SUMMARY
Objective
Examine the association of cognitive function with sex steroid and sex hormone binding globulin (SHBG) levels among elderly men.
Design
Prospective cohort study, The Osteoporotic Fractures in Men Study (MrOS), consisting of 5995 US community dwelling men 65 years or older.
Patients
1602 men chosen randomly from MrOS cohort for sex steroid level measurements by Mass Spectrometry (MS) at baseline. 2623 MrOS participants with sex steroids measured using RIA were also examined.
Measurements
Baseline and follow-up (4.5 years later) performance on two cognitive tests: Trails B (executive function and motor speed) and 3MS (global cognitive function). Baseline total testosterone and estradiol were measured by MS. Free testosterone (free-T) and free estradiol (free-E) were calculated. SHBG was measured by radioimmunoassay. Data were analyzed using linear regression.
Results
Baseline free-T and free-E levels were not associated with cognitive performance or change in cognition, following adjustment for age, education, race, health status and alcohol use. Baseline SHBG levels were inversely associated with follow-up trails B (p=0.03) and 3MS performance (p=0.02). Higher SHBG was associated with an increased risk of cognitive decline. Total sex steroid levels were not associated with cognitive performance.
Conclusions
Despite large numbers of participants and rigorous sex steroid measurements, we did not observe an association between cognition and either testosterone or estradiol levels. We conclude that endogenous sex steroids in the normal range are not related to executive function or global cognitive function in elderly men. High SHBG deserves further examination as a risk factor for cognitive decline.
Keywords: Cognition, Testosterone, Estradiol, SHBG, Aging
INTRODUCTION
In men, aging is accompanied by decreases in free testosterone (free-T) levels, with free-T falling by as much as 40% from age 40 to 70 years of age.1–4 Aging is also accompanied by declines in memory5, 6 and deterioration in psychomotor speed and cognitive flexibility.7 With evidence that free-T may have maintenance and neuroprotective actions in brain regions critical for these cognitive domains,8–11 the decrease in testosterone production in later decades of life may be an important contributor to cognitive aging.
Studies examining the relationship between sex steroids and cognition in older men have reported conflicting findings. In some, higher free (non protein bound) or bioavailable (loosely protein bound) T levels were associated with better cognitive performance;12–14 whereas, in others either no association15–21 or a negative association between free-T and cognitive performance has been reported.14 Total testosterone has been less closely associated with cognitive performance.12, 13, 20 One study noted that higher total and bioavailable estradiol levels were associated with worse cognitive performance on a measure of global cognition (MMSE); while on a measure of executive function (Trails B) there was a U shaped relationship.20 However, other studies have not corroborated these findings.12, 14, 15, 18
The disparate results may be due to differences in study populations and methodologies. Sex steroid’s effects on cognition may be moderated by age-related vulnerability of the brain to cognitive decline, e.g. testosterone levels may be associated with cognition in older but not younger men.12 Second, there may be critical testosterone levels below or above which cognition is impaired so that only men with very low or very high T levels might experience cognitive impairment.13 Finally, the timing of sex steroid measurements in relationship to cognitive testing may be important. Studies with sex hormone levels drawn years prior to cognitive testing15, 20 may fail to find an association because testosterone level at the time of cognitive testing is critical.
Unraveling the relationship between sex steroids and cognitive function in men as they age is an important public health issue. The Institute of Medicine issued a report in 2003 highlighting the need for more information about the association between sex steroids and cognitive aging.22 The present study examines data on sex steroids and cognitive performance in a large group of men aged 65 or older in the The Osteoporotic Fractures in Men (MrOS) study. The objectives of our study were to 1) determine the relation between cognitive function, measured specifically by the modified mini-mental status (3MS) and Trails B tests, and testosterone, estradiol and sex hormone binding globulin (SHBG) at a single point in time, 2) to determine if any single baseline measure of the sex steroid or SHBG was associated with future cognitive performance or cognitive decline during follow-up, and 3) to examine whether there was any evidence that a specific level (or threshold) of the sex steroid or SHBG was associated with cognition scores.
STUDY DESIGN AND METHODS
Participants
MrOS is a multi-center prospective study of risk factors for and consequences of vertebral and non-vertebral fractures in older men. The design of MrOS has been described in detail elsewhere.23, 24 Briefly, the MrOS cohort consists of 5995 community dwelling men aged 65 and older. Eligibility criteria were: (1) age ≥ 65 years, (2) ability to walk without the assistance of another, and (3) absence of bilateral hip replacements. Approximately 1000 participants were recruited at each of six academic medical centers: Birmingham, AL, Minneapolis, MN, Palo Alto, CA, Pittsburgh, PA, Portland, OR, and San Diego, CA. Recruitment efforts included community mailings and a variety of community outreach and educational activities.24 The Institutional Review Board at each center approved the study protocol, and written informed consent was obtained from all participants.
