Abstract
Neuronal inflammation is very common in Alzheimer’s disease (AD). This inflammation can be caused by infiltration of neutrophils across the blood brain barrier (BBB). Endothelial permeability changes are required for the infiltration of high molecular weight components to the brain. Deposition of toxic amyloid-β (Aβ) fibrils in the cerebral vasculature, as well as in brain neurons has been implicated in the development of AD. This study investigates the effect of Aβ fibrils on the permeability of the endothelium and the mechanism for the observed permeability changes. Aβ (1–40) and (1–42) fibrils, but not monomers, were found to increase permeability of bovine pulmonary arterial endothelial cells in a dose- and time-dependent manner as detected by transendothelial electrical resistance. This increase in permeability is only partially (25%) inhibited by catalase and is not associated with an increase in cytosolic Ca+2 or tyrosine phosphorylation. These results indicate that hydrogen peroxide is not the primary mediator for the permeability changes. Treatment of cells with both amyloid fibrils resulted in stress fiber formation, disruption and aggregation of actin filaments and cellular gap formation. The results of this study reveal that Aβ increases the permeability of endothelium by inducing change in the cytoskeleton network.
Keywords: Amyloid-β, Endothelial cells, Alzheimer’s disease, Permeability
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neuronal degenerative disorder of the central nervous system that ultimately results in the loss of cognitive function [1]. This disease is characterized by the accumulation of toxic amyloid-β (Aβ) proteins in the extracellular space of the brain and on the wall of blood vessels in the brain [2,3].
The endothelium of brain blood vessels provides a semi-permeable barrier between blood components and brain tissue. Maintenance of the integrity of this barrier is crucial for vessel wall homeostasis. Evidence for dysfunction in the endothelial barrier of the brain vascular system in AD subjects is provided by the prevalence of an increase in microvascular permeability and disruption of cerebral vascular endothelial cell (EC) tight junctions [4,5] and cerebrovascular inflammation in Alzheimer’s disease[6]. Impaired integrity of the blood brain barrier has also been reported in animal models of AD [7].
In animal models the impaired integrity of the BBB has been shown to be restored following immunization with antibodies against Aβ implicating vascular Aβ in the impaired BBB of these animals [8]. A role for Aβ in barrier dysfunction is further implied by studies involving the intracarotid infusion of Aβ into rats [9] and guinea pigs [10]. These studies demonstrate that infused Aβ penetrates the blood brain barrier and is found in the brain parenchyma. Amyloid β induced damage also increases the permeability of cerebral vessels and results in the transfer of T lymphocytes into brain [11].
The mechanism for Aβ induced barrier dysfunction has, however, not been elucidated. The barrier functions of ECs are known to be regulated by signal transduction systems of junctional and cytoskeletal proteins[12]. Elevated concentrations of reactive oxygen species (ROS) in the range of 50µM to100 µM alter the signal transduction process increasing the permeability of the endothelium and promoting leukocyte adhesion leading to transmigration of leukocytes to underlying tissue [13]. This process generates inflammation leading to impairment of the endothelial barrier function [12,14]. Amyloid-β fibrils are known to generate ROS causing lipid peroxidation and protein damage [15–17]. It is, however, not known to what extent Aβ induced changes in barrier function are associated with the production of ROS. In this study, we characterize the effect of Aβ proteins on vascular ECs that can be associated with changes in permeability and investigate the contribution of ROS to the observed changes in permeability.
MATERIALS AND METHODS
Synthetic Aβ (1–40), (40-1), (1–42) and (42-1) were obtained from BioSource International, Inc. (Camarillo, CA). Peptides were suspended in phosphate buffered saline, pH 7.4 without calcium and magnesium at a concentration of 250 µM and incubated at 37º C for 36 hrs to form fibrils. Under these conditions the (1–40) and (1–42) Aβ form fibrils but the (40-1) and (42-1) reverse sequence Aβ do not form fibrils.
Cell Culture
Bovine pulmonary arterial endothelial cells (BPAECs) were cultured in minimum essential medium (MEM) with 10% fetal calf serum. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2, 95% air. Cells grown in culture flasks were detached with 0.05% trypsin and resuspended in fresh medium and cultured on gold electrodes for electrical resistance determinations, or on glass coverslips for calcium and immunocytochemistry studies, or in 100-mm dishes for Western blotting.
Measurement of Transendothelial Electrical Resistance (TER)
TER was measured in an electrical cell-substrate impedance sensing system, Applied Biophysics, Inc. (Troy, NY) [18]. The cells were cultured on gold microelectrodes (8 electrodes per plate) until reaching −95% confluence. At this stage tight cell-cell junctions are formed. One hour before TER measurements, the cells were rinsed with serum-free MEM media and 0.38 ml of serum-free media was added to each electrode. Electrodes were placed into an electrical cell-substrate impedance incubator for 30 min-1 h to stabilize basal electrical resistance. The tightness of the junctions is reflected by their electrical resistance. The resistance is expressed as normalized resistance (1×103 Ohms) in time course experiments. Once cells attained stable TER, Aβ with or without SOD or catalase were added into electrodes and the TER was monitored for a designated time. A decrease in TER is an indication of weakened cell-cell junctions and increased permeability.
Measurement of Intracellular Ca2+ Concentration
BPAECs were plated on glass coverslips (Hitachi Instruments) pretreated with 0.1% gelatin solution and grown to ∼95% confluence in complete MEM. All procedures for Ca+2 measurement were carried out in basic media: 116 mM NaCl, 5.37 mM KCl, 26.2 mM NaHCO3, 1.8 mM CaCl2, 0.81 mM MgSO4,1.02 mM NaHPO4, 5.5 mM glucose, and 10 mM HEPES/HCl, pH 7.40. Cells were loaded with 5 µM Fura-2 AM (acetoxymethylester of Fura-2) in 1 ml of the above media in the presence of 0.1% bovine serum albumin and 0.03% pluronic acid at 37°C in a cell culture incubator, as recommended by the manufacturer's protocol. Cells were rinsed twice, and a coverslip was inserted diagonally into 1.0 cm acrylic cuvettes (Sarstedt, Newton, NC), which were filled with 3 ml of the incubation media at 37°C. Fura-2 fluorescence was measured with an Aminco-Bowman Series 2 luminescence spectrometer (SLM/Aminco, Urbana, IL) at excitation wavelengths of 340 and 380 nm and emission wavelength of 510 nm. Intracellular free calcium, [Ca2+]i, was calculated from the 340/380 ratio using a Ca2+ calibration curve and manufacturer software.
