Abstract
Background
The inter- and intra-subject variations of scintigraphy, which are used to identify colonic transit disturbances in irritable bowel syndrome (IBS), are unclear. The relationship between colonic transit and bowel functions is incompletely understood.
Aims
To assess inter-and intra-subject variations of scintigraphic colonic transit measurements in 86 IBS patients and 17 healthy subjects and to quantify the relationship between colonic transit and bowel symptoms in 147 IBS patients and 46 healthy subjects.
Methods
Data from participants with multiple colonic transit measurements were analyzed. Primary endpoints were colonic filling at 6h (CF6h) and geometric center (GC) at 24 and 48h for colonic transit. Bowel functions were assessed by daily stool diaries.
Results
Inter- and intra-subject variations were greater for small intestinal than colonic transit. Overall, inter- and intra-subject variations were relatively narrow for colonic transit (both GC24h and GC48h, with lower COV at 48h); there was little intra-subject variation in health and IBS-constipation over a period of ≤3 weeks and over 2.0 years (median, range 0.1, 11.0 years). Significant intra-individual differences in GC24h were observed only in IBS-D patients. Colonic transit was significantly associated with stool form (accounting for 19–27% of the variance), frequency (19%), and ease of stool passage (12%).
Conclusion
Despite inter-subject variation in scintigraphic colonic transit results, the intra-subject measurements are reproducible over time in healthy volunteers and patients with IBS; significant changes in colonic transit at 24h were observed only in IBS-D. Colonic transit is associated with stool form, frequency, and ease of passage.
Keywords: reproducibility, intra-subject, inter-subject, variation
Colonic transit time (CTT) refers to the time taken for chyme to pass through the colon. Measurement of CTT is frequently performed in clinical practice to identify colonic motor function abnormalities, for example, in patients with irritable bowel syndrome (IBS) [1] or constipation [2–4]. This method is also used to investigate pathophysiological mechanisms that lead to symptoms or syndromes [5, 6] and to evaluate the effect of treatment [7, 8].
The most widely applied techniques evaluate movement of radiopaque markers through the gut [3, 9] or appearing in stool [10]. A less commonly used method involves scintigraphy, which is valuable for the assessment of gastrointestinal function in humans [11]. Both provide noninvasive and quantitative assessments of colonic transit. In the most commonly applied radiopaque marker transit technique, subjects ingest radiopaque markers on three [9] or six [12] consecutive days, and a single abdominal x-ray is obtained on the subsequent day and, if necessary, on one or more days later [13]. With the most widely used and published scintigraphic method, radiolabeled charcoal particles [14] are delivered to the colon in a delayed-release, methacrylate-coated capsule [15], and gamma camera images are acquired at specified times during a 48-hour period.
Regardless of the method, there is considerable intra-subject variation in colonic transit [16–19], possibly because some studies administered the radiolabel in the meal rather than in a delayed-release capsule which delivers radiolabeled solid particles to the ileocolonic junction. Moreover, intra-subject variation has been studied mainly in healthy volunteers [20–23], with only 2 studies having evaluated the reproducibility of radiopaque marker CTT in disease states. Thus, Nam et al [4]reported an acceptable reproducibility in 22 patients with idiopathic constipation, but poor reproducibility in patients with colonic inertia or paradoxical puborectalis contraction. In another study by Bouchoucha et al [24] which included 30 healthy volunteers and 43 patients with IBS, 16 subjects underwent repeat colonic transit testing with radiopaque markers and, therefore, there are data on the reproducibility of radiopaque marker transit in some IBS patients. No significant differences were found between the two test results, but it is not clear whether these subjects were healthy controls or IBS patients.
Thus, the reproducibility of CTT measurements in disease states remains unclear. The aim of this study was to assess the inter- and intra-subject variations of scintigraphic colonic transit parameters in patients with IBS and healthy participants. A secondary aim was to quantify the relationship between colonic transit measurement and bowel functions. For the purpose of the current study in which the focus was on variation in IBS patients, we excluded the previously reported performance characteristics in 37 healthy participants [20].
METHODS
Data Source
Data were derived in a retrospective manner from a database of previously performed gastrointestinal transit studies conducted in patients with IBS and healthy volunteers (see Appendix for references). All the patients who participated in all the different studies were evaluated by the same clinical team (gastroenterologist, nurses and coordinators) in a single clinical research unit; all patients filled out the same bowel disease questionnaire [25], and the diagnosis of IBS was based on answers to standard, validated questions, as well as clinical evaluation including physical examination and review of the medical records to ensure they had IBS and other diseases had been excluded. The bowel symptom subgroup was based on standard, validated questions and responses in the questionnaire. Details are provided in the individual papers (see Appendix for list).
From this database, subjects participating in studies of pathophysiology or parallel-group design clinical trials were identified; some participated in more than one study, providing data to evaluate inter- as well as intra-subject variations. There were no drug trials in patients with IBS-M; therefore, short-term variability cannot be assessed in these patients. Only data obtained at baseline or after randomization to a placebo group were included. The same database was also used to assess the relationship between colonic transit and bowel functions (stool form, frequency and ease of passage). The latter evaluation also incorporated results from two additional studies [26, 27].
All participants had provided written consent in each of the previously conducted studies. The current analysis was approved by the Institutional Review Board at Mayo Clinic, Rochester, Minnesota. Patients who had withdrawn authorization to use their records for future research purposes had their data removed from the analysis, as required by the Mayo Clinic IRB for the current study. Participants’ phenotypes were based on the group designation in the original studies: diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), mixed-IBS (IBS-M) or healthy.
