Abstract
Background
A goal of Healthy People 2010 was to reduce health disparities. We determined the extent of reductions in geographic disparities in five breast cancer screening indicators.
Methods
We examined the extent of reductions in geographic disparities in five breast cancer screening indicators using data about women aged 40 and older from 200 counties in the 1988-2005 Surveillance, Epidemiology, and End Results Program database. County-level trends in five breast cancer indicators (in situ, stage I, lymph-node positive, locally advanced, and mortality) were summarized using the estimated annual percentage change. Observed county rates were smoothed using hierarchical Bayesian spatiotemporal methods to calculate measures of absolute and relative geographic disparity and their changes over time.
Results
For in situ breast cancer, absolute disparity increased 93.7% during 1988-2005. Relative disparity declined 61.5% during the entire study period. Absolute and relative disparity for stage I breast cancer declined 18.5% and 41.4%, respectively. Absolute disparity for lymph node-positive breast cancer declined 37.9% during the study period, while relative disparity declined 17.6%. Absolute disparity for locally advanced breast cancer declined 66.5% while relative disparity declined 17.8% during the study period. Absolute disparity in breast-cancer mortality declined 60.5%, while relative disparity declined 19.8%.
Conclusions
Absolute and relative geographic disparities narrowed over time for all breast cancer indicators except for in situ breast cancer.
Impact
Progress has been made toward reducing geographic disparities in breast cancer outcomes, particularly in advanced-stage breast cancer incidence and mortality rates, although disparities remain.
Keywords: geography, breast cancer, Bayesian models
Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer death among women in the United States with about 40,000 deaths expected in 2009 (1). Mammographic screening for breast cancer reduces the risk of breast-cancer mortality (2-5). The proportion of women aged 50-64 years who had a mammogram within two years more than doubled from 32% in 1987 to 79% in 2000 (6, 7); however recent studies show that mammography use has reached a plateau or even declined slightly (8).
Monitoring the effects of breast-cancer screening at the population level is vital to maximize its impact, particularly in light of changes in mammography use. Based on data from screening programs implemented in western European countries, the reduction in breast-cancer-related mortality among women with screen-detected cancers resulted from a predictable pattern of detection of tumors with smaller mass, at an earlier stage, and before they metastasize to lymph nodes (4, 9-11). In the United States, early-stage breast cancer incidence increased dramatically during the past few decades (12, 13), but declined during 2001-2004 (14). In addition, late-stage breast cancer declined over time, as did mortality rates (14).
While breast cancer incidence and mortality trends are generally monitored at the national level (14), little is known about small-area variation (geographic disparity) in these trends. Reducing disparities, including geographic disparities, is an overarching goal of the Healthy People 2010 initiative and of the National Cancer Institute’s (NCI) strategic plan (15, 16). Monitoring disparities in the abovementioned indicators of breast-cancer screening at small geographic levels (e.g., the county level) may help facilitate local health planning through interventions aimed at increasing screening and allocation of pertinent screening resources. Extensive geographic disparity in the effects of breast-cancer screening is expected since screening use varies geographically (17-19), but it is unclear if this disparity has changed over time. The purpose of our analysis was to describe temporal changes in geographic disparity and overall rates of five breast-cancer screening indicators across 200 counties using population-based breast cancer data during 1988-2005.
Methods
Data source
We used the 1988-2005 public-use county-level data from nine population-based Surveillance, Epidemiology, and End Results (SEER) programs to calculate the rate of five breast-cancer screening indicators in an ecological study design. We used 1988 as the first year of observation since this is the first year when detailed information about lymph node involvement, American Joint Commission on Cancer (AJCC) tumor-node-metastasis (TNM) staging, and tumor size are available in the SEER data. The SEER programs collect data about demographics (age, race, marital status, census tract, county), clinical characteristics of the tumor (stage at diagnosis, tumor biology), treatment (type of surgery, receipt of radiation therapy, lymph node dissection), and survival. During this time period, the SEER programs at the nine sites included in our study covered 200 counties and about nine percent of the United States population. The analyses were based on women age 40 or older diagnosed with first primary breast cancer or who died from breast cancer from 1988 to 2005.
Breast-cancer screening indicators
Indicators of early-stage breast cancer consist of in situ breast cancer and invasive breast cancers that were less than 2 cm at the time of diagnosis (T1 tumors). In situ breast cancer was identified from the fifth digit of the histology-behavior code of the SEER data, which is based on the International Classification of Diseases for Oncology. In situ and early-stage breast cancer have been shown to be indicators of reductions in mortality in European studies and correspond to the epidemiology of breast-cancer screening (4, 9-11).
The beneficial effect of screening in reducing mortality occurs as a result of identifying tumor early at a reduced tumor size, lower histologic grade, and reduced axillary lymph node involvement (4, 20). We used two indicators of advanced breast cancer: 1) the rate of lymph node-positive breast cancers, and 2) the rate of locally advanced breast cancer (LABC). We define LABC as tumors classified as T3 (tumors more than 5.0 cm in greatest diameter) or T4 (any size tumor with direct extension to the chest wall or skin, and inflammatory carcinoma) (21). The breast-cancer mortality rate was calculated for women who had breast cancer as the underlying cause of death on their death certificate. Women who were previously diagnosed with breast cancer but died from other causes were not included in the breast-cancer mortality rate (22).