Preparation of Cell Lysates and Western Blotting
BPAECs were grown on 100-mm culture dishes to ∼95% confluence in complete MEM. Subsequent incubations were carried out in serum-free media. Cells were incubated with 10 µg/ml of Aβ(40-1), Aβ(1–40), Aβ(42-1), and Aβ(1–42) for 1 hr. After incubation with Aβ, the reaction was stopped by rinsing with ice-cold PBS containing 1 mM orthovanadate. ECs were lysed with 0.5–1 ml of non-denaturing cell lyses buffer (20 mM Tris-HCl, pH 7.5,150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin and 5% protease inhibitor mixture (Roche Applied Science). The lysed cells were scraped off the culture dish and sonicated on ice with a probe sonicator (15s × 2) followed by centrifugation at 5000 × g in a microcentrifuge at 4°C for 5 min. Protein concentrations of the supernatants were determined using the Pierce protein assay kit. The supernatants, adjusted to 0.5–1 mg protein/ml, were denatured by boiling in 1× SDS sample buffer for 5 min and analyzed on 10% SDS-PAGE gels. Protein bands were transferred overnight (25 V, 4°C) onto polyvinylidene difluoride (Millipore) membranes, probed with primary antibody, anti-phosphotyrosine, clone 4G10 (Upstate Biotechnology Inc, NY, USA) and secondary antibody, peroxidase conjugated goat anti-mouse IgG (Upstate Biotechnology Inc, NY, USA) and immunodetected by using the enhanced chemiluminescence kit (Amersham Biosciences) according to the manufacturer's protocol. The blots were scanned (UMAX Power Look II) and quantified by the automated digitizing system UN-SCAN-IT GEL (Silk Scientific Corp.).
Immunofluorescence Microscopy
BPAECs grown on coverslips to∼95% confluence were treated with Aβ(1–40) and Aβ(1–42) for 6 hrs, and rinsed twice with PBS, and treated with 3.7% formaldehyde in PBS for 10 min at room temperature and then permeabilized for 4 min with 0.25% Triton X-100 prepared in 3.7% formaldehyde. After washing, cells were incubated for 30 min at room temperature in TBST blocking buffer containing 1% bovine serum albumin. Actin stress fibers were determined by staining cells on coverslips with Alexa Fluor phalloidin 568. Cells were visualized by Nikon Eclipse TE 2000-S immunofluorescence microscopy with a Hamamatsu digital camera (Japan) using a 60x oil immersion objective and MetaVue software (Universal Imaging Corp.).
RESULTS
Effect of Aβ(1–40) and Aβ(1–42)- fibrils on the permeability of BPAECs
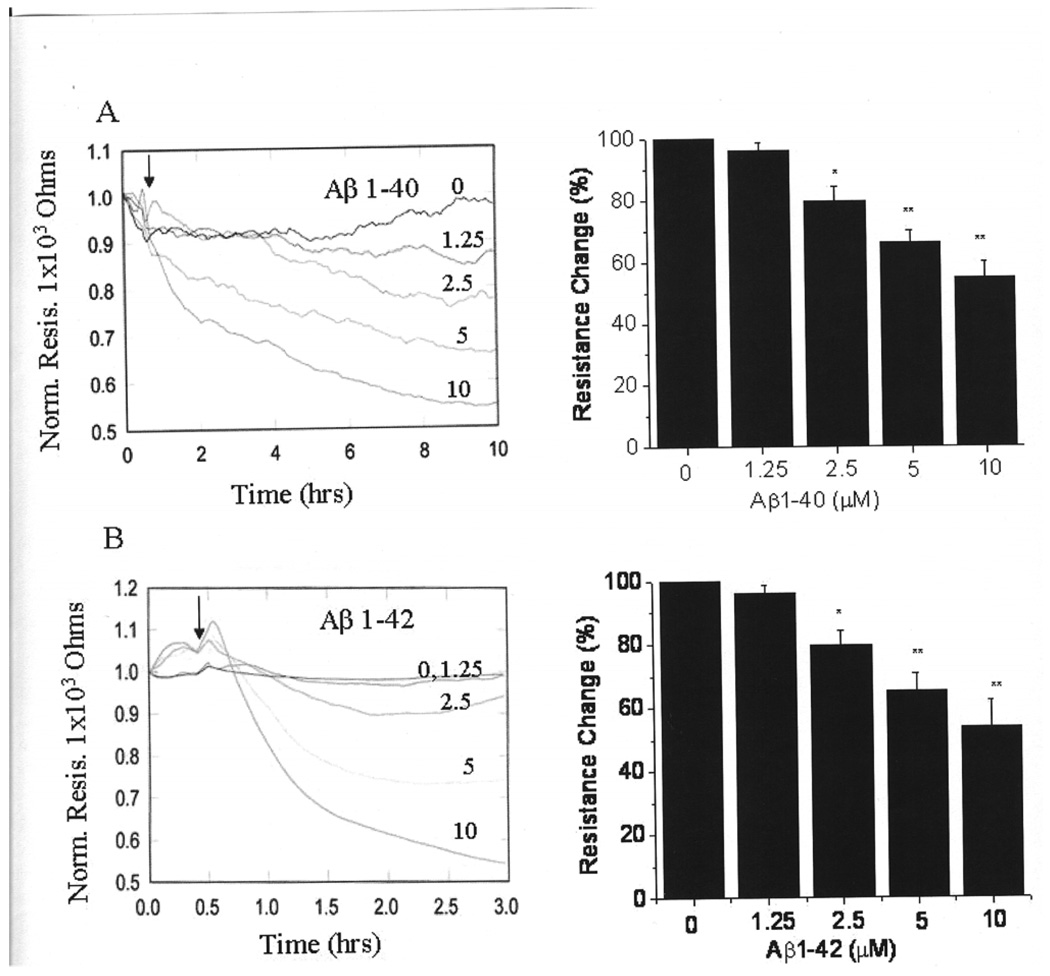
Both Aβ fibrils (1–40 and 1–42) formed by incubation of Aβ peptides for 36 hr at 37ºC decreased the TER of EC monolayers in a time- and concentration-dependent manner (Fig.1).The effect of the (1–42) fibrils (Fig. 1B) is, however, significantly faster than the effect of (1–40) fibrils (Fig. 1A). Thus, the decrease in TER with 10 µM fibrils had leveled off after 3 hrs for the (1–42) fibrils, while 10 hrs was required to produce a similar decrease in TER for the (1–40) fibrils. The decrease in the TER of cells by both types of Aβs is an indication of weakened cell-cell junctions and increased permeability.