Gastrointestinal Transit Studies
To evaluate gastrointestinal transit parameters, an adaption of our established scintigraphic method was used [14, 15, 28]. Briefly, 0.1mCi 111InCl3 was mixed with a slurry of 5 mg of activated charcoal. The mixture was allowed to evaporate to dryness, after which the radiolabeled charcoal was packed into a gelatin capsule. This capsule was coated with one layer of methacrylate (Eudragit L, The Dow Chemical Company) which dissolves in a pH-sensitive manner upon reaching the alkaline terminal ileum, thus allowing radiolabel to be transferred to the colon for quantitation of colon transit. The 111In containing capsule was administered following an overnight fast. After this capsule had emptied from the stomach, subjects ingested a 99mTc-labeled meal. Estimation of colonic filling with 99mTc at 6 hours (CF6h) served as a surrogate for small bowel transit. Subjects ingested standardized meals for lunch and dinner, 4 and 8 hours after the radiolabeled breakfast, respectively. Using a gamma camera, abdominal images with anterior and posterior cameras of 2 minutes duration were acquired immediately following ingestion of the radiolabeled meal and at specified time points during the subsequent 48 hours period.
Data Analysis
Transit measurements
99mTc counts were quantified within a 140 keV (±20%) window and 111In counts within a 247 keV (±20%) window. Count corrections were made for isotope decay, tissue attenuation and downscatter of the 111In in the 99mTc window. A variable region of interest program was employed to quantitate counts in the different segments of the gastrointestinal tract.
Primary endpoints were the CF6h and the geometric center (GC) at 24 and 48 hours. CF6h, an indirect measurement of small bowel transit, is the proportion of 99mTc-labeled chyme that has accumulated in the colon at 6 hours postprandially. The geometric center is the weighted average of the counts in the different segments of the colon, ascending colon (AS), transverse colon (TC), descending colon (DS) and rectosigmoid (RS), which are numbered as segments 1 to 4, respectively, and segment 5 is the expelled stool (S). The GC can be expressed as the sum of the multiplication of the proportion of 111In counts in each colonic segment at a given time by that segment’s weighting factor:
Therefore, a high GC implies fast colonic transit, whereas a low GC implies slow colonic transit.
Assessment of variation in colonic transit measurements
The following principles were applied to select data for analysis:
Inter-subject variation was estimated by comparing the first complete set of transit parameters (CF6h, GC24h and GC48h) among participants.
Short-term intra-subject variation was derived from the first complete set of baseline and post-placebo transit values within the same study protocol.
Long-term intra-subject variation was calculated by comparing the individual’s first complete set of transit values to the set of transit values from the latest estimation in a different study protocol for a given subject.
If a data set did not include all 3 transit parameters, we selected the first available set with 2 out of 3 transit values.
Relationship of colonic transit to bowel function
We also assessed the relationship between colonic transit (GC24h and GC48h) and bowel functions recorded through daily stool diaries, which included stool consistency (classified by the Bristol Stool Form Scale [29]), and stool frequency, and ease of passage which was rated on a 1 to 7 point scale where 1 signifies manual disimpaction and 7 signifies incontinence.
Statistical Analysis
Endpoints of small bowel and colonic transit are expressed as mean ± SEM. Bland-Altman plots [30] were constructed to visually assess the intra-subject variation between repeat transit test values. Inter- and intra-subjects coefficients of variation were calculated. The intra-subject COV was calculated as the SD of the within subject differences divided by the overall (grand) mean of the corresponding transit measurements; COV was then expressed as a percentage. The associations of subgroup status (constipation, diarrhea, mixed bowel pattern and healthy volunteer) and long-term changes in colonic transit values were assessed using analysis of variance (ANOVA). Means and 95% confidence intervals for the differences were computed using the pooled (across subgroups) variation from the ANOVA.
The relationship between bowel functions and colonic transit (predictor variable) were assessed using univariate linear regression models. The estimated regression coefficient for the GC value in each of the models (which corresponds to the slope of the regression line) provides an estimate of the change in stool form, frequency and ease of passage that would be expected per unit change in colonic geometric center values.
RESULTS
Subject Characteristics
From the original database, 103 eligible subjects (96 female) were identified who had participated in repeated measurements of CTT in different clinical trials (median 2.0 years, range 0.1–11.0 years apart). Seventeen were healthy volunteers and 86 had IBS. Participants’ characteristics are shown in Appendix Table 1A.
Short-term intra-subject variation was estimated in 38 patients (36 female, 29 IBS-C and 9 IBS-D) in whom small intestinal and colonic transit were assessed before and after (within 3 weeks) placebo-treatment in the same study.
To determine the relationships between colonic transit and bowel functions we used data from 193 subjects who documented bowel functions through a stool diary (Appendix Table 1B).
Inter-subject Variation of Transit Parameters
Coefficients of variation (COV) for all end points are summarized in Table 1. Ranges of inter-individual differences were wide for CF6h. For colonic transit, inter-individual variation was lower, and also consistently smaller for the geometric center at 48h compared to 24h. This was most apparent in patients with IBS-D (COV at 48h 18% vs 33% at 24h).
Table 1.