Statistical analysis
First, we identified changes in overall breast-cancer screening indicator rates over time in order to put in perspective changes in geographic disparities (23). It is possible for disparities to increase even when overall rates are declining. For example, the racial disparity in breast cancer mortality is growing even though overall breast cancer mortality is declining (24). Overall rates were age and race adjusted using the 2000 US standard population. Linear trends in rates from 1988 to 2005 were summarized using the estimated annual percentage change (EAPC). The EAPC was calculated by fitting a linear regression to the natural logarithm of the annual rates, using calendar year as a regression variable. Therefore, the model was
where x = calendar year and EAPC = 100 *(ea-1). Joinpoint regressions were performed to identify significant changes in rates over time. Joinpoint regression is based on permutation tests to identify an inflection point (hereafter called joinpoint) with a significant change in the slope of the trend (25, 26). For our analysis, a maximum of three joinpoints was allowed and a minimum of four points between two joinpoints was required.
Second, we smoothed the observed county rates using hierarchical Bayesian spatiotemporal methods in order to calculate measures of absolute and relative geographic disparity. Hierarchical Bayesian methods were used because county rates are strongly affected by the annual number of breast cancers in each county and may be very unreliable if based on few breast cancers. Moreover, rates that are close in proximity are not independent of each other (spatial correlation). The smoothed rates are close to the observed rates when based upon a large number of breast cancers or population size. However, in counties with lower incidence of breast cancers or with smaller population size, the rates can be strongly affected each year. In this case, the observed rate was smoothed toward the rates of the adjacent counties. Observed county rates were age adjusted using the 2000 US standard population when age-race-county-year specific data included fewer than five breast cancer cases. Rates were age and race adjusted to this population when age-race-county-year specific data included at least five breast cancer cases. We used three racial groups: white, Black, and Other race. Specifically, we used the Knorr-Held model to obtain the yearly, smoothed county rates during 1988-2005 (27). This model contained four random terms,
where θij is the county-year-specific rate; β0 is the intercept; μi and vi are the spatially structured and unstructured random terms, respectively; δj is the temporal random term; and ϕij is the spatiotemporal random term. Specification of the structured spatial random effect was derived from an intrinsic conditional autoregressive model (iCAR) in which adjacent counties were assumed to have similar disease risk (28). The other three random effects were assumed to be independent of counties and with exchangeable normal priors. Markov Chain Monte Carlo methods were adopted to fit the models. The spatial adjacency matrix was created in ArcGIS (ESRI, Redlands, CA) using an add-in adjacency tool (29).
Third, we calculated trends in geographic disparity for each of the breast-cancer screening indicators. The NCI defines cancer health disparities as adverse differences in cancer incidence (new cases), cancer prevalence (all existing cases), cancer death (mortality), cancer survivorship, and burden of cancer or related health conditions that exist among specific population groups in the United States (30). Geographic disparity can be measured in two different ways, depending on whether one is concerned with measuring the relative or absolute distribution of these indicators across counties. The most frequent method of communicating information about disparities in epidemiology and public health is in relative terms (e.g., relative risk). Risk difference, a measure of absolute disparity, is used less frequently. We used measures of both relative disparity (Mean Log Deviation [MLD]) and absolute disparity (the between-group variance [BGV]) because of potential differences in findings between both measures (23, 31). Whereas many measures of geographic disparity are available, for unordered groups (such as counties, in our instance) the MLD and BVD are recommended (32). This approach uses population-weighted measures that account for changes over time in the underlying distribution of the county populations and measure absolute and relative disparity as differences from the population average (i.e., overall rate) for each of the five breast-cancer screening indicators. Both measures weight the county rates by their population size and are more sensitive than other measures of absolute and relative disparity to deviations further from the overall rate (31). The BGV is calculated by squaring the differences in county rates from the population average and weighting by population size. The MLD summarizes the disproportionality between county rates and population size (expressed on the natural logarithm scale). Extending the risk difference approach to unordered groups, the yearly absolute disparity of BGV is defined as . Extending the relative risk approach to unordered groups, the yearly relative disparity of MLD is written as , where, rj = yj/μ, pj is county j’s population fraction, yj is the county j’s age-(race) adjusted rate and μ is the overall age-race-adjusted rate across all 200 SEER counties. In the current study, was defined as model-based predicted county-year-specific rates, and μ was obtained from a summary after multiplying the smoothed county rate by its population fraction. Theoretically, BGV and MLD are no less than 0 and larger values indicate greater disparities. If there is no disparity, then the BGV and the MLD are 0.