Figure 1. Decrease in the permeability of endothelial cells by Aβ proteins.
The endothelial cells were grown in gold microelectrodes to 95% confluence in MEM. Before the experiment, media in the microelectrodes was replaced with 0.38 ml serum free MEM. Electrodes were placed into an electrical cell-substrate impedance folder for 30 min to stabilize basal electrical resistance. After stabilization of resistance 20 µl Aβ were added and the TER was monitored as mentioned in the materials and methods section. A: Varying concentrations Aβ (1–40) (1.25 to 10 µM) fibrils were added into microelectrodes and the TER was monitored for 10 hrs. The changes in the TER at the end of 10 hrs was measured and compared with control. B: Varying concentrations of Aβ (1–42) fibrils were added into microelectrodes and the TER was monitored for 3 hrs. The decrease in the TER was measured at the end of 3 hrs and compared with control. Values are mean of 4 independent experiments. * Significantly different from control p< 0.05. ** Significantly different from control p< 0.001.
Effect of H2O2 and inverted sequence Aβ monomers on the Permeability of BPAECs
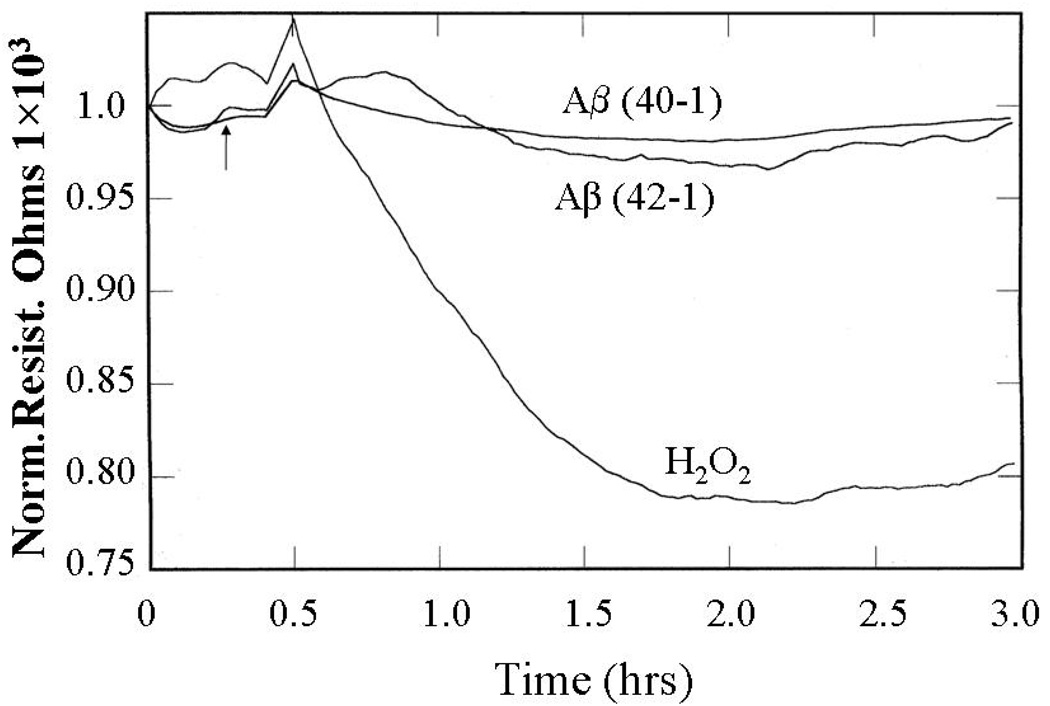
The effect of hydrogen peroxide, a positive control, and the inverted sequence amyloids Aβ (40-1) and (42-1), which do not form fibrils, on the TER was investigated, As shown in Figure 2 the TER of the confluent endothelial cell monolayer was decreased following the addition of 100 µM hydrogen peroxide in a time dependent manner. The decrease in TER is an indication of increased permeability of the BPAECs monolayers. Treatment of cells with Aβ 40-1 or 42-1 of inverted sequence, which remain as monomers did not change the TER of ECs. Aβ (1–42) has a greater tendency to form fibrils than Aβ (1–40) [19]. This finding indicates that fibril formation is required for the decrease in TER and, thus, explains the more effective decrease in TER by Aβ (1–42) than Aβ (1–40) fibrils (Fig.1).
Figure 2. Effect of Aβ monomers and hydrogen peroxide on endothelial barrier function.
