison to the measured responses of one individual temporal bone, TB3. This bone was chosen for comparison with the model since its natural frequencies are close to the mean values from the five temporal bones that were used to tune the FE middle-ear model (Table 2). This bone also exhibits response characteristics that reflect the overall trends of the four other temporal bones in the normalization analysis of Fig. 5. As discussed earlier, it was important to compare the model with the response from a representative individual ear rather than with the mean response, since in the latter case the detailed response characteristics tend to be lost due to averaging. The figure also shows simulated middle-ear responses with a negligible level of damping, in order to highlight the resonance characteristics. This was done by reducing the loss factors, η, from 0.15–0.25 (Table 1) to 0.01, for all tendons and ligaments and also by removing the resistive cochlear load.
Figure 7.
Comparison of simulated AC and BC responses (with nominal and reduced damping) and representative measured responses from a temporal bone (TB3): (a) umbo AC response, vumbo∕pec; (b) Umbo/LP AC velocity ratio, vumbo∕vlp; (c) umbo BC response, Δvumbo∕vbase; (d) umbo/LP BC velocity ratio, Δvumbo∕Δvlp. In addition, FE-simulated stapes responses (nominal and reduced damping) are also shown in (a) and (c).
Figures 7a, 7c show the simulated and measured umbo responses for AC, vumbo∕pec, and for BC, Δvumbo∕vbase, respectively. Figures 7b, 7d show the velocity ratios between the umbo and LP for AC, vumbo∕vlp, and for BC, Δvumbo∕Δvlp, respectively. The figures also show simulated responses for the stapes footplate, which were calculated by taking the ratio of the normal velocity at the center of the stapes footplate, vstapes, to the acoustic pressure applied at TM, pec. The simulated umbo AC response in Fig. 7a shows a high degree of correspondence with the temporal bone measurement data. The result shows the presence of three major resonance peaks, which are associated with the three modes at f0, f1, and f2 identified earlier in the normal mode analysis. These resonance peaks become especially clear in the simulated response with reduced damping. Comparing Fig. 7a with Fig. 5a suggests that these three middle-ear modes, at f0, f1, and f2, correspond to the three resonance peaks observed earlier in the frequency-normalized AC responses at , 1.5, and 2.3.
Figure 7a also shows the simulated AC response at the stapes footplate, vstapes∕pec. It can be seen that the stapes response is also mainly characterized by the three middle-ear modes, f0, f1, and f2. In other words, the modal response characteristics of the middle ear observed at umbo are also observable at stapes. This is reasonable since the overall motion of the middle-ear structural system, characterized by the three normal modes, is also what drives the stapes. This in turn reinforces the validity of the present approach, in which the modal characteristics of the middle ear are investigated primarily through the motions of the malleus handle (i.e., the umbo and LP), rather than the stapes.
Figure 7b shows the umbo∕LP velocity ratio, vumbo∕vlp, in response to AC excitation. Again, the response characteristics exhibited by the measured data are well captured by the FE model. At frequencies below the first mode at f0, the umbo response magnitude is significantly higher than that of the LP, which is characteristic of the hinging motion associated with the first mode shown in Fig. 6a. Above f0, both the magnitude and phase transition to approximately zero at f1, indicating a translational motion of the malleus handle, which is associated with the characteristic pivoting motion of the second mode shown in Fig. 6b. These characteristics can also be seen in the reduced damping case where the malleus handle motion at f0 becomes essentially that of the first mode (rotational malleus handle motion) and the motion at f1 becomes that of the second mode (translational malleus handle motion).
The simulated umbo BC response shown in Fig. 7c, Δvumbo∕vbase, also exhibits good agreement with the overall characteristics of the measured response. Again, the BC response in this frequency range is also characterized mainly by the three normal modes at f0, f1, and f2. This is also observed for the simulated stapes BC responses, which exhibit similar modal response characteristics. [The stapes BC response is calculated using Eq. 1, but in the normal direction of the stapes footplate. Accordingly, the base velocity for the stapes, vbase,stapes, is the component of the base velocity, vbase, in the normal direction of the stapes footplate.] By comparing the BC responses in Fig. 7c to the AC responses in Fig. 7a, however, it can be observed that the resonance peak of the second mode at f1, compared to that of the first mode at f0, is more prominent in BC than in AC. This is particularly recognizable in the reduced damping case.