Inter-subject variation across groups
| All | Healthy | IBS-C | IBS-D | IBS-M | ||
|---|---|---|---|---|---|---|
| CF6h, % | Mean ± SEM | 51.4±3.0 | 58.4±7.2 | 48.4±4.5 | 51.9±5.0 | 52.4±13.3 |
| N | 95 | 17 | 45 | 28 | 5 | |
| COV | 56 | 50 | 62 | 51 | 57 | |
| GC24h | Mean ± SEM | 2.42±0.10 | 2.50±0.25 | 1.95±0.11 | 3.06±0.17 | 2.41±0.28 |
| N | 103 | 17 | 48 | 33 | 5 | |
| COV | 42 | 42 | 40 | 33 | 26 | |
| GC48h | Mean ± SEM | 3.44±0.12 | 3.54±0.29 | 2.79±0.14 | 4.33±0.15 | 4.51±0.23 |
| N | 96 | 15 | 48 | 29 | 4 | |
| COV | 34 | 32 | 35 | 18 | 10 | |
CF, colonic filling. GC, geometric center. COV, coefficient of variation.
Intra-subject Variation of Transit Parameters
Bland-Altman plots (Figures 1 and 2) show the intra-subject variation for repeat measurements, either after a short (within 3 weeks) or a long interval (median [range] of 2.0 [0.1,11.0] years).
Figure 1.
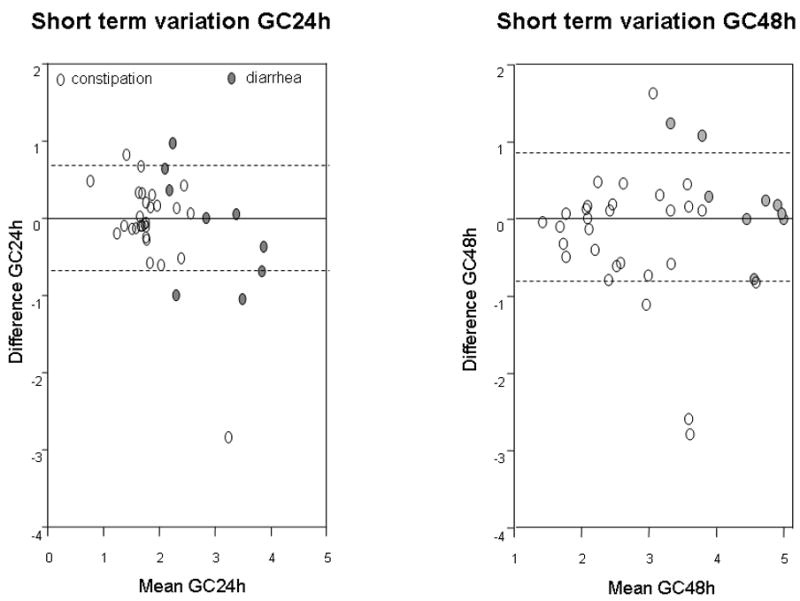
Bland-Altman plots showing short-term intra-subject variation of colonic transit at 24h and 48h in IBS patients with diarrhea or constipation. Plot shows 1 standard deviation as the interrupted lines. Note most data are well within 1 SD which is ~0.7 GC units (y axis)
Figure 2.
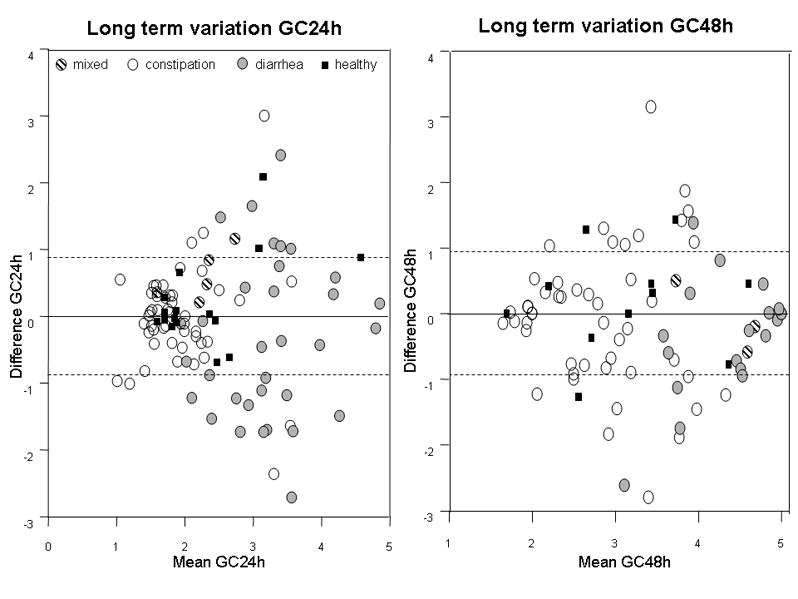
Bland-Altman plots showing long-term intra-subject variation of colonic transit at 24h and 48h for participants in different subgroups. Plot shows 1 standard deviation as the interrupted lines. Note most data are well within 1 SD which is ~0.9 GC units (y axis). Note that the greatest variation occurs in IBS patients with diarrhea and the variation is greater at 24h than at 48 h.
Short-term intra-subject variation
This reproducibility was assessed in 38 patients with IBS-C and IBS-D. The intra-individual COV in CF6h was very high (Appendix Table 2). However, colonic transit was reproducible. The mean difference between replicate transit assessments, performed within a 3-week period (Figure 1), was −0.08 ± 0.11 for GC24h, and differences were within 1 geometric center unit for all except for one subject with diarrhea. Variation was slightly greater at the 48h endpoint, as the mean difference was −0.14 ± 0.13. However, even at 48h, these differences were within 1 geometric center unit for 84% of patients, indicating high test reproducibility. Moreover, differences between initial and repeat assessments were not related to the average GC24h or GC48h during initial and repeat studies (see Figure 1).