Participants completed two study visits, which averaged 4.5 years apart. At baseline, participants completed questionnaires regarding medical history, highest level of schooling completed, mental functioning (included mood-oriented items) and physical health (Medical Outcomes Study 12-Item Short Form [SF-12]), and lifestyle characteristics and had fasting morning serum collected.23 Participants were asked about prescription drugs they took daily or almost daily over the past month including benzodiazepines, narcotics, corticosteroids, selective serotonin reuptake inhibitors, trazodone, or tricyclics. Participants completed cognitive tests at the baseline and follow-up visit.
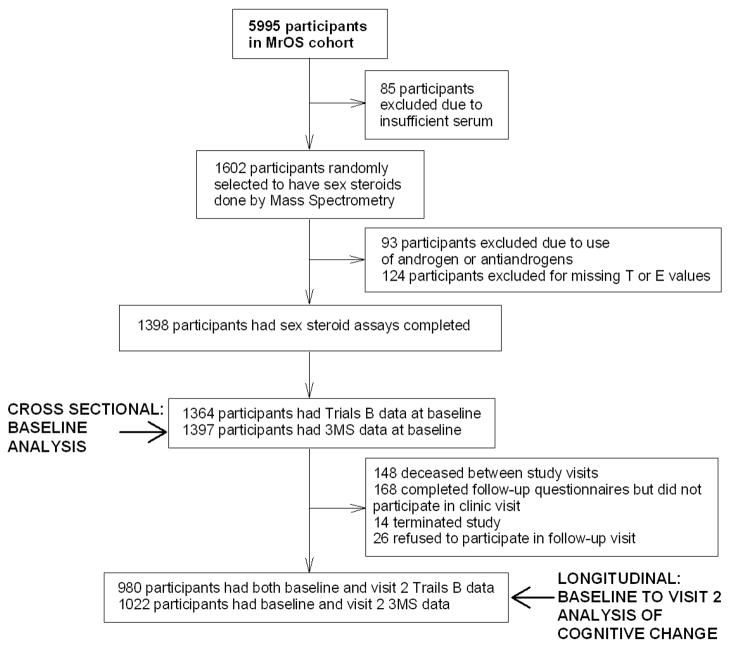
In this analysis we excluded participants taking exogenous androgens or antiandrogens, as our goal was to examine the association between endogenous sex steroid levels and cognition. Only men with complete data for specified sex hormone levels and cognitive test results were eligible (Fig.).
Figure 1.
Design for the MrOS sex steroids and cognitive performance study
Cognitive measures
To assess cognitive function, participants completed Part B of the Trail Making Test (Trails B)25 and the Modified Mental State Examination (3MS).26 Trails B is a test of executive function and motor speed.27, 28 Executive function includes those brain processes that guide action in accordance with internally generated ideas (i.e. planning and strategizing). The participant sequentially connects a series of dots shifting between numbers and letters (e.g. 1 to A to 2 to B…). Time to complete the test is measured in seconds and faster times indicate better performance. If the subject did not complete the trail within 5 minutes or made 5 mistakes, the test was stopped and a score of 300 seconds was recorded.
3MS is a screening measure for dementia that includes questions in a variety of cognitive domains. It includes the Mini Mental Status Exam (MMSE). 29 The maximum score for 3MS is 100 and higher scores indicate better performance.
Sex steroid assays
Primary sex steroid cohort (Mass Spectometry cohort)
Baseline fasting morning blood was collected for all participants. Serum was prepared immediately after phlebotomy and stored at −70°C. A random sample of 1602 men was chosen to have sex steroids measured by Gas Chromatographic Mass Spectometry (GC/MS). Total serum testosterone and estradiol were measured using a combined GC negative ionization tandem mass spectrometry (GC/NCI/MS/MS) and liquid chromatographic electrospray tandem mass spectrometry (LC/ESI/MS/MS) bioanalytical method (Taylor Technology; Princeton, NJ, USA). A 1/(concentration)2 weighted least squares regression procedure was used to fit a linear function to the calibration data. The lower limit of detection for estradiol is 0.625 pg/mL (2.29 pmol/l), and for testosterone is 25.0 pg/mL (0.09 nmol/l). Duplicate aliquots from each participant’s serum were assayed, and the two results were averaged. The intra-assay coefficient of variation (CV) for testosterone was 2.5%, interassay CV 6.0%; the estradiol intra-assay CV was 6.4%, interassay CV 10.1%. Serum SHBG concentrations were measured using an Immulite Analyzer with chemiluminescent substrate (Diagnostic Products Corp.; Los Angeles, CA, USA). The standard curve ranged from 0.2 to 180 nm/l. The SHBG intra-assay CV was 4.4%, interassay CV 6.0%. Albumin values for the free hormone calculations were obtained from baseline serum using routine colorimetric methods (interassay CV 2.0%). Calculation of the free fractions of testosterone and estradiol use the method described by Södergard et al.30
Secondary sex steroid cohort (RIA cohort)
To be able to compare our results to previous studies which used radioimmunoassay (RIA) to measure sex steroids, we also evaluated the association between sex steroids and cognition in 2623 participants who were selected using a stratified sampling design to have sex steroids measured by RIA. Strata were clinic site (each of six sites), race (white or nonwhite), and availability of a complete set of skeletal imaging procedures (to be used in future analyses of the effects of sex steroids on skeletal change).4 Sex hormones were measured using solid-phase 125I RIA (Testosterone; Diagnostic Products Corp., Los Angeles, CA) or ultra sensitive RIA (Estradiol; Diagnostic Systems Laboratory Inc., Webster, TX).4 1139 participants had sex steroid levels measured by both RIA and GC/MS. Testosterone levels by RIA and GC/MS were very highly correlated (r= 0.92). Estradiol levels were modestly correlated (r=0.52).