Bayesian spatiotemporal models, including the county-year-specific breast-cancer screening indicator rates, year-specific overall breast-cancer screening indicator rates, and both measures of geographic disparity, were implemented in WinBUGS (Ver.1.4.3, Medical Research Council, UK). After running 20,000 iterations as burn-in, 20,000 more samples were used to obtain parameter estimates, including county-year-specific breast cancer indicator rates and their absolute and relative disparity measures. Model fit was evaluated using the Deviance Information Criteria, with lower values indicating better fit (33).
Fourth, we used joinpoint regressions to identify significant changes in both absolute and relative disparity measures for each of the five breast-cancer screening indicators. Standard errors of the absolute and relative disparity measures were based on the 95% credible interval obtained from the Bayesian models. Again, the estimated annual percentage change was calculated for each joinpoint. We also calculated the period change in absolute and relative disparity between 1988 and 2005.
Fifth, we identified priority counties where disparities remained high. Setting priorities was based on concepts developed in other studies (34, 35) and CDC’s state cancer profiles (www.statecancer profiles.cancer.gov), namely trends over time, 2005 estimated county rate, and precision of the estimate. Specifically, we calculated the difference between the estimated county rate and the age-race-adjusted rate across all 200 counties. Also, we calculated the ratio of the difference between the estimated 1988 county rate and the estimated 2005 county rate and the difference between the 1988 and the 2005 age-race-adjusted rate across all 200 counties. This ratio describes the change over time of the estimated county rate relative to the change of the rate of all 200 counties. Both the difference measure and the ratio measure were categorized into three groups based on their distribution across the 200 counties, resulting in nine classes of counties. The highest priority counties had an estimated rate that was at least 10 per 100,000 population higher in 2005 than the overall rate and a decline in rate from 1988 to 2005 that was at least two times lower than the overall rate. The lowest priority counties had an estimated rate that was at least 10 per 100,000 population lower than the overall rate in 2005 and a decline in rate from 1988 to 2005 that was at least two times faster than the overall rate. We did not use the measures of absolute disparity and the relative disparity to prioritize counties, because they do not indicate if a rate is below or above the overall rate. All data were managed in SAS 9.1 (SAS Institute, Inc., Cary, NC).
Results
Table 1 displays the number of breast cancers and adjusted county rates for each of the five breast cancer indicators. The median and mean numbers of breast cancers per county per year vary because of the skewed distribution. A number of counties have a large number of breast cancers.
Table 1.
Number of breast cancers and adjusted rates per county per year for five indicators, 1988-2005
| Breast cancer indicator | Number per county per year | Adjusted rate per county per year (per 100,00 population) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Minimum | Median | Mean | Maximum | Minimum | Median | Mean | Maximum | |
| In situ | 48,763 | 0 | 2 | 13.5 | 349 | 0 | 31.1 | 35.2 | 494.1 |
| Stage I | 114,015 | 0 | 6 | 31.7 | 584 | 0 | 101.1 | 100.9 | 785.2 |
| Lymph node positive | 68,686 | 0 | 3 | 19.1 | 380 | 0 | 62.7 | 65.1 | 1,832.7 |
| LABC | 25,696 | 0 | 1 | 7.1 | 187 | 0 | 19.8 | 22.9 | 1,574.5 |
| Mortality | 55,774 | 0 | 3 | 15.5 | 404 | 0 | 49.2 | 53.2 | 1,102.1 |
LABC: Locally advanced breast cancer.
In situ and stage I breast cancers
From 1988 to 2005, 48,763 in situ breast cancers were diagnosed (Table 1). The overall in situ rate increased 6.3 percent per year from 1988 to 2000, after which it remained stable until 2005 (Figure 1, Table 2). From 1988 to 1993, the absolute disparity for in situ breast cancer incidence remained stable (Figure 2, Table 2), then increased 15.6 percent per year from 1993 to 1999 and then declined 5.3 percent per year from 1999 to 2005. Relative disparity declined 5.5 percent per year during the entire study period. Absolute disparity for in situ incidence increased 93.7 percent from 1988 to 2005, while relative disparity declined 61.5 percent.
Figure 1.
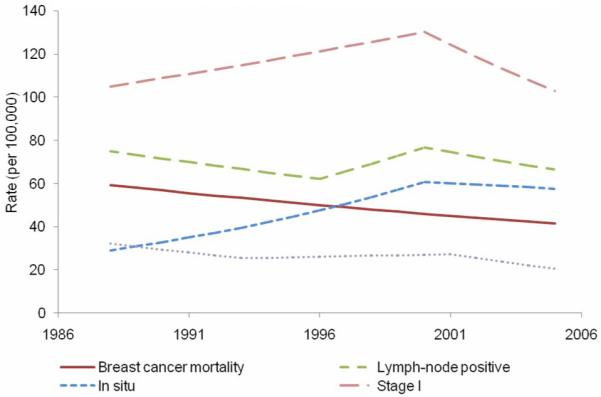
Adjusted, observed breast cancer indicator rates over time based on joinpoint analysis, 1988-2005.
Table 2.