Endothelial cells were grown in gold microelectrodes as mentioned in figure 1. Cells were stimulated with 10µM Aβ monomers (40-1 or 42-1) or 100µM hydrogen peroxide and monitored TER as mentioned in the materials and methods section
Effect of SOD and catalase on Aβ(1–40) and Aβ(1–42) mediated vascular permeability
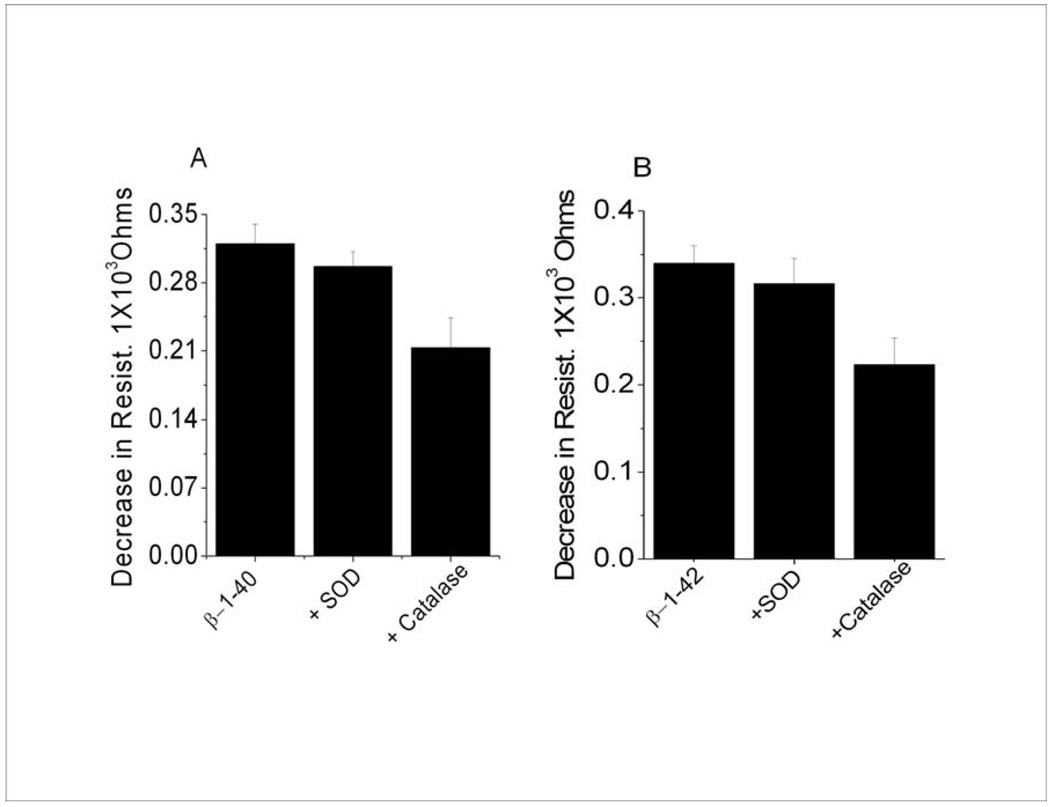
The neural and vascular toxicity of Aβ fibrils has been shown to be associated at least in part by the generation of ROS such as superoxide and hydrogen peroxide [20]. ROS are known to alter the TER of endothelial cell monolayers [21–23]. To determine whether Aβ mediated permeability (Fig. 1) is dependent on ROS, the effect of superoxide dismutase (SOD) and catalase, a hydrogen peroxide scavenger, on the changes of TER were investigated. As shown in Figure 3, SOD had no significant effect on the decrease of TER by Aβ (1–40) or Aβ (1–42). Furthermore, catalase only partially (∼25%) inhibited the decrease of TER by both amyloid fibrils.
Figure 3. Effect of SOD and catalase on Aβ mediated endothelial resistance.
Cells were grown in electrodes as described in Fig 1. A, SOD (100 U) and catalase (100U) were added prior to addition of 10 µM Aβ (1–40). B, SOD and catalase were added prior to addition of 10 µM Aβ (1–42). The decrease in the resistance was calculated by the subtraction of the resistance obtained at the end of experimental period (Fig.1) from the beginning of normalized resistance.
Effect of Aβ on cellular calcium levels
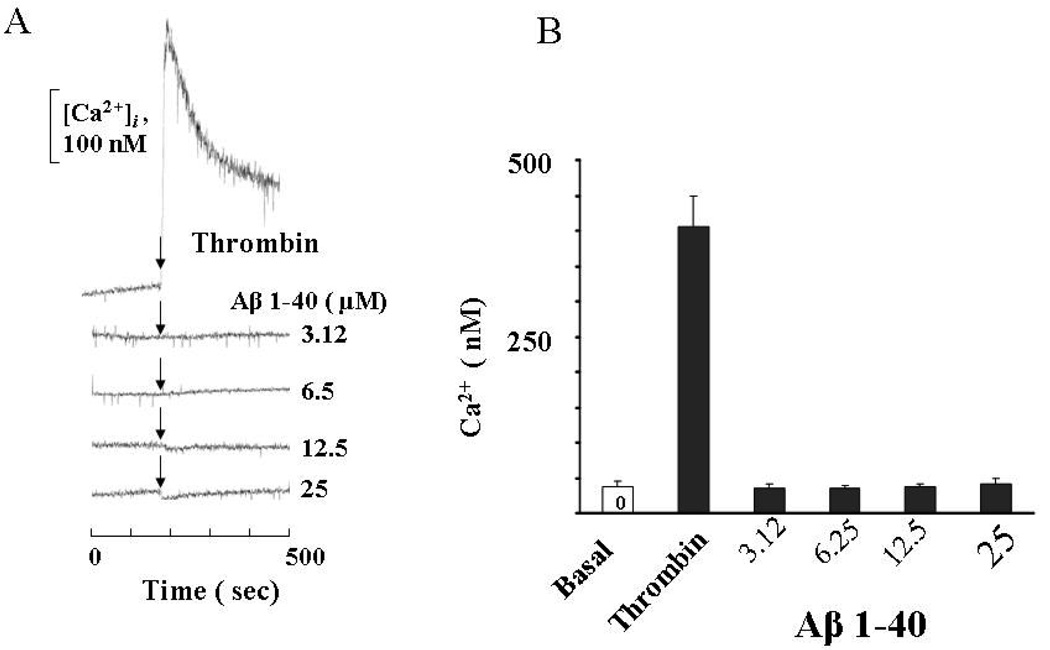
Cytosolic calcium levels were measured (Fig. 4) to understand the mechanism for decreasing the TER of ECs by Aβ. The increase in cytosolic calcium in response to ROS and other edemagenic agents has been shown to be associated with endothelial barrier dysfunction [12,24,25]. In our experiments, thrombin was used as a positive control to check the release of Ca2+ into cytosol of EC monolayers. Treatment of confluent ECs with Aβ (1–40) did not have any effect on cytosolic Ca+2 (Fig.4). The effect of Aβ fibrils formed from (1–42) also had no effect on Ca+2 (data not shown). The decrease in the TER of EC monolayers by Aβ fibrils is, therefore, not primarily associated with the release of calcium.