Long-term intra-subject variation
Long-term variation in CF6h exceeded 20% in 27 of 38 patients (Appendix Table 3). In contrast, assessments of colonic transit were consistent at least up to 6 years, suggesting reproducibility was high. The overall mean differences in GC at 24h and 48h were −0.12 ± 0.09 and −0.21 ± 0.11 respectively. Coefficients of variation were consistently smaller for GC48h compared to GC24h. As shown in the Bland-Altman plot (Figure 2), transit was more variable in patients with IBS-D than in patients with IBS-C or IBS-M or normal healthy volunteers. Among IBS-D patients, 55% at 24h and 22% at 48h had measurements of colonic transit that varied by more than 1 GC unit. The difference in GC24h between measurements differed significantly from zero in patients with IBS-D, but not for any other subgroup (Figure 3). There was no significant variation in GC at 48h in any group.
Figure 3.
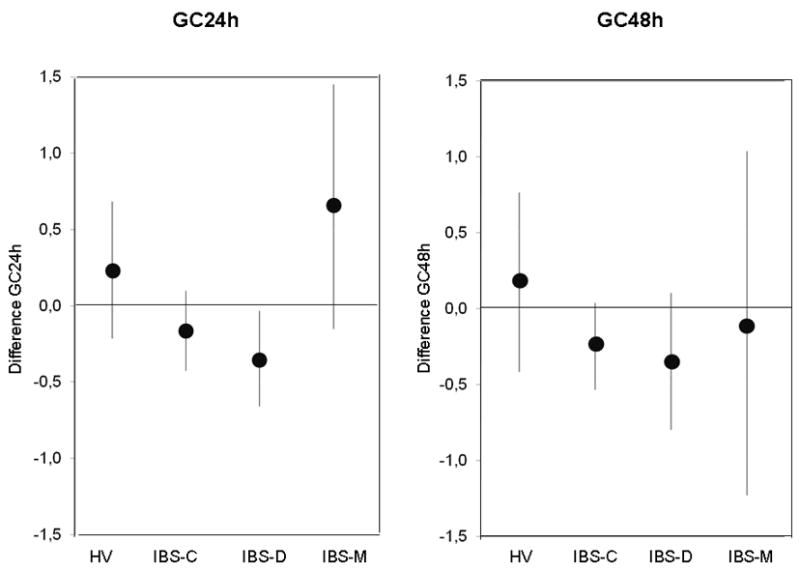
Change in geometric center in different subgroups expressed as means and 95% confidence intervals. Note that the 95% confidence interval for IBS-D subgroup does not cross the zero line, indicating a significant difference in colonic transit for the IBS-D subgroup, but not for the other groups.
The change in geometric center between two repeat transit assessments was not influenced by the time interval between measurements, up to 6 years (Figure 4); the number of replicate studies >6 years apart was too small to assess the impact of time beyond 6 years.
Figure 4.
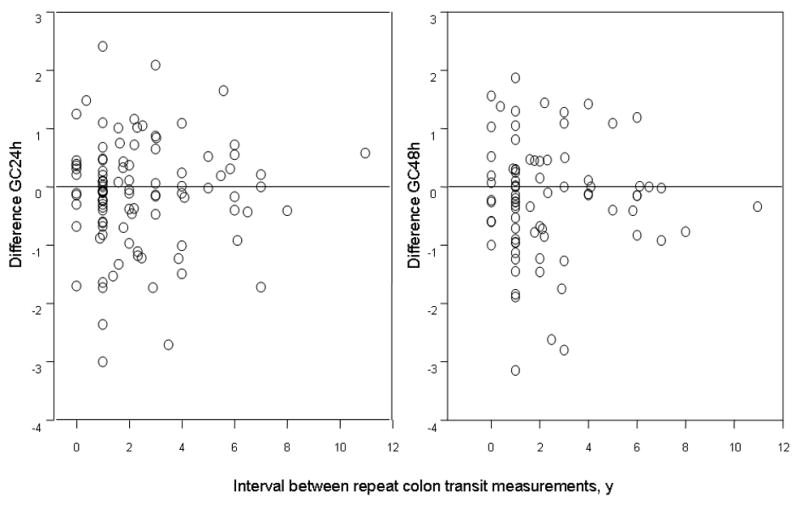
Effect of time interval in years between consecutive measurements on colonic transit measurements. There does not appear to be a difference in the variation of colonic transit when the interval is between 1 and 6 years. The numbers of participants studied > 6 years apart is too small to assess the variation beyond 6 years.
Correlation between Colonic Transit and Bowel Function
Figure 5 shows linear regression plots for the relationships between colonic transit and stool form, frequency, and ease of passage. In general, an increase in colonic GC24h by 1 unit was associated with a 0.58 unit change in stool form, a change of 0.523 bowel movements per day, and a change in ease of passage of 0.23 on a 7 point scale. At 48h, a 1 point difference in geometric center was associated with a 0.65 unit change in stool form. Colonic transit was significantly correlated with changes in stool form (accounting for 19–27% of the variance), frequency (19%), and ease of stool passage (12%).
Figure 5.