Trends (years and estimated annual percentage change) in five breast cancer indicator rates, absolute disparity (Between-Group Variance) and relative disparity (Mean Log Deviation), 1988-2005
| Trend 1 |
Trend 2 |
Trend 3 |
Percentage of change, 1988-2005 |
DIC | ||||
|---|---|---|---|---|---|---|---|---|
| Breast cancer indicator Disparity measure |
Years | EAPC | Years | EAPC | Years | EAPC | ||
| In situ incidence | 1988-2000 | 6.3* | 2000-2005 | −1.1 | ||||
| BGV | 1988-1993 | 2.4 | 1993-1999 | 15.6* | 1999-2005 | −5.3* | 93.7 | 21,819 |
| MLD | 1988-2005 | −5.5* | −61.5 | 21,819 | ||||
| Stage I incidence | 1988-2000 | 1.8* | 2000-2005 | −4.6* | ||||
| BGV | 1988-2005 | −1.2 | −18.5 | 29,381 | ||||
| MLD | 1988-1995 | −6.1* | 1995-2005 | −0.9 | −41.4 | 29,381 | ||
| Lymph node positive | 1988-1996 | −2.3* | 1996-2000 | 5.4* | 2000-2005 | −2.9* | ||
| BGV | 1988-1993 | −8.9* | 1993-2005 | 0.1 | −37.9 | 27,184 | ||
| MLD | 1988-2005 | −1.1* | −17.6 | 27,184 | ||||
| LABC incidence | 1988-1993 | −4.7* | 1993-2001 | 0.9 | 2001-2005 | −6.8* | ||
| BGV | 1988-1993 | −10.4* | 1993-1998 | −0.7 | 1998-2005 | −7.0* | −66.5 | 19,611 |
| MLD | 1988-2002 | −2.8* | 2002-2005 | 7.1 | −17.8 | 19,611 | ||
| Mortality rate | 1988-2005 | −2.0* | ||||||
| BGV | 1988-2005 | −5.3* | −60.5 | 25,907 | ||||
| MLD | 1988-2005 | −1.3* | −19.8 | 25,907 | ||||
p<0.05; LABC: locally advanced breast cancer; EAPC: Estimates annual percent change; BGV: Between-Group Variance (absolute disparity); MLD: Mean Log Deviation (relative disparity). Because BGV and MLD were calculated during the same winBUGS run, the DIC values are from the same model.
Figure 2.
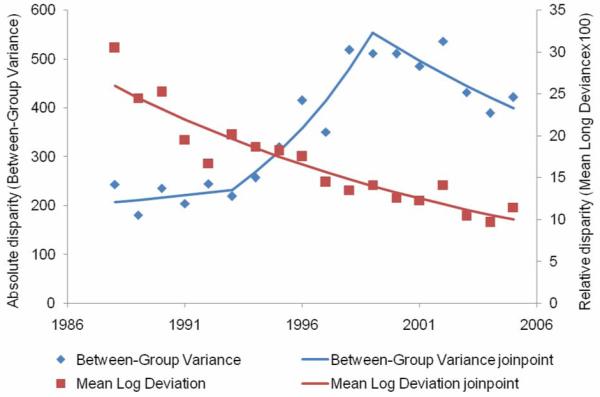
Absolute and relative geographic disparity over time for in-situ breast cancer, 1988-2005.
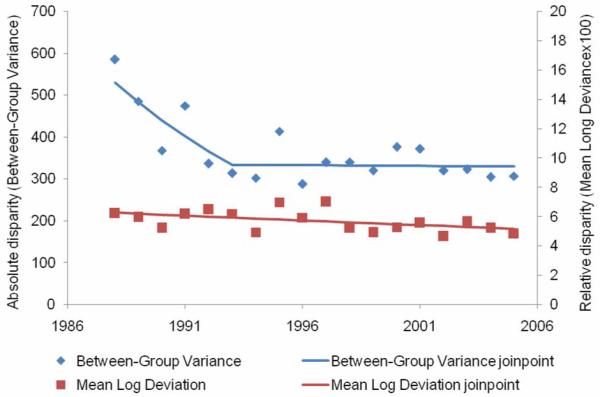
From 1988 to 2005, 114,015 stage I breast cancers were diagnosed. The overall stage I rate increased 1.8 percent per year from 1988 to 2000, after which it declined 4.6 percent per year. No significant changes were observed in absolute disparity for stage I breast cancer rates on a yearly basis (Figure 3, Table 2) although there was an 18.5 percent decline after 1988. Relative disparity declined 6.1 percent per year from 1988 to 1995 and declined 41.4 percent over the entire study period.
Figure 3.
Absolute and relative geographic disparity over time for stage I breast cancer, 1988-2005.