Figure 4. Effect of Aβ(1–40) on cytosolic calcium level.
Cells were grown on coverslips to 95% confluence and loaded with fura-2 AM (5µM) for 15 min in a cell culture incubator. Cells were washed and challenged with thrombin, as a positive control, and varying concentration of Aβ (1–40). The intracellular concentration of Ca+2 was then measured as described in the materials and methods section. Values are mean of 3 independent experiments.
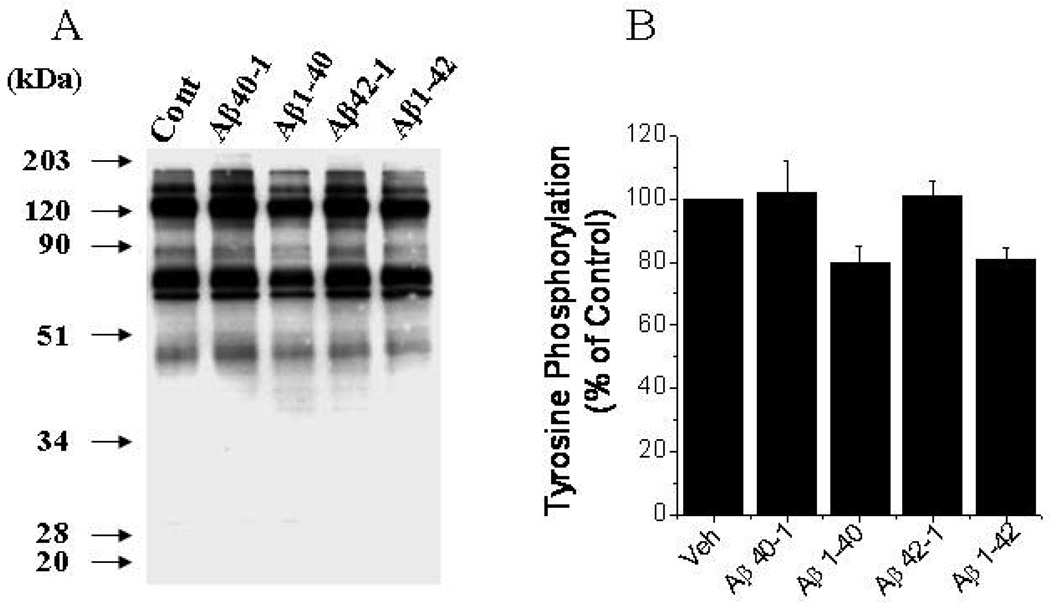
Total protein tyrosine phosphorylation in Aβ treated endothelial cells
Endothelial barrier functions are regulated by phosphorylation of adherens and tight junction proteins [12,26]. ROS are known to increases the tyrosine phosphorylation of various proteins involved in endothelial barrier functions [12] [14]. Several studies have shown that cellular gap formation in endothelial monolayers due to oxidative stress is dependent on an increase in the phosphorylation of junctional proteins [27,28]. Therefore, total protein tyrosine phosphorylation in an endothelial lysate was assessed by the immunocytochemistry method. Treating ECs with both Aβ proteins for 60 min did not increase the phosphorylation of proteins (Fig.5). In fact, a decrease in protein tyrosine phosphorylation was observed with Aβ(1–40) and Aβ(1–42) fibrils. These results indicate that the increase in permeability of EC monolayers by Aβ is not due to altering the phosphorylation of junctional proteins.
Figure 5. Effect of Aβ fibrils on total protein tyrosine phosphorylation in ECs.
Cells were grown on 100 mm dishes to 95% confluence and treated with Aβ (1–40 or (1–42) for 1 hr. Cell lysates were subjected to 10% SDS-PAGE and probed with ant-phosphotyrosine antibody as described in the materials and methods section. B. Phosphorylation of total tyrosine groups (% control) was calculated by image analysis of blot. Values are mean of 3 independent experiments.