Figure 7d shows the umbo∕LP velocity ratio for BC, Δvumbo∕Δvlp, which also exhibits a good level of agreement between the model and the measured data. At the natural frequency of the second mode, f1, the magnitude and phase are close to zero, which indicates the translational motion of the malleus handle associated with the second mode in Fig. 6b. However, in contrast to the AC response case in Fig. 7b, the umbo∕LP magnitude ratio at f0 for the BC case indicates that the umbo and LP are moving by comparable amounts, and therefore are not exhibiting the rotational malleus handle motion associated with the first mode shown in Fig. 6a. Only when the damping is reduced, as shown by the simulation curve, does the malleus handle motion at f0 match what is expected of the first mode, in which the umbo moves significantly more than the LP. This indicates that, for BC, the contribution of the first mode to the malleus handle motion is relatively weak, compared to that of the second mode.
DISCUSSION
Comparisons with published middle-ear response data
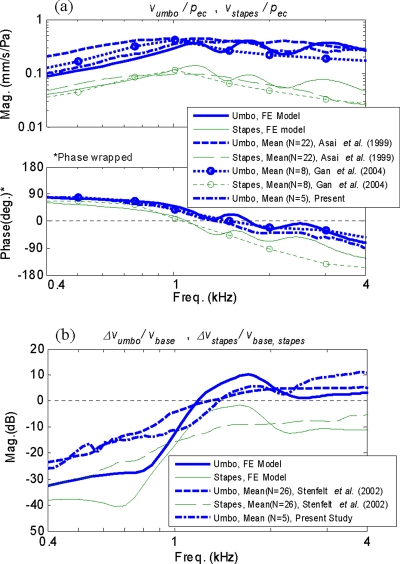
Figure 8 compares the middle-ear responses obtained from the FE model with mean response data from previous temporal bone measurements from the literature. Figure 8a shows the magnitude and phase of the FE-simulated AC responses obtained at both the umbo and the stapes, compared with the measured mean data from Asai et al. (1999) with sample size N=22, and Gan et al. (2004) with N=8. [Magnitude responses are only presented for Asai et al. (1999) since the phase data were not reported in their study.] The figure also shows the mean of the umbo response data from the current temporal bone measurement (N=5). As shown in Fig. 8a, the FE-simulated results follow the general trends exhibited by the published mean data, except that the simulated results contain detailed resonance features that are missing from the mean data. The umbo responses show especially good agreement with the mean response data for both the magnitude and the phase, in the frequency range shown. The stapes responses are also in generally good agreement, especially at frequencies below 2 kHz. However, the rates of magnitude and phase roll-off at frequencies above 2 kHz for the mean stapes response data are seemingly higher than those exhibited by the simulated result, although the overall trend is similar. The cause of this difference is currently unknown.
Figure 8.
Comparison of the FE-simulated AC and BC responses with published mean temporal bone data (Asai et al., 1999; Gan et al., 2004; Stenfelt et al., 2002): (a) AC responses at umbo and stapes (magnitude and phase); (b) BC responses at umbo and stapes (magnitude only). Measured mean umbo response data (N=5) from the present study are also shown.
Figure 8b shows the comparison between the FE-simulated BC responses and the mean temporal bone measurement data by Stenfelt et al. (2002), at both the umbo and the stapes. Only magnitudes are shown in the figure since Stenfelt et al. (2002) did not report the corresponding phase data. As shown by the figure, the overall responses at both the umbo and the stapes are in general agreement between the FE-simulated responses and the mean data, except again that the mean data are much smoother than the FE model response, due to averaging (N=26). The current mean temporal bone data are also seen to be consistent with the data from Stenfelt et al. (2002).