Statistical analysis
The analysis quantified the relationship between sex steroid levels and cognitive performance as measured by Trails B and 3MS. We hypothesized that free serum sex steroids would be the most accurate reflection of brain levels as unbound hormones can cross the blood brain barrier.31 We first excluded men who were taking androgens or antiandrogens at baseline (n=93), with missing free-T and/or free-E values (n=124), or with missing values for cognitive testing at baseline (n=34 for Trails B and n=1 for 3MS). Pearson correlation coefficients were calculated between sex steroids and cognitive performance. We then applied non-parametric generalized additive models (GAMs) to sex steroid variables and cognition scores (adjusted for age and study site) in order to identify and characterize the appropriate functional form (i.e. linear vs. nonlinear). We generated plots showing the measured data, the fitted model and the trend. These plots illustrated how well the data fit the model and also allowed the visual assessment of any non-linear trends. The GAM model did not suggestany statistically significant nonlinear relationship; therefore we explored the association between sex steroids and cognition further with linear regression. Before applying linear regression models, residuals were examined for non normality visually using quantile-quantile (Q-Q) and histogram plots. Generalized Linear Models were constructed by using sex steroids in quartiles and adjusted only for age. Age-adjusted least-squares means and 95% CIs were calculated.
The dependent variables were baseline, follow-up and change in cognitive test score and the independent variables were the continuous values of free-T, free-E, and SHBG. We also examined the association using quartiles of sex steroids as the independent variable. Because the results were similar, we present only the significance values for the analyses using the continuous sex steroid values. We repeated the above analyses using total sex steroid levels as independent variables.
We next examined whether free-T, free-E, or SHBG were risk factors for cognitive decline. Because the definition of cognitive decline by 3MS is not established, we defined decline on the 3MS as either a 5 or more34 or 10 or more32 decrease in score. For Trails B, decline was considered to be the worst decile of change between visits.33 For the MrOS cohort, this translated into a greater than 49 second increase in Trails B performance. Men who were considered to have cognitive decline at baseline (Trails B performance >250 seconds or 3MS score<80) were excluded from these analyses. We used log binomial regression to estimate regression parameters, and used a robust variance estimation procedure to get their standard errors and perform significance testing. The independent variable, continuous sex hormones, were fitted to estimate the adjusted risk ratios (expressed per 1 standard deviation as the unit change in sex steroid or SHBG) and 95% confidence intervals of significant cognitive decline. We repeated the log-binomial regression models using quartile of sex steroid or SHBG. Because the results were similar, we present the data using the continuous sex steroid values.
We considered all the demographic (age, clinic site, race, BMI, highest level of education obtained), lifestyle (alcohol use, smoking), physical and mental health (SF-12), physical activity (Physical Activity Scale for the Elderly [PASE]), self-reported healthcare provider diagnosed medical conditions (Parkinson’s disease, stroke, non-skin cancer), and medication (benzodiazepines, narcotics, corticosteroids, selective serotonin reuptake inhibitors, trazodone, or tricyclics) variables known to influence sex steroid measures and cognition test scores as potential confounding factors. During descriptive analyses, we determined which confounding factors varied according to both sex steroid level and cognitive score. These identified confounders were added into the model individually in the multivariable analysis and compared with the crude model that contained only baseline sex steroid quartiles. We took 10% change in the measure of association to identify confounding variables. For SHBG analyses, we also covaried by free-T and free-E to evaluate the independent effect of SHBG.