Cytoskeletal protein changes
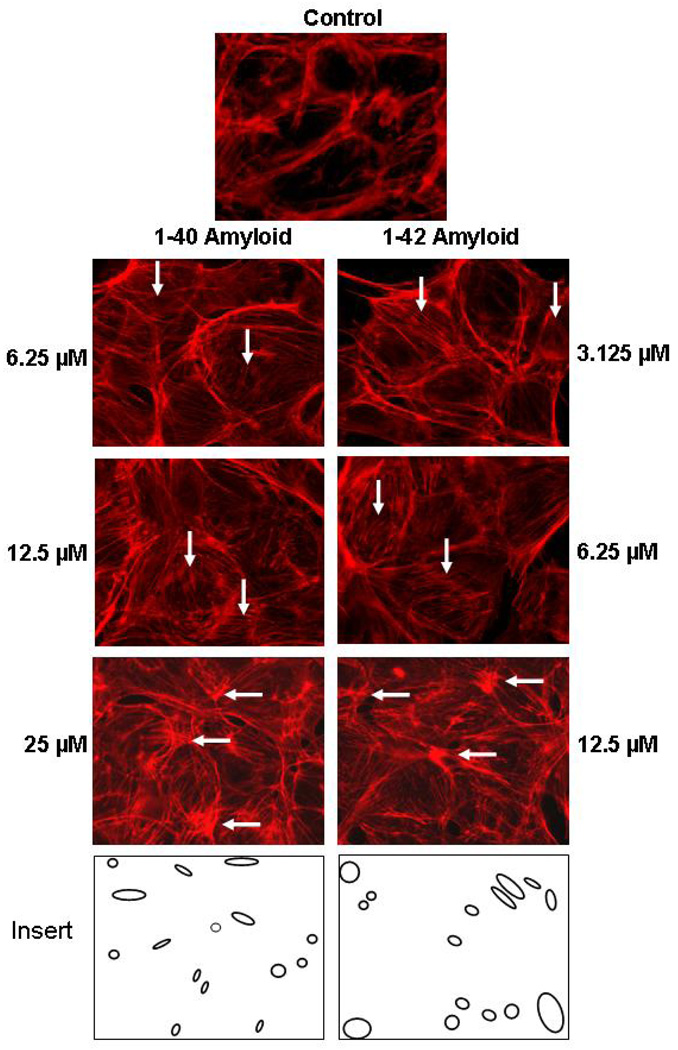
The endothelial barrier functions are maintained by a highly dynamic network of actin filaments through their association with actinin, myosin, and tropomyosin. The effect of Aβ on the cytoskeleton network was investigated to determine if the change in barrier function by Aβ involves the cytoskeleton. Treatment of ECs with varying concentrations of Aβ for 6 hrs and staining with Alexa fluor Phalloidin to visualize F actin by immunofluorescence microscopy is shown in Figure 6. Lower concentrations of amyloid (3.125–6.25µM for Aβ(1–42) and 6.25 –12.5µM for Aβ(1–40)) resulted in an increase in stress fiber formation (see vertical arrows in Fig. 6), while higher concentrations (12.5 µM for Aβ(1–42) and 25 µM for Aβ(1–40)) resulted in aggregation of actin filaments (parallel arrows in Fig. 6), which produce gaps in the cell monolayer (Fig. 6). The formation of stress fibers, actin cytoskeleton aggregation and gap formation at lower concentrations for Aβ (1–42) than for Aβ(1–40) is consistent with a reduction in TER at lower concentrations for Aβ (1–42) (Fig. 1). These results show that Aβ causes cytoskeleton remodeling in endothelial cell. The initial step in this remodeling that involves an increase in stress fibers corresponds to the Aβ concentration that produces the decrease in electrical resistance. The formation of these stress fibers are expected to alter the interactions between adjacent ECs that are responsible for the barrier function retaining the endothelial cells intact. The higher concentrations of Aβ resulting in aggregation and the disruption of the actin network may indicate the beginning of a necrosis affect on the ECs.
Figure 6. Amyliod induced actin fiber rearrangement in ECs.
Cells were grown on glass coverslips to 95% confluence. Cells were incubated with varying concentrations of Aβ (1–40) or (1–42) for 6 hr. F-actin cytoskeletal organization was visualized following the fixation with 3.7% formaldehyde and staining with Alexa Fluor phalloidin 568. Stained cells were examined as mentioned in the materials and methods section. (↓) Indication of stress fiber formation, (→) Indication of aggregated actin filaments, (insert) gaps formation relative to control image.
DISCUSSION
Amyloid peptides (1–40) and (1–42) are formed from the cleavage of the amyloid β precursor protein (AβPP) by β and γ secretases [29]. They are found primarily in the brain, but are also found at low nanomolar concentrations in the human plasma. [30]. These plasma amyloids can be sequestered from the luminal side to the abluminal side of the blood brain barrier for fibrils formation [9] [10].Deposition of fibrils formed by these peptides in the brain has been implicated in the development of AD. In addition, Aβ deposits are found in brain capillaries, small arteries and arterioles producing cerebral amyloid angiopathy (CAA) [31–33] in spite of an anti-amyloidogenic effect of BBB by preferential sequestration of the soluble Aβ-apoJ complex and excluding pro-amyloidogenic isoforms of apoE3 and apoE4 [34]. About 80 to 90% of Alzheimer’s subjects are diagnosed with CAA, implying a role for vascular Aβ in AD subjects.
Pulmonary arterial endothelial cells produce tight junctions that restrict the passage of substances across the pulmonary endothelium. This barrier is, however not as restrictive as the BBB with very tightly junctional proteins linking the endothelial cells. In addition BBB ECs shares a common basement membrane with astrocytes and pericytes making even tighter contacts. These same differences may explain why we were unable to grow human brain endothelial cells to confluence on gold electrodes. We therefore were forced to use bovine pulmonary arterial endothelial cells (BPAECs) for the present in vitro study of barrier function.
Neuronal inflammation is commonly found in most Alzheimer’s subjects [35,36]. It usually occurs when pro-inflammatory mediators interact with the endothelium resulting in adhesion and a compromised BBB that permits the migration of blood neutrophils and plasma proteins into the brain [6,35,37–40]. In AD subjects the large pool of Aβ fibrils, consisting primarily of Aβ(1–40), in the vascular system [32] suggest that Aβ may play a role in the breeching of the BBB that facilitates the migration of neutrophils considered a major source for inflammation in AD subjects.
In this investigation, we studied the effect of Aβ fibrils (1–40) and (1–42) on barrier dysfunction by measuring TER of bovine pulmonary arterial endothelial cells. Exposure of these ECs to Aβ fibrils (1–40) and (1–42), but not monomers in the concentration range of (1–10 µM) decreased the TER indicating that Aβ fibrils increase endothelial permeability (Fig.1).