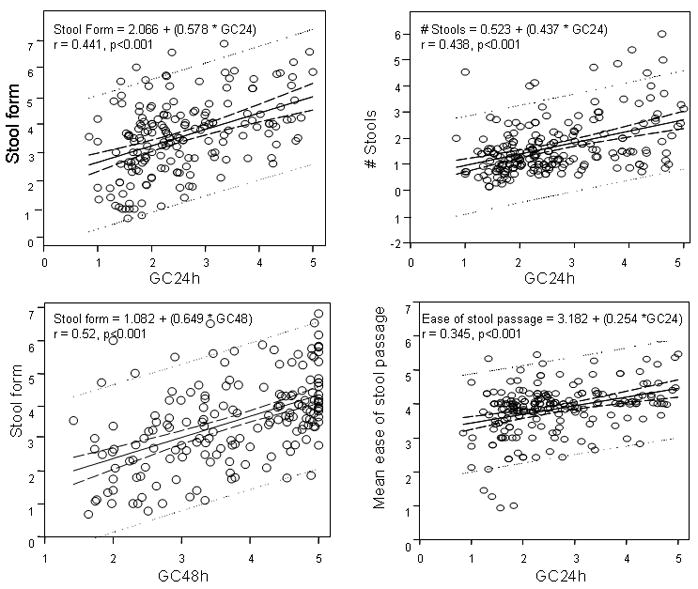
Relationship between bowel function and colonic transit: the regression formulae are shown for the relationships between colonic transit at 24h and stool form, frequency and ease of passage and the relationship between colonic transit at 48h and stool form. The full lines represent the regression. Confidence and prediction interval lines our represented by the interrupted and dotted lines respectively.
DISCUSSION
We have evaluated the performance characteristics of scintigraphic colonic transit measurements and assessed the clinical relevance of transit on bowel functions. Our results show that scintigraphic assessment of colonic transit is highly reproducible over the short- and long-term in patients with IBS and in healthy volunteers. Our study did not compare results of scintigraphy with those of radiopaque marker transit measurement in this cohort of patients. We previously demonstrated that, in health volunteers, transit through the ascending colon was faster when measured by the radiopaque marker method than with scintigraphy, and ranged from 2.8 to 18.2 h. The difference in ascending colon transit times estimated by the two methods was statistically significant (radiopaque markers, mean 9.9 hours and 111In-labeled particles 11.9 hours), and the transit of radiopaque markers through the whole colon was also somewhat faster than that of 111In-labeled particles (mean 26.2 and 35.7 h respectively, P = 0.067 [31]). Similarly, in patients with constipation, estimated transit of radiopaque markers through the ascending and transverse colons was considerably shorter than the transit of radioisotopically-labeled particles during simultaneous studies [32].
As previously noted [20, 33], inter-subject variation was considerable for small bowel transit. For colonic transit, however, inter-subject variation was similar in healthy and different IBS subgroups. This variation may reflect inherent biological variation among subjects rather than the technique employed [21]. For healthy volunteers, coefficients of inter-individual variation were slightly higher, though comparable to those previously published by Cremonini et al [20] in a different cohort of 37 healthy volunteers. The slightly higher COV may be attributable to different male-female ratios in the two study groups, as inter-individual variation appears to be smaller in men [34], and the current analysis includes a smaller proportion of men than the earlier study.
For colonic transit, the intra-individual COV was generally comparable at GC48h and GC24h, except for IBS-D where the intra-individual variation was lower at 48h than 24h. We believe that this observation reflects a ceiling effect, since the GC48h in patients with IBS-D and rapid colonic transit is often close to the maximum score of 5. Indeed, 10 of 33 patients had a GC48h ≥4.9. Since the upper limits of normal in 175 healthy volunteers for GC48h (i.e., 5.0) is higher than for GC24h (i.e., 4.07), it is easier to identify rapid colonic transit with the GC24h. Thus, GC24h should remain the primary end point for assessing colonic transit in clinical trials.
Over the short-term, colonic transit parameters were very reproducible within subjects, even in patients with IBS-C and IBS-D. Indeed, the mean intra-individual differences are comparable to a group of 51 healthy volunteers who underwent repeat scintigraphic colonic transit measurements within 14 days [21]. While reproducibility after a short time interval is of limited clinical importance, it is critical in planning pre- and post-treatment transit measurements to study drug effects [35, 36].
Extending previous studies in healthy volunteers [20] and patients with chronic idiopathic constipation [4], colonic transit measurements are also very reproducible within subjects when repeated up to 6 years later; long-term reproducibility was not influenced by the duration of time between two measurements. These observations provide insights into the natural history of diseases that affect colonic motor function. Our findings suggest that, in contrast to symptoms, colonic transit is relatively stable over time in IBS patients [37]. However, there are caveats to these apparent differences between stability of symptoms and colonic transit. First, the criteria used to categorize IBS patients in the longitudinal study assessing symptoms differed from our study. Second, in the symptoms study, the diagnosis was exclusively based on questionnaires; patients were not interviewed or examined by a physician to exclude an organic etiology of symptoms. Third, our analysis only included subjects that fulfilled criteria for the same IBS subgroup at both times of colonic transit assessment. Thus, it does not account for those that might present with an altered symptom phenotype over time and would not, therefore, have been eligible for the subsequent transit study. There may also be differences between subjects with IBS symptoms in the general population (participating in a questionnaire study) and those seeking medical care or treatment for their symptoms in a physician’s office [38, 39] or patients volunteering to participate in clinical trials.
Although we have demonstrated high reproducibility of colonic transit over time, we have also observed a significant intra-individual variation in patients with IBS-D, with a general trend towards a decrease of GC24h; thus showing that, on average, transit time was slower on repeat testing. The significance of this novel finding requires further study. On the other hand, these data document that colonic transit in patients with IBS-C is consistent and does not vary significantly over time, justifying the use of the transit measurement to evaluate natural history and responses to therapy.