Finally, to enable comparisons of results to previous studies using RIA, we evaluated the association between cognitive performance and free sex steroid levels as measured by RIA. Because MrOS participants whose serum was used for sex steroid measurement by RIA were selected using a stratified sampling design, we included the strata [clinic site (each of six sites), race (white or nonwhite), and availability of a complete set of skeletal imaging procedures (to be used in future analyses of the effects of sex steroids on skeletal change)] as covariates in the analyses. To determine the robustness of our findings we performed sensitivity analyses. We compared those with the lowest free-T levels (lowest 2.5%) to those with higher free-T levels to determine if the lowest T levels impart a specific increased risk. Lastly, we examined the association between sex steroids and SHBG in the oldest men (those >74 years of age). SAS v9.1 software (Statistical Analysis System, Cary, NC) was used for all analyses.
RESULTS
Study population
Sex steroid data by MS were available on 1602 participants. After exclusions based on medical conditions or medication use, missing values for free-T or free-E, or missing values for cognitive assessments (Fig.), 1364 and 1397 participants were included in the baseline cross-sectional Trails B and 3MS analyses, respectively. Deaths (N=140), lack of participation in the follow-up clinic visit (N=202), or missing Trails B score at the follow-up visit (N=42) resulted in further sample size reductions, so that 980 and 1022 participants were included in the longitudinal Trails B and 3MS analyses. Men who completed cognitive testing at both visits had significantly higher baseline scores than those who did not (p < 0.0001). In addition, men who completed cognitive testing at both visits had slightly higher free-T at baseline than men who did not [p = 0.01; 0.28 ng/dL (N= 980) vs. 0.26 ng/dL (N = 384)]. The study participants were aged 73.6 years on average and generally well-educated, with approximately 52% having received college or higher degrees.
Men in the higher free-T quartiles were younger, had lower BMI’s, were more educated, and had higher self-rated health and physical summary scores (all p values<0.001). Race was not evenly distributed among free-T quartiles (p=0.04; Table 1); however, the differences were not systematic. Participants in the higher free-E quartiles tended to be younger (p=0.08), heavier (p<0.001) and less likely to have smoked (p<0.02) or to consume alcohol (p<0.04; data available from corresponding author upon request). Men in the highest quartile of SBHG were younger (p<0.0001), weighed less (p<0.0001), and were less likely to smoke (p<0.03; data available from corresponding author upon request).
Table 1.
Baseline Characteristics by Free Testosterone* Quartile
| Quartiles of Free T (range in nmol/l) |
|||||
|---|---|---|---|---|---|
| Baseline Characteristics | Q1 (lowest) | Q2 | Q3 | Q4 (highest) | P value† |
| N = 371 | N = 296 | N = 382 | N = 315 | ||
| (0.00 – 0.22) | (0.23 – 0.26) | (0.27 – 0.32) | (0.33 – 1.36) | ||
| Age group [Number (%§)] | 0.0002 | ||||
| 65 – 69 yrs | 87(23.5) | 85(28.7) | 124(32.5) | 119(37.8) | |
| 70 – 74 yrs | 100(27.0) | 87(29.4) | 101(26.4) | 89(28.3) | |
| 75 – 79 yrs | 99(26.7) | 85(28.7) | 86(22.5) | 69(21.9) | |
| BMI [Mean (SD)] | 28.8 (4.2) | 27.6 (3.7) | 27.0 (3.2) | 26.2 (3.2) | <0.0001 |
| Caucasian race [Number (%§)] | 346 (93.3) | 263 (88.9) | 352 (92.2) | 281 (89.2) | 0.12 |
| College graduate [Mean (SD)] | 167 (45.0) | 145 (49.0) | 212 (55.5) | 187 (59.4) | 0.0007 |
| Self-rated health [Number (%§)] | < 0.0001 | ||||
| Excellent | 108 (29.1) | 111 (37.5) | 117 (30.6) | 131 (41.6) | |
| Fair/Poor/Very poor | 75 (20.2) | 39 (13.2) | 69 (18.1) | 26 (8.3) | |
| Physical Activity Scale for the Elderly (PASE) [Mean (SD)] | 138.7 (67.4) | 145.6 (70.0) | 151.3 (72.8) | 152.3 (64.9) | 0.03 |
| Physical Summary Scale [Mean (SD)] | 46.6 (11.4) | 49.6 (10.0) | 48.2 (10.5) | 51.1 (8.7) | <0.0001 |
| Mental Summary Scale [Mean (SD)] | 55.5 (7.3) | 55.5 (6.9) | 55.8 (6.4) | 56.1 (6.1) | 0.78 |
| Ever smoked [Number (%§)] | 241 (65.0) | 198 (66.9) | 235 (61.5) | 184 (58.4) | 0.13 |
| Alcohol consumption [Number (%§)] | 0.29 | ||||
| None | 137 (36.9) | 98 (33.2) | 116 (30.4) | 92 (29.2) | |
| ≥14 drinks/week | 48 (12.9) | 34 (11.5) | 45 (11.8) | 45 (14.3) | |
| Free T nmol/l [Mean (SD)] | 0.17 (0.05) | 0.25 (0.01) | 0.29 (0.02) | 0.40 (0.10) | <0.0001 |
| Free E pmol/l [Mean (SD)] | 1.7 (0.6) | 1.9 (0.5) | 2.0 (0.6) | 2.4 (0.9) | <0.0001 |
| Total T nmol/l [Mean (SD)] | 8.6 (3.0) | 12.3 (2.5) | 15.2 (2.8) | 21.1 (5.6) | <0.0001 |
| Total E pmol/l [Mean (SD)] | 68.2 (26.4) | 78.2 (22.1) | 84.4 (25.1) | 104.2 (37.6) | <0.0001 |
| SHBG nM [Mean (SD)] | 45.2 (20.4) | 46.0 (18.7) | 50.7 (17.7) | 56.9 (19.6) | <0.0001 |
Baseline characteristics by Free Estradiol and SHBG quartiles are available from corresponding author upon request
P values for categorical variables were compared using chi-square tests for categorical variables; p values for continuous variables with normal distributions were calculated using one-way ANOVA; p values for variables with skewed distributions were calculated using Kruskal Wallis tests.