The effect of non-monomeric amyloids on endothelial barrier function has recently been reported [40]. That study, however, emphasized the nature of the amyloid aggregate and showed that the dominant effect is due to small aggregates. The nature of the amyloid species in our study is limited to a comparison of monomers (no effect) and the difference between aggregates formed from (1–40) and (1–42) amyloids. We observed enhanced affects on the barrier function with Aβ(1–42), which can be attributed to a greater tendency to form fibrils. However, in our study we have a distribution of aggregates and fibrils. Consistent with the results of Gonzalez et al.[40], the enhanced effect that we observe (Fig. 1) for Aβ(1–42) can be attributed to greater toxicity of the (1–42) aggregates than the (1–40) aggregates of a similar size distribution and not to the formation of larger fibrils.
Our study instead of being directed at the nature of the Aβ aggregate, has investigated the mechanism for Aβ induced changes in barrier function. An increase in vascular permeability that does not involve EC death (necrosis or apoptosis) can be generated by ROS. Hydrogen peroxide, a ROS produced in vivo, is known to increase the vascular endothelial permeability (Fig.2) [28]. The mechanism for hydrogen peroxide-induced changes in permeability of ECs involves signal transduction and redox-regulated transcription factor pathways [12]. Hydrogen peroxide induces protein kinases and cytosolic calcium, which increases the endothelial permeability through the tyrosine phosphorylation of junctional proteins [28,41] and the regulation of myosin light chain kinase [12,24,42]. An effect of Aβ on endothelial permeability was expected to involve ROS, because of studies that have shown that the toxicity of Aβ is associated with the generation of ROS [43]. Our studies, however, rule out a primary role for ROS generation in Aβ induced permeability. We, thus find (Fig.3) that SOD which reacts with superoxide has no effect on Aβ induced permeability and catalase produces only a 25% decrease in the Aβ induced permeability.
The catalase effect suggests a secondary role for ROS. However, the cytosolic calcium (Fig. 4) and the tyrosine phosphorylation results indicate that the amyloid induced ROS effect implied by the catalase experiment does not involve the same pathway involved in hydrogen peroxide mediated permeability changes. The absence of any increase in cytostolic calcium and an actual decrease in phosphorylation instead of the expected increase in phosphorylation also rules out an edemagenic function of Aβ where the increase in permeability coincides with the phosphorylation of adherens and tight junction proteins [26].
Our studies instead implicate changes in the cytoskeletal proteins of endothelial cells. It is known that the cytoskeleton network is maintained by linking the actin filaments directly to tight junctional proteins and via actin binding proteins which interact with catenins to other junctional proteins [12,14]. A dramatic effect on the organization of the F-actin filaments, formation of stress fibers and intracellular gap formation in the presence of β(1–40) and β(1–42) (Fig.6) is indicative of cytoskeleton rearrangement. The damage to these actin and junctional protein bonds results in the dissociation and redistribution of proteins, which affect the cell to cell barrier functions.
This finding is consistent with Amyloid-β induced morphological changes of astrocytes produced by inducing reorganization of the cytoskeleton [44]. Multiple pathways for an effect of ROS on the cytoskeleton have been proposed[12], which may help to explain the partial inhibition by catalase (Fig. 3) despite the absence of a an effect on calcium and tyrosine phosphorylation (Fig. 4 &Fig. 5 ).
Cytotoxic compounds and certain drugs, which cause tissue necrosis also change the permeability of the endothelium [45–47]. Amyloid-β fibrils known to have a cytotoxic effect [48,49] and to cause cell apoptosis [50,51] can, therefore, be expected to increase the permeability. However, apoptosis and cell death generally requires concentrations > 10 µM and longer incubation times (24 to 72 hrs) [49,50] . In our experiments the permeability changes were observed at concentrations < 10µM and within an hour of incubation indicating that necrotic effects of Aβ should not be involved in the permeability changes that we have observed. However the gross cytoskeleton changes observed at the highest Aβ concentrations may be indicative of the beginning of necrosis. The cytoskeletal changes observed under these conditions are actually similar to the reported changes observed during necrosis. These reported cytoskeletal changes also did not produce an increase in calcium, although they were associated with an increase in MAP kinases, which have also been reported to increase with Aβ [50,51].
Our results implicate the cytoskeleton in the amyloid induced barrier dysfunction. However, additional studies are necessary to delineate the actual mechanism for this process. The possible roles of the different MAP kinases need to be investigated. In addition modulation of cellular thiol redox status, which is regulated by the redox status of glutathione, can alter signal transduction pathways of EC barrier function.
In conclusion, the Aβ proteins found in the circulatory system and in brain cells have been shown to alter the endothelium barrier function resulting in increased permeability by modifying the cytoskeletal proteins. These modifications can increase the adhesion and diapedesis of monocytes into the subendothelium causing inflammation that is seen in Alzheimer’s subjects.
ACKNOWLEDGEMENTS
This research was supported by Intramural Research Program of the National Institutes of Health, National Institute on Aging.