Primary resonance frequency differences between AC and BC
The mean primary BC resonance frequency, , was 1.72(±0.34) kHz for the five temporal bones, while the mean AC primary resonance frequency, , was 1.26(±0.20) kHz (Table 2). The AC resonance frequency of 1.26(±0.20) kHz in this study is comparable to the general expected range of 0.8–1.2 kHz (Silman and Silverman, 1991) and is also consistent with the 1.17(±0.27) kHz value from Wada et al. (1998). For the mean BC resonance frequency, no previous studies are known that explicitly identify this value. However, the characteristics of the BC response data from Stenfelt et al. (2002), in Fig. 8, suggest a BC resonance frequency in the 1.5–2.0 kHz range.
The results obtained in this study suggest that difference in the primary resonance frequencies between AC and BC, which has not been clearly explained in the past, can be attributed to the modal characteristics of the middle ear. The temporal bone measurement data, together with the FE analysis, reveal the presence of two distinct middle-ear structural modes within the frequency range below 2 kHz. The first mode, whose natural frequency f0 is typically at about 1.2 kHz, is recognizable as the primary AC resonance mode, and so . The most dominant mode in the BC case is not the first mode, but rather the second mode, whose natural frequency f1 is typically around 1.7 kHz, so . Therefore, the difference in the primary resonance frequency between AC and BC can be said to result from a difference in the characteristic vibration mode that is dominantly excited by the two forms of excitation.
The reason why the second mode at f1 is dominant in BC rather than the first mode at f0 is not completely clear at this point and requires further investigation. It is speculated that this may be due to a difference in the degree of coupling of the BC excitation to each of the two modes. Generally speaking, a specific vibration mode is excited most effectively when the excitation vector is aligned with the direction of the eigenvector, or mode shape, associated with the mode. For the human middle-ear structure investigated in this study, the preferential vector direction of the second mode may be better matched to the vector direction of the BC excitation than the first mode. It appears intuitively reasonable that the second mode, which is characterized by a translation-like, pivoting ossicular motion, would be excited efficiently by the BC excitation given in the direction that is generally aligned with the motional direction of the mode.
Now, what if the BC excitation were given in directions other than this particular direction? It is possible that from some excitation directions the second mode may not be as efficiently excited, such as if the BC excitation were given in a direction orthogonal to the characteristic motion of the second mode. In that case, one might expect the first mode at f0=1.2 kHz to increase in prominence relative to the second mode at f1=1.7 kHz. However, this contradicts available evidence (Carhart, 1971; Tonndorf, 1972; Linstrom et al., 2001) that indicate the peak BC resonance effect to be at around 2 kHz, which is more in line with the second mode at f1=1.7 kHz than the first mode at f0=1.2 kHz. Therefore, it is unlikely that in situ BC excitations occur primarily in these alternate directions. However, the issues surrounding in situ BC excitation in live subjects are beyond the scope of this study and would require future investigation. In any case, the basic finding that the human middle ear exhibits a dual ossicular mode structure (i.e., the presence of the two ossicular modes) is independent of the particular type of excitation given to the system.
Presence of the second ossicular mode
One of the key findings in this study is the presence of the second ossicular mode at f1, which is described as the pivoting mode in this study. In addition to being the primary resonance mode for BC, the results indicate that this mode is also excited in the AC case, resulting in the second resonance peak at f=f1, just above the first peak at f=f0. The presence of this mode and its role in AC and BC hearing mechanics have not been recognized in the past. Decraemer and Khanna (1994) observed that the measured malleus handle motion contains a significant translational component in addition to a rotational component at some frequencies. Goode et al. (1994) also observed in their temporal bone measurements that the magnitude of the LP response approaches that of the umbo at around 1.6 kHz, indicating a translational motion of the malleus handle. The current findings suggest that the translational malleus handle motions observed in these previous studies are likely attributed to the motional contribution of the second mode.