Refers to percentage of men in each quartile with that characteristic
Cognitive performance and sex steroid characteristics of participants
Baseline performance on Trails B and 3MS was related to age (all p values <0.0001); older men had worse cognitive performance. Participants did not show a decline in Trails B performance between visits (4.5 years apart); in fact there was a slight, nonsignificant improvement in performance, which is presumably a practice effect.(Table 2) 3MS performance declined between visits (Table 2). 57 (4.2%) participants achieved the maximum possible score (at floor) for Trails B. 59 (4.2%) obtained the maximum score (at ceiling) for 3MS. Participants at floor or ceiling were included in the analyses. Pearson correlations between serum levels of sex steroids and SHBG (free-T and SHBG: r = 0.16; free-E and SHBG: r = −0.14) and between free-T and free-E (r = 0.53) were modest.
Table 2.
Cognitive performance at baseline and follow-up in men who had testing performed at both visits
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Trails B mean completion time(sec) (SD) | 124.59 (51.91) | 121.96 (56.60) | 0.09 |
| 3MS score, mean (SD) | 94.05 (5.23) | 92.30 (7.17) | 0.0001 |
Association between baseline sex steroids and Trails B
There was no association between baseline free-T level and Trails B performance at either the baseline or follow-up visits in the age or multiple-variable adjusted models (Table 3A). Baseline free-T level was also not associated with change in Trails B performance between visits. Total-T showed no significant associations with Trails B performance at baseline, at follow-up, or with change in performance from baseline to follow up (all p values ≥0.63; data available from corresponding author upon request).
Table 3.
| Table 3A. Association Between Sex Steroids and Trails B performance | ||||||
|---|---|---|---|---|---|---|
| Quartile 1 (lowest) Baseline Free Testosterone | Quartile 2 Baseline Free Testosterone | Quartile 3 Baseline Free Testosterone | Quartile 4 (highest) Baseline Free Testosterone | Age adjusted p value† | Multiple-variable adjusted p value†§ | |
| (0.00 – 0.22 nMol/l) | (0.23 – 0.26 nMol/l) | (0.27 – 0.32 nMol/l) | (0.33 – 1.36 nMol/l) | |||
| N = 371* | N = 296* | N = 382* | N = 315* | |||
| Baseline Trails B mean completion time (sec), age adjusted (95% CI) | 153.2 (147.1,159.4) | 146.2 (139.1, 153.3) | 147.7 (141.4, 153.9) | 140.1 (133.1, 147.1) | 0.11 | 0.44 |
| Follow-up Trails B mean completion time (sec), age adjusted (95% CI) | 143.8 (135.4, 152.3) | 142.2 (133.8, 150.7) | 139.5 (130.4, 148.5) | 138.0 (129.0, 147.0) | 0.22 | 0.48 |
| Change in mean Trails B completion time, baselineto follow up (sec), age adjusted (95% CI) | 2.3 (−5.3, 10.0) | 2.8 (−4.8, 10.3) | 2.9 (−5.2, 11.0) | 3.2 (−4.8, 11.3) | 0.59 | 0.52 |
| Quartile 1 (lowest) Baseline Free Estradiol | Quartile 2 Baseline Free Estradiol | Quartile 3 Baseline Free Estradiol | Quartile 4 (highest) Baseline Free Estradiol | Age adjusted p value† | Multiple- variable adjusted p value†§ | |
| (0.1 – 1.5 pMol/l) | (1.5 – 1.9 pMol/l) | (1.9 – 2.3 pMol/l) | (2.3 – 9.6 pMol/l) | |||
| N = 342* | N = 334* | N = 346* | N = 342* | |||
| Baseline Trails B mean completion time (sec), age adjusted (95% CI) | 142.6 (136.1, 149.2) | 151.8 (145.2, 158.4) | 147.0 (140.4, 153.5) | 149.3 (142.6, 156.0) | 0.40 | 0.46 |