REFERENCES
- 1.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 2.de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 3.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 4.Wisniewski HM, Vorbrodt AW, Wegiel J. Amyloid angiopathy and blood-brain barrier changes in Alzheimer's disease. Ann N Y Acad Sci. 1997;826:161–172. doi: 10.1111/j.1749-6632.1997.tb48468.x. [DOI] [PubMed] [Google Scholar]
- 5.Claudio L. Ultrastructural features of the blood-brain barrier in biopsy tissue from Alzheimer's disease patients. Acta Neuropathol. 1996;91:6–14. doi: 10.1007/s004010050386. [DOI] [PubMed] [Google Scholar]
- 6.Giri R, Selvaraj S, Miller CA, Hofman F, Yan SD, Stern D, Zlokovic BV, Kalra VK. Effect of endothelial cell polarity on beta-amyloid-induced migration of monocytes across normal and AD endothelium. Am J Physiol Cell Physiol. 2002;283:C895–C904. doi: 10.1152/ajpcell.00293.2001. [DOI] [PubMed] [Google Scholar]
- 7.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein DL, Biron KE, Ujiie M, Pfeifer CG, Jeffries AR, Jefferies WA. Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer disease. Faseb J. 2006;20:426–433. doi: 10.1096/fj.05-3956com. [DOI] [PubMed] [Google Scholar]
- 9.Jancso G, Domoki F, Santha P, Varga J, Fischer J, Orosz K, Penke B, Becskei A, Dux M, Toth L. Beta-amyloid (1–42) peptide impairs blood-brain barrier function after intracarotid infusion in rats. Neurosci Lett. 1998;253:139–141. doi: 10.1016/s0304-3940(98)00622-3. [DOI] [PubMed] [Google Scholar]
- 10.Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer's amyloid beta in guinea pigs. Neurosci Lett. 1996;206:157–160. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- 11.Farkas IG, Czigner A, Farkas E, Dobo E, Soos K, Penke B, Endresz V, Mihaly A. Beta-amyloid peptide-induced blood-brain barrier disruption facilitates T-cell entry into the rat brain. Acta Histochem. 2003;105:115–125. doi: 10.1078/0065-1281-00696. [DOI] [PubMed] [Google Scholar]
- 12.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 13.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 16.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 17.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer's disease amyloid beta peptide. Biochim Biophys Acta. 2007;1768:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 20.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 21.Pearse DB, Shimoda LA, Verin AD, Bogatcheva N, Moon C, Ronnett GV, Welsh LE, Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium. 2003;10:309–317. doi: 10.1080/10623320390272307. [DOI] [PubMed] [Google Scholar]
- 22.Usatyuk PV, Natarajan V. Regulation of reactive oxygen species-induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am J Physiol Lung Cell Mol Physiol. 2005;289:L999–L1010. doi: 10.1152/ajplung.00211.2005. [DOI] [PubMed] [Google Scholar]
- 23.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 24.Siflinger-Birnboim A, Lum H, Del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol. 1996;270:L973–L978. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 25.Garcia JG, Schaphorst KL, Shi S, Verin AD, Hart CM, Callahan KS, Patterson CE. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. Am J Physiol. 1997;273:L172–L184. doi: 10.1152/ajplung.1997.273.1.L172. [DOI] [PubMed] [Google Scholar]
- 26.Verin AD. Tyrosine phosphorylation and endothelial cell barrier regulation. Am J Pathol. 2005;166:955–957. doi: 10.1016/S0002-9440(10)62316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery. 2002;132:180–185. doi: 10.1067/msy.2002.125305. [DOI] [PubMed] [Google Scholar]
- 28.Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:L150–L158. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 30.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 31.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 32.Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic BV. Cerebrovascular transport of Alzheimer's amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59:1483–1497. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 38.Rhodin JA, Thomas T. A vascular connection to Alzheimer's disease. Microcirculation. 2001;8:207–220. doi: 10.1038/sj/mn/7800086. [DOI] [PubMed] [Google Scholar]
- 39.Rhodin JA, Thomas TN, Clark L, Garces A, Bryant M. In vivo cerebrovascular actions of amyloid beta-peptides and the protective effect of conjugated estrogens. J Alzheimers Dis. 2003;5:275–286. doi: 10.3233/jad-2003-5403. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Velasquez FJ, Kotarek JA, Moss MA. Soluble aggregates of the amyloid-beta protein selectively stimulate permeability in human brain microvascular endothelial monolayers. J Neurochem. 2008;104:500–513. doi: 10.1111/j.1471-4159.2008.05618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kevil CG, Okayama N, Alexander JS. H(2)O(2)-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol. 2001;281:C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 43.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 44.Salinero O, Moreno-Flores MT, Ceballos ML, Wandosell F. beta-Amyloid peptide induced cytoskeletal reorganization in cultured astrocytes. J Neurosci Res. 1997;47:216–223. [PubMed] [Google Scholar]
- 45.Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- 46.Kurose I, Wolf R, Miyasaka M, Anderson DC, Granger DN. Microvascular dysfunction induced by nonsteroidal anti-inflammatory drugs: role of leukocytes. Am J Physiol. 1996;270:G363–G369. doi: 10.1152/ajpgi.1996.270.2.G363. [DOI] [PubMed] [Google Scholar]
- 47.Campbell WN, Fitzpatrick M, Ding X, Jett M, Gemski P, Goldblum SE. SEB is cytotoxic and alters EC barrier function through protein tyrosine phosphorylation in vitro. Am J Physiol. 1997;273:L31–L39. doi: 10.1152/ajplung.1997.273.1.L31. [DOI] [PubMed] [Google Scholar]
- 48.Folin M, Baiguera S, Tommasini M, Guidolin D, Conconi MT, De Carlo E, Nussdorfer GG, Parnigotto PP. Effects of beta-amyloid on rat neuromicrovascular endothelial cells cultured in vitro. Int J Mol Med. 2005;15:929–935. [PubMed] [Google Scholar]
- 49.Xu J, Chen S, Ku G, Ahmed SH, Chen H, Hsu CY. Amyloid beta peptide-induced cerebral endothelial cell death involves mitochondrial dysfunction and caspase activation. J Cereb Blood Flow Metab. 2001;21:702–710. doi: 10.1097/00004647-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Hsu MJ, Hsu CY, Chen BC, Chen MC, Ou G, Lin CH. Apoptosis signal-regulating kinase 1 in amyloid beta peptide-induced cerebral endothelial cell apoptosis. J Neurosci. 2007;27:5719–5729. doi: 10.1523/JNEUROSCI.1874-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao M, Nguyen TV, Pike CJ. Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci. 2005;25:1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]