The clinical relevance of a change in colonic transit is illustrated by the relationship with bowel function; the strongest correlation being with stool form, as shown previously in healthy subjects treated with medications to accelerate or delay colonic transit [40]. The associations with the number of daily bowel movements and with the ease of passage were less robust. Colonic transit was significantly correlated with changes in stool form (accounting for 19–27% of the variance), frequency (19%), and ease of stool passage (12%).
This study has a number of strengths, including the large sample size and the inclusion of >100 patients with IBS and healthy volunteers. Weaknesses to consider include the retrospective manner in which data was obtained, the small number of patients in the IBS-M subgroup, and the incomplete set of small bowel transit values for some subjects. However, this parameter has been previously shown to be of limited diagnostic value [20, 33].
In summary, although there is an inherent inter-individual variation of colonic motor function which reflects the true differences in colonic physiology rather than problems with measurement technique, we have demonstrated high test reproducibility of colon transit in IBS and health using scintigraphy. In addition, we have demonstrated the relationship between colonic transit and bowel functions. In conclusion, this study validates the use of scintigraphy for assessing colonic transit in clinical or research settings.
Acknowledgments
Dr. Camilleri’s work in IBS is supported in part by RO1 grant DK-54681 from National Institutes of Health.
APPENDIX
Table 1.
| Table 1A. Participants’ characteristics in assessment of inter- and intra-subject variation in transit | ||||
|---|---|---|---|---|
| Healthy volunteers | IBS-C | IBS-D | IBS-M | |
| N | 17 | 48 | 33 | 5 |
| Age, years | 37.6 ± 2.9 | 38.5 ± 1.5 | 38.1 ± 2.5 | 39.4 ± 4.6 |
| BMI, kg/m2 | 25.0 ± 1.0 | 25.3 ± 0.6 | 27.8 ± 1.1 | 26.9 ± 1.5 |
| CF6h, % | 58.4 ± 7.2 | 48.4 ± 4.5 | 51.9 ± 5.0 | 52.4 ± 13.3 |
| GC24h | 2.50 ± 0.25 | 1.95 ± 0.11 | 3.06 ± 0.18 | 2.41 ± 0.28 |
| GC48h | 3.54 ± 0.29 | 2.79 ± 0.14 | 4.33 ± 0.15 | 4.51 ± 0.23 |
| Table 1B. Participants’ characteristics in assessment of relationship of transit and bowel functions | ||||
|---|---|---|---|---|
| Healthy volunteers | IBS-C | IBS-D | IBS-M | |
| N | 46 | 75 | 68 | 4 |
| Age, years | 34.6 ± 1.3 | 38.6 ± 1.1 | 43.8 ± 1.7 | 42.1 ± 6.4 |
| BMI, kg/m2 | 24.1 ± 0.5 | 25.7 ± 0.5 | 28.6 ± 1.0 | 27.4 ± 2.0 |
| Stool form | 3.7 ± 0.1 | 2.9 ± 0.2 | 4.2 ± 0.2 | 4.5 ± 0.4 |
| Stool frequency | 1.3 ± 0.1 | 1.3 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.3 |
| Ease of passage | 4.0 ± 0.03 | 3.5 ± 0.1 | 4.1 ± 0.1 | 4.6 ± 0.15 |
BMI, body mass index. CF, colonic filling. GC, geometric center.
BMI, body mass index.
Table 2.
Short-term intra-subject variation across groups
| All | IBS-C | IBS-D | ||
|---|---|---|---|---|
| CF6h, % | Mean ± SEM | 44.8 ± 5.8 | 44.8 ± 5.8 | - |
| Difference ± SD | −17.5 ± 42.8 | −17.5 ± 42.8 | - | |
| N | 4 | 4 | 0 | |
| COV | 96 | 96 | - | |
| GC24h | Mean ± SEM | 2.08 ± 0.12 | 1.81 ± 0.09 | 2.91 ± 0.25 |
| Difference ± SD | −0.08 ± 0.65 | −0.07 ± 0.0.64 | −0.12 ± 0.71 | |
| N | 37 | 28 | 9 | |
| COV | 31 | 35 | 24 | |
| GC48h | Mean ± SEM | 3.09 ± 0.17 | 2.68 ± 0.14 | 4.40 ± 0.20 |
| Difference ± SD | −0.14 ± 0.83 | −0.26 ± 0.86 | 0.25 ± 0.61 | |
| N | 38 | 29 | 9 | |
| COV | 27 | 32 | 14 | |
CF, colonic filling. GC, geometric center. COV, coefficient of variation.
Table 3.