| Follow-up Trails B mean completion time (sec), age adjusted (95% CI) | 136.0 (127.4, 144.6) | 145.7 (136.8, 154.5) | 138.4 (129.9, 147.0) | 145.8 (137.1, 154.5) | 0.72 | 0.69 |
| Change in mean Trails B completion time, baseline to follow up (sec), age adjusted (95% CI) | 6.8 (−1.0, 14.6) | 0.3 (−7.7, 8.2) | 2.2 (−5.4, 9.9) | 1.4 (−6.5, 9.2) | 0.27 | 0.26 |
| Table 3B. Association Between Sex Steroids and 3MS performance | ||||||
|---|---|---|---|---|---|---|
| Quartile 1 (lowest) Baseline Free Testosterone | Quartile 2 Baseline Free Testosterone | Quartile 3 Baseline Free Testosterone | Quartile 4 (highest) Baseline Free Testosterone | Age adjusted p value† | Multiple- variable adjusted p value†§ | |
| (0.00 – 0.21 nMol/l) | (0.22 – 0.26 nMol/l) | (0.27 – 0.31 nMol/l) | (0.32 – 1.36 nMol/l) | |||
| N = 322* | N = 365* | N = 338* | N = 372* | |||
| Baseline 3MS score, age adjusted mean (95% CI) | 91.6 (90.8, 92.3) | 92.2 (91.5, 92.9) | 92.5 (91.8, 93.3) | 92.3 (91.6, 93.0) | 0.77 | 0.33 |
| Follow-up 3MS score, age adjusted mean (95% CI) | 89.2 (88.2, 90.2) | 89.6 (88.6, 90.5) | 90.1 (89.1, 91.1) | 89.7 (88.7, 90.7) | 0.68 | 0.85 |
| Change in 3MS score, baseline to follow up, adjusted mean (95% CI) | −3.3 (−4.1, −2.6) | −3.1 (−3.9, −2.4) | −2.9 (−3.7, −2.1) | −3.0 (−3.8, −2.2) | 0.69 | 0.50 |
| Quartile 1 (lowest) Baseline Free Estradiol | Quartile 2 Baseline Free Estradiol | Quartile 3 Baseline Free Estradiol | Quartile 4 (highest) Baseline Free Estradiol | Age adjusted p value† | Multiple- variable adjusted p value†§ | |
| (0.1–1.5 pMol/l) | (1.5–1.9 pMol/l) | (1.9–2.3 pMol/l) | (2.3 – 9.6 pMol/l) | |||
| N = 350* | N = 342* | N = 351* | N = 354* | |||
| Baseline 3MS score, age adjusted mean (95% CI) | 92.6 (91.9, 93.3) | 91.9 (91.2, 92.6) | 92.0 (91.3, 92.7) | 91.9 (91.2, 92.6) | 0.16 | 0.20 |
| Follow-up 3MS score, age adjusted mean (95% CI) | 90.0 (89.0, 91.0) | 89.3 (88.3, 90.3) | 89.5 (88.5, 90.4) | 89.6 (88.6, 90.6) | 0.83 | 0.75 |
| Change in 3MS score, baseline to follow up, adjusted mean (95% CI) | −3.2 (−3.9, −2.4) | −2.8 (−3.5, −2.0) | −3.1 (−3.8, −2.3) | −3.5 (−4.3, −2.7) | 0.31 | 0.32 |
Note: Lower Trails B score indicates better performance. Higher 3MS score indicates better performance.
Refers to N for visit 1. Number decreases for follow-up visit. For Trails B: free-T quartiles at follow-up: 248, 274, 229, and 247; free E: 248, 248, 251, 251. For 3MS: free-T quartiles at follow-up: 256, 277, 237, and 252; free-E: 252, 260, 256, 254.
P value calculated based on continuous sex steroid measures entered as independent variables in linear regression models.
Multiple-variable adjusted model adjusted for age group, education level (complete high school or less vs. complete college or more), race, self-rated health and alcohol consumption
Baseline free-E levels (Table 3A) and total-E levels (all p values ≥0.10; data available from corresponding author upon request) were not associated with Trails B performance at either visit or with change in performance.
Relationship between baseline sex steroids and 3MS
Baseline free-T levels were not associated with 3MS performance at baseline or follow-up or with change in 3MS performance (Table 3B). Baseline total-T levels were also not associated with 3MS performance (all p values ≥0.29; data available from corresponding author upon request). Neither baseline free-E (Table 3B) nor total-E levels (all p values ≥0.10; data available from corresponding author upon request) were associated with either 3MS performance or change in 3MS performance.