Lymph-node positive breast cancer, locally-advanced breast cancer, and breast cancer mortality
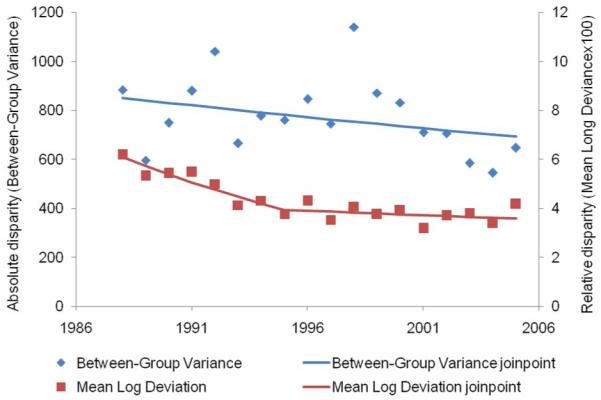
From 1988 to 2005, 68,686 breast cancers were lymph-node positive (Table 1). The overall age-and-race-adjusted lymph-node positive breast cancer rate declined from 1988 to 1996 (2.3 percent per year), increased from 1996 to 2000 (5.4 percent per year), and then declined again until 2005 (2.9 percent per year). The absolute disparity in lymph-node positive breast cancer rate declined 8.9 percent per year from 1988 to 1993, and remained stable thereafter for an overall decline of 37.9 percent during the study period (Figure 4, Table 2). The relative disparity declined by 1.1 percent per year and 17.6 percent over the entire study period.
Figure 4.
Absolute and relative geographic disparity over time for lymph node-positive breast cancer, 1988-2005.
From 1988 to 2005, there were 25,699 LABC cases diagnosed (Table 1). The overall age-and-race-adjusted LABC rate declined 4.7 percent per year from 1988 to 1993 (Figure 5, Table 2), after which the LABC rate remained stable and then declined 6.8 percent per year from 2001 to 2005. The absolute disparity in LABC rates declined 10.4 percent per year from 1988 to 1993, remained stable from 1993 to 1998, and then further declined 7.0 percent per year after 1998. Overall, the absolute disparity in LABC rate declined 66.5 percent from 1988 to 2005. The relative disparity in LABC declined 2.8 percent per year from 1988 to 2002 and then remained stable after 2002 for an overall decline of 17.8 percent since 1988.
Figure 5.
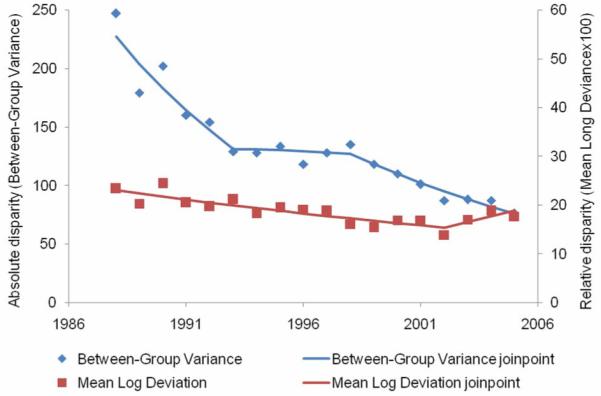
Absolute and relative geographic disparity over time for locally advanced breast cancer, 1988-2005.
From 1988 to 2005, there were 55,774 breast cancer deaths (Table 1). During this time period, the overall breast-cancer mortality rate decreased 2.0 percent per year (Figure 6, Table 2). The absolute disparity in breast-cancer mortality declined 5.3 percent per year for an overall decline of 60.5 percent since 1988. The relative disparity in breast-cancer mortality rates declined 1.3 percent per year for an overall decline of 19.8 percent since 1988.
Figure 6.
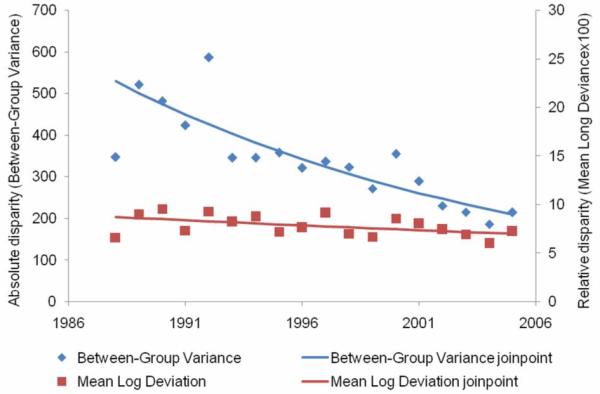
Absolute and relative geographic disparity over time for breast cancer mortality, 1988-2005.
Table 3 displays priority counties for lymph-node positive breast cancer, which was selected because of the lack of change in the absolute disparity measure and the very small change in relative disparity measure over time. The nine classes of counties were based on the 1988-2005 changes in the estimated county lymph-node positive breast cancer rate relative to the age-race-adjusted lymph node-positive rate across the 200 counties and the difference in estimated county rate and the age-race-adjusted lymph node-positive rate across the 200 counties in 2005. Fifty-one counties were classified as hotspots or probable hotspots, which accounted for 25.5 percent of the 200 counties. Interestingly, there were no counties classified as hotspots or probable hotspots in the Atlanta area, Hawaii, the Detroit area, the San Francisco/Oakland area, and Connecticut. In Iowa, 32 counties (32.3% of 99 Iowa SEER counties) were (probable) hotspots, in New Mexico 8 counties (24.2% of 33 New Mexico counties), in Utah 8 counties (27.6% of 29 Utah counties), and in the Seattle/Puget Sound area 3 counties (23.1% of 13 area counties). The number of (probable) hotspots was overrepresented in the New Mexico, Utah, and Seattle/Puget Sound area based on the number of counties in these areas in SEER. Forty-one counties were considered to be of the lowest priority (Table 3).