Long-term intra-subject variation across groups
| All | Healthy | IBS-C | IBS-D | IBS-M | ||
|---|---|---|---|---|---|---|
| CF6h, % | Mean ± SEM | 53.0 ± 3.8 | 54.1 ± 4.8 | 49.6 ± 6.5 | 56.7 ± 15.2 | 62.5 |
| Difference ± SD | 6.2 ± 31.7 | 8.7 ± 38.1 | 0.3 ± 23.1 | 19.0 ± 35.1 | −11.0 | |
| N | 38 | 17 | 15 | 5 | 1 | |
| COV | 60 | 70 | 47 | 62 | - | |
| GC24h | Mean ± SEM | 2.43 ± 0.09 | 2.30 ± 0.21 | 1.93 ± 0.09 | 3.22 ± 0.13 | 2.23 ± 0.18 |
| Difference ± SD | −0.12 ± 0.93 | 0.20 ± 0.68 | −0.16 ± 0.76 | −0.35 ± 1.19 | 0.65 ± 0.38 | |
| N | 101 | 16 | 47 | 33 | 5 | |
| COV | 38 | 30 | 39 | 37 | 17 | |
| GC48h | Mean ± SEM | 3.28 ± 0.11 | 3.15 ± 0.27 | 2.82 ± 0.11 | 4.34 ± 0.13 | 4.33 ± 0.30 |
| Difference ± SD | −0.22 ± 0.98 | 0.17 ± 0.81 | −0.25 ± 1.06 | −0.35 ± 0.90 | −0.10 ± 0.55 | |
| N | 80 | 11 | 47 | 19 | 3 | |
| COV | 30 | 26 | 38 | 21 | 13 | |
CF = colonic filling; GC = geometric center; COV = coefficient of variation.
Publications of previously performed gastrointestinal transit studiesconducted in patients with IBS and healthy volunteers from which data were derived in a retrospective manner for the current study
Prather C, Camilleri M, Zinsmeister A, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000 Mar;118(3):463–8.
Viramontes B, Malcolm A, Camilleri M, Szarka L, McKinzie S, Burton D, et al. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol. 2001 Dec;281(6):G1468–76.
Delgado-Aros S, Chial H, Camilleri M, Szarka L, Weber F, Jacob J, et al. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointest Liver Physiol. 2003 Apr;284(4):G558–66.
Samsom M, Szarka L, Camilleri M, Vella A, Zinsmeister A, Rizza R. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol. 2000 Jun;278(6):G946–51.
* Camilleri M, McKinzie S, Fox J, Foxx-Orenstein A, Burton D, Thomforde G, et al. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004 Oct;2(10):895–904.
* Kim H, Camilleri M, McKinzie S, Lempke M, Burton D, Thomforde G, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003 Apr;17(7):895–904.
Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008 Jul;6(7):772–81.
Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005 Aug;3(8):784–91.
Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006 May;290(5):G942–7.
Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, et al. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil. 2006 Oct;18(10):911–8.
Park MI, Ferber I, Camilleri M, Allenby K, Trillo R, Burton D, et al. Effect of atilmotin on gastrointestinal transit in healthy subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006 Jan;18(1):28–36.
Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006 Sep;18(9):831–8.
* Camilleri M, Vazquez-Roque MI, Burton D, Ford T, McKinzie S, Zinsmeister AR, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007 Jan;19(1):30–8.
* Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007 Sep;133(3):761–8.
Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000 Jul;119(1):41–50.
Coulie B, Bouras BE, Zinsmeister AR, Burton DD, Camilleri M. A simplified approach to assess proximal colonic emptying in humans. Gastroenterology. 1998 Apr;114:A737. (Abstract)
Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, et al. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. 2003 May;1(3):211–8.
* Cremonini F, Camilleri M, McKinzie S, Carlson P, Camilleri CE, Burton D, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005 Mar;100(3):652–63.
Bouras E, Camilleri M, Burton D, Thomforde G, McKinzie S, Zinsmeister A. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001 Feb;120(2):354–60.
Bharucha A, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, et al. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000 Nov;47(5):667–74.
* Manini ML, Camilleri M, Goldberg M, Sweetser S, McKinzie S, Burton D, Wong S, Kitt MK, Li Y-P, Zinsmeister AR. Effects of TD-5108 on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil (submitted for publication)
* Odunsi ST, Camilleri M, McKinzie S, Burton D, Ryks M, Carlson P, Nadeau A, usciglio IA, Lamsam J, Singh R, Zinzmeister AR. Bile acids, colonic transit and effect of bile acid binding in unselected irritable bowel syndrome with diarrhea (in preparation)
Footnotes
DISCLOSURES
The authors have no competing interests.