Relationship to the mid-frequency BC limit peak
This study was originally motivated by a desire to understand the origin of the prominent peak feature at 1.5–2 kHz in the BC limit data (Fig. 1). The working hypothesis has been that this feature can be attributed to a structural resonance of the middle ear. The findings from the present study support this hypothesis since the middle-ear structural system is observed to resonate at around 1.7 kHz on average in response to a BC excitation, due to the presence of the second ossicular mode whose natural frequency, f1, occurs at that frequency. However, further studies are perhaps desirable before it is possible to conclusively link the middle-ear BC resonance to the mid-frequency BC limit peak.
CONCLUSION
The modal dynamic characteristics of the middle ear were investigated through temporal bone measurements and FE analysis. The results indicate that the apparent difference in the primary resonance frequency between AC and BC is due to the presence of two distinct ossicular resonance modes whose natural frequencies are in the 1–2 kHz range. Although the first middle-ear mode, whose natural frequency is typically at around 1.2 kHz, is the primary resonance in AC, it is the second mode, near 1.7 kHz, that is dominantly excited in the BC response. The second mode is also excited in AC, resulting in a second resonance peak in the AC response. The FE simulation shows that the ossicular motion associated with the second mode occurs as a pivoting motion of the malleus-incus complex, with its rotational axis running through the incus ligament in an approximately superior-inferior orientation. This motion is fundamentally different from the classical ossicular hinging motion associated with the first mode. The present results indicate that this little-recognized second ossicular mode is likely the source of the translational component of the malleus handle motion that has been observed in some previous studies. The current findings also further support the hypothesis that a middle-ear structural resonance is responsible for the prominent peak feature seen at 1.5–2 kHz in the BC limit data.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Stefan Stenfelt for his advice on temporal bone experiments. This work was sponsored by the Air Force Office of Scientific Research (AFOSR) under a STTR funding (Contract No: FA9550-06-C-0039).
References
- Aibara, R., Welsh, J. T., Puria, S., and Goode, R. L. (2001). “Human middle-ear sound transfer function and cochlear input impedance,” Hear. Res. 10.1016/S0378-5955(00)00240-9 152, 100–109. [DOI] [PubMed] [Google Scholar]
- Asai, M., Huber, A. M., and Goode, R. L. (1999). “Analysis of the best site on the stapes footplate for ossicular chain reconstruction,” Acta Oto-Laryngol. 10.1080/00016489950181396 119, 356–361. [DOI] [PubMed] [Google Scholar]
- Berger, E. H., Kieper, R. W., and Gauger, D. (2003). “Hearing protection: surpassing the limits to attenuation imposed by the bone-conduction pathways,” J. Acoust. Soc. Am. 10.1121/1.1605415 114, 1955–1967. [DOI] [PubMed] [Google Scholar]
- Carhart, R. (1971). “Effects of stapes fixation on bone-conduction response,” in Hearing Measurement: A Book of Readings, edited by Ventry I. M., Chailkin J. B., and Dixon R. F. (Appleton-Century-Crofts, New York: ), pp. 116–129. [Google Scholar]
- Decraemer, W. F., and Khanna, S. M. (1994). “Modeling the malleus vibration as a rigid body motion with one rotational and one translational degree of freedom,” Hear. Res. 72, 1–18. [DOI] [PubMed] [Google Scholar]