Association between SHBG and cognitive performance
In the multiple-variable adjusted model, increasing baseline SHBG levels were associated with worsening performance on Trails B and 3MS at follow-up (p=0.03 and p=0.02, respectively; Table 4A and 4B). When the association was corrected for free-T and free-E, it became stronger (p=0.006 and p=0.01; Table 4A and 4B). Trails B and 3MS performance worsened as SHBG levels increased. There was no association between baseline SHBG level and baseline cognitive performance or change in cognitive performance in the multiple-variable adjusted model.
Table 4.
| Table 4A. Association Between SHBG and Trails B Performance | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (lowest) SHBG | Quartile 2 SHBG | Quartile 3 SHBG | Quartile 4 (highest) SHBG | Age adjusted p value† | Multiple-variable adjusted p value†§ | Adjusted for free-T and free-E†§ | |
| (12.2–35.7 nM) | (35.8–46.6 nM) | (46.7–59.2 nM) | (59.3–179.0 nM) | ||||
| N = 343* | N = 339* | N = 342* | N = 340* | ||||
| Baseline Trails B mean completion time (sec), age adjusted‡ (95% CI) | 147.2 (140.3, 154.2) | 142.4 (135.7, 149.2) | 150.6 (144.0, 157.2) | 149.3 (143.0, 155.6) | 0.19 | 0.10 | 0.02 |
| Follow-up Trails B mean completion time (sec), age adjusted‡ (95% CI) | 138.0 (128.9, 147.0) | 133.8 (124.8, 142.8) | 142.1 (133.5, 150.8) | 146.8 (138.6, 155.1) | 0.05 | 0.03 | 0.006 |
| Change in mean Trails B completion time, baseline to follow up (sec), age adjusted‡ (95% CI) | −2.5 (−10.6, 5.6) | 1.2 (−6.9, 9.4) | 3.4 (−4.4, 11.1) | 6.4 (−1.0, 13.9) | 0.14 | 0.25 | 0.27 |
| Table 4B. Association Between SHBG and 3MS Performance | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (lowest) SHBG | Quartile 2 SHBG | Quartile 3 SHBG | Quartile 4 (highest) SHBG | Age adjusted p value† | Multiple-variable adjusted p value†§ | Adjusted for free-T and free-E†§ | |
| (12.2–35.7 nM) | (35.8–46.5 nM) | (46.7–59.1 nM) | (59.2–179.0 nM) | ||||
| N = 350* | N = 350* | N = 347* | N = 350* | ||||
| Baseline 3MS score, age adjusted mean ‡ (95% CI) | 92.5 (91.8, 93.3) | 91.8 (91.1, 92.6) | 92.1 (91.4, 92.8) | 92.1 (91.4, 92.8) | 0.54 | 0.56 | 0.50 |
| Follow-up 3MS score, age adjusted mean‡ (95% CI) | 90.4 (89.3, 91.4) | 89.9 (88.8, 90.9) | 89.8 (88.8, 90.8) | 88.8 (87.9, 89.8) | 0.02 | 0.02 | 0.01 |
| Change in 3MS score, baseline to follow up, adjusted mean‡ (95% CI) | −2.8 (−3.6, −2.0) | −2.8 (−3.6, −2.0) | −2.8 (−3.5, −2.0) | −3.7 (−4.5, −3.0) | 0.06 | 0.07 | 0.01 |
Note: Lower Trails B score indicates better performance. Lower 3MS score indicates worse performance.
Refers to N for visit 1. Number decreases for follow-up visit. For SHBG and Trails B: 248, 251, 250, 249. For SHBG and 3MS : 257, 253, 256, 256.
P value calculated based on continuous sex steroid measures entered as independent variables in linear regression models.
Multiple-variable adjusted model adjusted for age group, education level (complete high school or less vs. complete college or more), race, self-rated health and alcohol consumption
Mutliple-variable adjusted means (95% CI) are as follows: Baseline Trails B (sec) Q1 162.4 (151.9, 172.8), Q2 159.9 (149.2, 170.7), Q3 166.8 (156.4, 177.3), Q4 166.4 (156.3, 176.6); Follow-up Trials B (sec) Q1 152.8 (139.7, 165.9), Q2 151.5 (137.9, 165.1), Q3 158.2 (145.0, 171.3), Q4 163.1 (150.0, 176.1); Change in mean Trails B completion time Q1 0.3 (−12.2, 12.7), Q2 3.9 (−9.0, 16.8), Q3 5.3 (−7.1, 17.7), Q4 8.2 (−4.2, 20.6); Baseline 3MS score Q1 89.7 (88.5, 90.8), Q2 88.7 (87.5, 89.8), Q3 89.1 (88.0, 90.3), Q4 89.1 (88.0, 90.3); Follow-up 3MS score Q1 86.6 (85.0, 88.1), Q2 85.7 (84.1, 87.3), Q3 85.8 (84.3, 87.4), Q4 85.0 (83.5, 86.5); Change in mean 3MS score Q1 −4.1 (−5.3, −2.8), Q2 −4.1 (−5.4, −2.8), Q3 −4.0 (−5.3, −2.8), Q4 −4.9 (−6.2, −3.7).