Table 3.
Nine classes of priority counties based on county-level changes in lymph-node positive breast cancer incidence rates, 1988-2005
| Classes | Ratio of 1988-2005 changes in county rate versus overall rate* |
Difference between county rate and overall rate in 2005 |
Number of counties |
|---|---|---|---|
| Class 1: Definite hotspots | < − 2.0 | >10/100,000 | 35 |
| Class 2: Probable hotspots | < − 2.0 | ± 10/100,000 | 16 |
| Class 3: Moderate priority | < − 2.0 | < − 10/100,000 | 6 |
| Class 4: Moderate priority | ± 2.0 | >10/100,000 | 13 |
| Class 5: Moderate priority | > 2.0 | >10/100,000 | 5 |
| Class 6: Lower priority | ± 2.0 | ± 10/100,000 | 30 |
| Class 7: Lower priority | ± 2.0 | < − 10/100,000 | 36 |
| Class 8: Lower priority | > 2.0 | ± 10/100,000 | 18 |
| Class 9: Lowest priority | > 2.0 | < − 10/100,000 | 41 |
ratio: < − 2.0: The county rate change from 1988 to 2005 was at least 2 times slower than the overall predicted change during this time period; ratio: > 2.0: The county rate change from 1988 to 2005 was at least 2 times faster than the overall predicted change during this time period.
Discussion
The purpose of this study was to examine trends over time in absolute and relative geographic disparity in five breast-cancer screening indicators using 1988-2005 population-based SEER data. Disparities narrowed since 1988 for all indicators except for in situ breast cancer. Important progress has been made toward achieving the Healthy People 2010 and NCI strategic objectives for reducing disparities, particularly in locally advanced breast cancer incidence and mortality rates. Despite trends over time, geographic disparities in breast-cancer screening indicators remained. This suggests that not all counties studied benefitted from early detection of breast cancer.
The age-and-race-adjusted in situ breast cancer rate as well as absolute disparity increased dramatically from 1988 to 2000, while relative disparity declined during the entire study period. This suggests that the in situ breast cancer rate for some counties increased faster, particularly from 1993 to 1999 (15.6 percent per year), while the rates for other counties lagged behind. Increased mammography use likely contributed to the increase in incidence of in situ breast cancers from 1988 to 2000 (36). It is also likely that there was geographic variation in mammography adoption during this time period. There was a sudden change in absolute disparity starting in 1999, which may have been related to geographic variation in saturation of mammography use (37, 38) and/or declines in the use of hormone replacement therapy (39). Between 2000 and 2005, nationwide use of screening mammography fell by 4% overall among women aged 40 years or older and by almost 7% among women aged 50-64 years of age (8). Starting in 1999, both absolute and relative disparity in in situ breast cancer rates declined. Continued monitoring of geographic disparities in in situ breast cancer rates is warranted in light of recent changes in mammography and hormone replacement therapy use.
For stage I breast cancer, the overall age-and-race-adjusted rate increased until 2000, after which it declined. During the entire study period, absolute and relative disparity declined, suggesting that the stage I incidence rates for some counties changed more rapidly than incidence rates for other counties but that the variation across counties diminished over time and became more similar to the overall stage I breast cancer rate.
Overall, declines were observed for lymph node-positive and LABC rates from 1988 to 2005. This is particularly important since both are indicators of reductions in breast-cancer mortality (4, 9-11). However, absolute disparity plateaued for lymph node-positive breast cancer and LABC starting in 1993, but then further declined for LABC starting in 1998. Mammography use likely played a role in the changes in both rates over time, since early detection is associated with smaller tumor size, lower histologic grade, and lower likelihood of lymph node invasion (4). The changes in disparities over time may be the result, therefore, of increases in county mammography rates similar to overall increases in mammography use, but there was no “catching up” by counties starting out with lower mammography rates in terms of the prevention of lymph node metastasis.
For mortality, the overall rate declined linearly over time as did absolute and relative disparity. This suggests that all SEER counties progressed toward the reduction in the overall age-and-race-adjusted mortality rate thereby making progress toward the Healthy People 2010 goal and one of the NCI’s key strategic objectives (16). It is expected that reductions in disparity in county mammography use and implementation of evidence-based treatment contributed to the reduction in geographic disparity in breast-cancer mortality, just as they accounted for changes in the overall breast cancer mortality rate over time (40).