Assessing bowel functions
References
- 1.Parkman HP, Miller MA, Fisher RS. Role of nuclear medicine in evaluating patients with suspected gastrointestinal motility disorders. Semin Nucl Med. 1995 Oct;25(4):289–305. doi: 10.1016/s0001-2998(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 2.Bouchoucha M, Devroede G, Arhan P, Strom B, Weber J, Cugnenc PH, et al. What is the meaning of colorectal transit time measurement? Dis Colon Rectum. 1992 Aug;35(8):773–82. doi: 10.1007/BF02050328. [DOI] [PubMed] [Google Scholar]
- 3.Sadik R, Stotzer P, Simren M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008 Mar;20(3):197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 4.Nam Y, Pikarsky A, Wexner S, Singh J, Weiss E, Nogueras J, et al. Reproducibility of colonic transit study in patients with chronic constipation. Dis Colon Rectum. 2001 Jan;44(1):86–92. doi: 10.1007/BF02234827. [DOI] [PubMed] [Google Scholar]
- 5.Cann P, Read N, Brown C, Hobson N, Holdsworth C. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983 May;24(5):405–11. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993 Oct 7;329(15):1073–8. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha A, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, et al. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000 Nov;47(5):667–74. doi: 10.1136/gut.47.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke MC, Chase JW, Gibb S, Catto-Smith AG, Hutson JM, Southwell BR. Standard medical therapies do not alter colonic transit time in children with treatment-resistant slow-transit constipation. Pediatr Surg Int. 2009 Jun;25(6):473–8. doi: 10.1007/s00383-009-2372-4. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987 Jan;92(1):40–7. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 10.Read NW, Miles CA, Fisher D, Holgate AM, Kime ND, Mitchell MA, et al. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980 Dec;79(6):1276–82. [PubMed] [Google Scholar]
- 11.Odunsi ST, Camilleri M. Selected interventions in nuclear medicine: gastrointestinal motor functions. Semin Nucl Med. 2009 May;39(3):186–94. doi: 10.1053/j.semnuclmed.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003 Jan;38(1):36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 13.Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int. 2009 Jul;25(7):559–72. doi: 10.1007/s00383-009-2387-x. [DOI] [PubMed] [Google Scholar]
- 14.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997 Nov;38(11):1807–10. [PubMed] [Google Scholar]
- 15.Proano M, Camilleri M, Phillips S, Brown M, Thomforde G. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990 Jun;258(6 Pt 1):G856–62. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 16.Rao SS, Kuo B, McCallum RW, Chey WD, Dibaise JK, Hasler WL, et al. Investigation of Colonic and Whole Gut Transit with Wireless Motility Capsule and Radioopaque Markers in Constipation. Clin Gastroenterol Hepatol. 2009 Feb 3; doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Graff J, Brinch K, Madsen J. Gastrointestinal mean transit times in young and middle-aged healthy subjects. Clin Physiol. 2001 Mar;21(2):253–9. doi: 10.1046/j.1365-2281.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 18.Lundin E, Graf W, Garske U, Nilsson S, Maripuu E, Karlbom U. Segmental colonic transit studies: comparison of a radiological and a scintigraphic method. Colorectal Dis. 2007 May;9(4):344–51. doi: 10.1111/j.1463-1318.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- 19.Price JM, Davis SS, Wilding IR. Characterization of colonic transit of nondisintegrating tablets in healthy subjects. Dig Dis Sci. 1993 Jun;38(6):1015–21. doi: 10.1007/BF01295715. [DOI] [PubMed] [Google Scholar]
- 20.Cremonini F, Mullan B, Camilleri M, Burton D, Rank M. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002 Oct;16(10):1781–90. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 21.Degen L, Phillips S. Variability of gastrointestinal transit in healthy women and men. Gut. 1996 Aug;39(2):299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Choi M, Choi H, Cho Y, Oh J, Lee I, et al. Measurement of colonic transit using a delayed-release capsule containing radio-opaque markers. Scand J Gastroenterol. 2008;43(5):545–50. doi: 10.1080/00365520701850204. [DOI] [PubMed] [Google Scholar]
- 23.Wyman J, Heaton K, Manning A, Wicks A. Variability of colonic function in healthy subjects. Gut. 1978 Feb;19(2):146–50. doi: 10.1136/gut.19.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchoucha M, Odinot J, Devroede G, Landi B, Cugnenc P, Barbier J. Simple clinical assessment of colonic response to food. Int J Colorectal Dis. 1998;13(5–6):217–22. doi: 10.1007/s003840050164. [DOI] [PubMed] [Google Scholar]
- 25.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 26.Grudell AB, Camilleri M, Jensen KL, Foxx-Orenstein AE, Burton DD, Ryks MD, et al. Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health. Am J Physiol Gastrointest Liver Physiol. 2008 May;294(5):G1114–9. doi: 10.1152/ajpgi.00051.2008. [DOI] [PubMed] [Google Scholar]
- 27.Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1299–306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri M, Colemont L, Phillips S, Brown M, Thomforde G, Chapman N, et al. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989 Aug;257(2 Pt 1):G284–90. doi: 10.1152/ajpgi.1989.257.2.G284. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997 Sep;32(9):920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–10. [PubMed] [Google Scholar]
- 31.Proano M, Camilleri M, Phillips SF, Brown ML, Thomforde GM. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–62. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 32.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–15. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 33.Argenyi E, Soffer E, Madsen M, Berbaum K, Walkner W. Scintigraphic evaluation of small bowel transit in healthy subjects: inter- and intrasubject variability. Am J Gastroenterol. 1995 Jun;90(6):938–42. [PubMed] [Google Scholar]
- 34.McLean RG, Smart RC, Lubowski DZ, King DW, Barbagallo S, Talley NA. Oral colon transit scintigraphy using indium-111 DTPA: variability in healthy subjects. Int J Colorectal Dis. 1992 Dec;7(4):173–6. doi: 10.1007/BF00341215. [DOI] [PubMed] [Google Scholar]
- 35.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000 Jul;119(1):41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 36.Verne GN, Davis RH, Robinson ME, Gordon JM, Eaker EY, Sninksy CA. Treatment of chronic constipation with colchicine: randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2003 May;98(5):1112–6. doi: 10.1111/j.1572-0241.2003.07417.x. [DOI] [PubMed] [Google Scholar]
- 37.Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007 Sep;133(3):799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Heaton KW, O’Donnell LJ, Braddon FE, Mountford RA, Hughes AO, Cripps PJ. Symptoms of irritable bowel syndrome in a British urban community: consulters and nonconsulters. Gastroenterology. 1992 Jun;102(6):1962–7. doi: 10.1016/0016-5085(92)90320-x. [DOI] [PubMed] [Google Scholar]
- 39.Kettell J, Jones R, Lydeard S. Reasons for consultation in irritable bowel syndrome: symptoms and patient characteristics. Br J Gen Pract. 1992 Nov;42(364):459–61. [PMC free article] [PubMed] [Google Scholar]
- 40.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut. 1996 Jul;39(1):109–13. doi: 10.1136/gut.39.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