- Fay, J. P., Puria, S., and Steele, C. R. (2006). “The discordant eardrum,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0603898104 103, 19743–19748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, R. Z., Feng, B., and Sun, Q. (2004). “Three-dimensional finite element modeling of human ear for sound transmission,” Ann. Biomed. Eng. 10.1023/B:ABME.0000030260.22737.53 32, 847–859. [DOI] [PubMed] [Google Scholar]
- Goode, R. L., Killion, M., Nakamura, K., and Nishihara, S. (1994). “New knowledge about the function of the human middle ear: Development of an improved analog model,” Am. J. Otol. 15, 145–154. [PubMed] [Google Scholar]
- James, M. L., Smith, G. M., Wolford, J. C., and Whaley, P. W. (1994). Vibration of Mechanical and Structural Systems (HarperCollins, New York: ). [Google Scholar]
- Koike, T., Wada, H., and Kobayashi, T. (2002). “Modeling of the human middle ear using the finite-element method,” J. Acoust. Soc. Am. 10.1121/1.1451073 111, 1306–1317. [DOI] [PubMed] [Google Scholar]
- Linstrom, C. J., Silverman, C. A., Rosen, A., and Meiteles, L. Z. (2001). “Bone conduction impairment in chronic ear disease,” Ann. Otol. Rhinol. Laryngol. 110, 437–441. [DOI] [PubMed] [Google Scholar]
- McElveen, J. T., Goode, R. L., Miller, C., and Falk, S. A. (1982). “Effect of mastoid cavity modification on middle ear sound transmission,” Ann. Otol. Rhinol. Laryngol. 91, 526–532. [DOI] [PubMed] [Google Scholar]
- O’Connor, K. N., and Puria, S. (2008). “Middle-ear circuit model parameters based on a population of human ears,” J. Acoust. Soc. Am. 10.1121/1.2817358 123, 197–211. [DOI] [PubMed] [Google Scholar]
- Reinfeldt, S., Stenfelt, S., and Håkansson, B. (2007). “Examination of bone-conducted transmission from sound field excitation measured by thresholds, ear-canal sound pressure, and skull vibrations,” J. Acoust. Soc. Am. 10.1121/1.2434762 121, 1576–1587. [DOI] [PubMed] [Google Scholar]
- Shin, M., Baek, J. D., Steele, C. R., and Puria, S. (2008). “Stapes biomechanics: Is there an optimal stimulation axis?,” Association for Research in Oto-Laryngology, Mid-Winter Meeting, Phoenix, AZ.
- Silman, S., and Silverman, C. A. (1991). Auditory Diagnosis (Academic, San Diego: ), Chap. 3, p. 79. [Google Scholar]
- Sim, J. H., Puria, S., and Steele, C. R. (2007). “Calculation of inertial properties of the malleus-incus complex from micro-CT imaging,” J. Mech. Mater. Struct. 2, 1515–1524. [Google Scholar]
- Stenfelt, S., Hato, N., and Goode, R. L. (2002). “Factors contributing to bone conduction: The middle ear,” J. Acoust. Soc. Am. 10.1121/1.1432977 111, 947–959. [DOI] [PubMed] [Google Scholar]
- Tonndorf, J. (1972). “Bone conduction,” in Foundations of Modern Auditory Theory, edited by Tobias J. V. (Academic, New York: ), Vol. II, pp. 197–237. [Google Scholar]
- Tonndorf, J., and Khanna, S. M. (1972). “Tympanic-membrane vibrations in human cadaver ears studied by time-averaged holography,” J. Acoust. Soc. Am. 10.1121/1.1913236 52, 1221–1233. [DOI] [PubMed] [Google Scholar]
- Voss, S. E., Rosowski, J. J., Merchant, S. N., and Peake, W. T. (2000). “Acoustic responses of the human middle ear,” Hear. Res. 10.1016/S0378-5955(00)00177-5 150, 43–69. [DOI] [PubMed] [Google Scholar]
- Wada, H., Koike, T., and Kobayashi, T. (1998). “Clinical applicability of the sweep frequency measuring apparatus for diagnosis of middle ear diseases,” Ear Hear. 19, 240–249. [DOI] [PubMed] [Google Scholar]
- Zwislocki, J. (1957). “In search of the bone-conduction threshold in a free sound field,” J. Acoust. Soc. Am. 10.1121/1.1909058 29, 795–804. [DOI] [Google Scholar]
- Zwislocki, J. (1962). “Analysis of the middle-ear function. Part I: Input impedance,” J. Acoust. Soc. Am. 10.1121/1.1918382 34, 1514–1523. [DOI] [Google Scholar]