Studies are needed to more fully understand the geographic disparity of the breast cancer indicators examined in order to replicate the findings. Established risk factors for breast cancer in women include older age, a family history of breast cancer, greater height, adult weight gain, high birth weight, alcohol intake, high mammographic density, postmenopausal hormone use, and certain reproductive factors, including earlier menarche, older age at first pregnancy, shorter duration of breastfeeding, lower parity, longer interval between births, and greater body mass index in postmenopausal women (41). Changes in some of these risk factors, such as hormone replacement use, may have contributed to changes in breast cancer incidence at the population level (42). Unfortunately, much less is known if changes in some of these risk factors contributed to changes in geographic disparity in population-based breast cancer rates. The prevalence of these risk factors should vary across space and time in order to affect changes in geographic disparities in the breast cancer indicators observed. Future research should focus on examining trends in disparities related to breast cancer risk factors, particularly in light of changes in disparities observed in the breast cancer indicators. Moreover, future studies should examine remaining disparities in specific counties that were identified, such as those with lymph-node positive breast cancer rates that changed little over time and remained elevated in 2005.
Our study has some limitations. First, we restricted our data to women aged 40 and older to focus on the age group that accounts for the majority of breast cancer cases and effectiveness of breast-cancer screening. Second, we used county as the smallest geographic entity within a state since it is the smallest geographic unit with the social, political and legal responsibility for providing a broad range of services, including health-related services. It also is recognized that comparisons across counties presents a number of challenges in that one county in one state (e.g., San Francisco) is likely to be comparable to several counties in another state (e.g., Iowa) in terms of size and population density. However, a strength of our Bayesian analysis is that we took into account the spatial relationships among counties, which have been ignored in many ecological analyses. These types of analyses provided many useful features, including the smoothed, posterior estimates of the county breast cancer rates we used in the current analysis. Because of our use of the smoothed county rates, disparity measures were less likely affected by extreme rates that were based on few breast cancers or small population size. Consequently, Bayesian methods appear to be more appropriate. Our analysis went beyond describing changes in geographic disparities across the 200 counties to identify also several priority counties.
We recognize that the breast-cancer screening indicators are not independent of each other. For example, 53.7 percent of women diagnosed with LABC had positive lymph nodes and some women (3.3 percent) who were diagnosed with breast cancer earlier during the study period subsequently died. Regardless of the overlap, we observed important differences across the indicators. Moreover, there are specific biological reasons for focusing on each of the breast cancer indicators (4).
In conclusion, we observed important declines in absolute and relative geographic disparity over time, particularly in LABC incidence and breast cancer mortality rates. This suggests important progress toward achieving Healthy People 2010 and NCI objectives to reduce geographic disparities. However, disparities in county rates remained for in situ, stage I, lymph node-positive breast cancer, and breast cancer mortality indicating a need for continued monitoring and further study to determine where to target efforts to reduce remaining disparities.
Acknowledgments
This research was supported in part by grants from the National Cancer Institute (CA109675, CA91842). We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for allowing the use of the Health Behavior and Outreach Core.
References
- 1.American Cancer Society . Cancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 2.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography: a meta-analysis. JAMA. 1995;273:149–254. [PubMed] [Google Scholar]
- 3.van den Akker-van Marle E, de Koning H, Boer R, van der Maas P. Reduction in breast cancer mortality due to the introduction of mass screening in the Netherlands: comparison with the United Kingdom. J Medical Screening. 1999;6:30–34. doi: 10.1136/jms.6.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Tabar L, Duffy SW, Vitak B, Chen HH, Prevost TC. The natural history of breast carcinoma. What have we learned from screening? Cancer. 1999;86:449–462. [PubMed] [Google Scholar]
- 5.Feig SA. Effect of service screening mammography on population mortality from breast carcinoma. Cancer. 2002;95:451–457. doi: 10.1002/cncr.10764. [DOI] [PubMed] [Google Scholar]
- 6.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 7.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 8.Breen N, Cronin K, Meissner H, Taplin S, Tangka F, Tiro JA, McNeel T. Reported drop in mammography. Cancer. 2007;109:2405–2409. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 9.Norden T, Thurfjell E, Hasselgren M, Lindgren A, Norgren A, Bergstrom R, Holmberg L. Mammographic screening for breast cancer. What cancers do we find? Eur J Cancer. 1997;33:624–628. doi: 10.1016/s0959-8049(96)00482-0. [DOI] [PubMed] [Google Scholar]
- 10.Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30:187–210. [PubMed] [Google Scholar]
- 11.Dongen JA. The usefulness of screening data for studying the biology of breast cancer. Eur J Cancer. 1997;33:519–520. doi: 10.1016/s0959-8049(96)00505-9. [DOI] [PubMed] [Google Scholar]
- 12.Chu KC, Tarone RE, Kessler LG, Ries LA, Hankey BF, Miller BA, Edwards BK. Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571–1579. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- 13.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer . Breast Cancer Facts & Figures 2007-2008. American Cancer Society; Atlanta, GA: 2007. [Google Scholar]
- 15.U.S. Department of Health and Human Services . Healthy People 2010. With understanding and improving health and objectives for improving health. U.S. Government Printing Office; Washington, D.C.: 2000. [Google Scholar]
- 16.National Cancer Institute . The NCI strategic plan for leading the nationa to eliminate the suffering and death due to cancer. National Cancer Institute, US Department of Health and Human Services; Washington, DC: 2006. [Google Scholar]
- 17.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis: possible relationship to cancer screening. Cancer. 1991;67:1545–1559. doi: 10.1002/1097-0142(19910301)67:5<1454::aid-cncr2820670533>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Howe HL, Katterhagen JG, Yates J, Lehnherr M. Urban-rural differences in the management of breast cancer. Cancer Causes and Control. 1992;3:533–539. doi: 10.1007/BF00052750. [DOI] [PubMed] [Google Scholar]
- 19.Schootman M, Jeffe DB, Baker EA, Walker MS. Effect of area poverty rate on cancer screening across US communities. J Epidemiol Community Health. 2006;60:202–7. doi: 10.1136/jech.2005.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day NE, Williams DRR, Khaw KT. Breast cancer screening programmes: the development of a monitoring and evaluation system. Br J Cancer. 1989;59:954–958. doi: 10.1038/bjc.1989.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, Sullivan BO, Sobin LH, Yarbro JW. AJCC cancer staging manual. 5th ed. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 22.Tabar L, Duffy SW, Yen MF, Warwick J, Vitak B, Chen HH, Smith RA. All-cause mortality among breast cancer patients in a screening trial: support for breast cancer mortality as an end point. J Med Screen. 2002;9:159–62. doi: 10.1136/jms.9.4.159. [DOI] [PubMed] [Google Scholar]
- 23.Houweling T, Kunst A, Huisman M, Mackenbach J. Using relative and absolute measures for monitoring health inequalities: experiences from cross-national analyses on maternal and child health. Int JEquity Health. 2007;6:15. doi: 10.1186/1475-9276-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSantis C, Jemal A, Ward E, Thun M. Temporal trends in breast cancer mortality by state and race. Cancer Causes and Control. 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Fay M, Feuer E, Midthune D. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Joinpoint regression program. Version 3.2. Bethesda, MD: [Google Scholar]
- 27.Knorr-Held L. Bayesian modelling of inseparable space-time variation in disease risk. Statistics in Medicine. 2000;19:2555–2567. doi: 10.1002/1097-0258(20000915/30)19:17/18<2555::aid-sim587>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Besag J, York J, Mollie A. Bayesian image restoration with two applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–59. [Google Scholar]
- 29.United Stated Geological Survey [Last accessed: August 12, 2009];Adjacency for WinBUGS tool. http://www.umesc.usgs.gov/management/dss/adjacency_tool.html.
- 30.National Cancer Institute [Accessed October 26, 2009];Cancer health disparities. 2009 Online at: http://www.cancer.gov/cancertopics/factsheet/cancer-health-disparities.
- 31.Harper S, Lynch J. Methods for measuring cancer disparities: a review using data relevant to Healthy People 2010 cancer-related objectives. National Cancer Institute; Washington, DC: 2006. [Google Scholar]
- 32.Harper S, Lynch J. Measuring health inequalities. In: Oakes J, Kaufman J, editors. Methods in social epidemiology. Jossey-Bass; San Francisco: 2006. pp. 134–168. [Google Scholar]
- 33.Spiegelhalter D, Best N, Carlin B, van der Linde A. Bayesian measures of model complexity and fit (with discussion) J R Stat Soc Series B Methodol. 2002;64:583–616. [Google Scholar]
- 34.Li W, Kelsey JL, Zhang Z, et al. Small-area estimation and prioritizing communities for obesity control in Massachusetts. Am J Public Health. 2009;99:511–9. doi: 10.2105/AJPH.2008.137364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Land T, Zhang Z, Keithly L, Kelsey JL. Small-area estimation and prioritizing communities for tobacco control efforts in Massachusetts. Am J Public Health. 2009;99:470–9. doi: 10.2105/AJPH.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773–80. doi: 10.1158/1055-9965.EPI-07-0546. [DOI] [PubMed] [Google Scholar]
- 37.Jemal A, Ward E, Thun M. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schootman M, Aft R. Rural-urban differences in radiation therapy for ductal carcinoma in-situ of the breast. Breast Cancer Res Treat. 2001;68:117–25. doi: 10.1023/a:1011915323038. [DOI] [PubMed] [Google Scholar]
- 39.Hausauer A, Keegan T, Chang E, Glaser S, Howe H, Clarke C. Recent trends in breast cancer incidence in US white women by urban/rural and poverty status. BMC Medicine. 2009;7:31. doi: 10.1186/1741-7015-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990-8: comparison of observed with predicted mortality. Br Med J. 2000;321:665–9. doi: 10.1136/bmj.321.7262.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152:950–64. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 42.Krieger N. Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol. 2008;37:627–37. doi: 10.1093/ije/dyn055. [DOI] [PubMed] [Google Scholar]