However, when free-T and free-E were added to the model, higher SHBG was associated with worse baseline Trails B performance (p=0.02; Table 4A) and more decline on 3MS performance between visits (p=0.01; Table 4B). The results are similar when further adjusted for insulin levels.
Effects of sex steroids and SHBG on cognitive decline
We examined whether free sex steroid and SHBG levels were risk factors for cognitive decline (Table 5), defined as a 49 second or more increase in Trails B performance33 or either a 5 or greater34 or 10 or greater point decline on 3MS.32 Free-T and free-E levels were not associated with cognitive decline as measured by Trails B or 3MS. However, every SD increase in SHBG was associated with a 1.18-fold higher risk of cognitive decline on Trails B (95% CI 1.01, 1.37) and a 1.07 (for 5 point decline) to 1.25-fold (for 10 point decline) higher risk of cognitive decline on 3MS (95% CI 0.97, 1.19 for 5 point decline and 95% CI 1.06, 1.49 for 10 point decline) after multiple-variable adjustment. The risks increased slightly after further adjustment for free-T and free-E [RR 1.23 (95% CI 1.07, 1.41) for Trails B; RR 1.13 (95% CI 1.02, 1.26) for 3MS by 5 point decline; and RR 1.32 (95% CI 1.11, 1.58) for 3MS by 10 point decline]. This translated into those in the highest SHBG quartile having a 1.8 fold increased risk of cognitive decline on Trails B (RR 1.81; 95% CI 1.05–3.14) and a 2-fold increased risk of a 10 point decline on 3MS (RR 2.02, 95% CI 1.0–4.07) compared to those in the lowest quartile. Adjustment for insulin levels did not alter the results.
Table 5.
Risk of cognitive decline according to sex hormone or SHBG quartile
| Baseline Sex Steroid or SHBG | |||
|---|---|---|---|
| RR (95% CI) | Free Testosterone | Free Estradiol | SHBG |
| Relative Risk of clinically important decline (>49 seconds) in Trials B per SD change in sex steroid or SHBG | |||
| Age- adjusted | 0.89 (0.74,1.07) | 0.97 (0.82, 1.16) | 1.19 (1.03, 1.38) |
| Multiple-variable adjusted* | 0.87 (0.73,1.05) | 0.95 (0.80, 1.14) | 1.18 (1.01, 1.37) |
| Multiple-variable and free T, free E adjusted | N/A | N/A | 1.23 (1.07, 1.41)† |
| Relative Risk of clinically important decline in 3MS (−5 as the cut point) per SD change in sex steroid or SHBG | |||
| Age- adjusted | 0.94 (0.83, 1.07) | 1.06 (0.97, 1.17) | 1.08 (0.98, 1.19) |
| Multiple-variable adjusted* | 0.94 (0.82, 1.07) | 1.07 (0.97, 1.18) | 1.07 (0.97, 1.19) |
| Multiple-variable and free T, free E adjusted | N/A | N/A | 1.13 (1.02, 1.26)† |
| Relative Risk of clinically important decline in 3MS (−10 as the cut point) per SD change in sex steroid or SHBG | |||
| Age-adjusted | 1.06 (0.87, 1.28) | 1.14 (0.99, 1.31) | 1.23 (1.06, 1.44) |
| Multiple-variable adjusted* | 1.07 (0.87, 1.31) | 1.15 (0.99, 1.35) | 1.25 (1.06, 1.49) |
| Multiple-variable and free T, free E adjusted | N/A | N/A | 1.32 (1.11, 1.58)† |
Footnote for Table 5:
Note: For Trails B analyses, the size of the analytic sample is 944, after 36 men with baseline completion time ≥ 251 seconds were excluded. The size of the 3MS analytic sample is 1001, after 21 men with baseline 3MS score < 80 were excluded.
Multiple-variable RR adjusted for age groups, race, education level (complete high school or less vs. complete college or more), self-reported health and alcohol consumption
These RR per SD change in SHBG translate into the following relative risks when highest SHBG quartile is compared to lowest SHBG quartile: 1.81 (95% CI 1.05, 3.14) fold increased risk of clinically important decline on Trails B; 1.39 (95% CI 0.99, 1.96) fold increased risk of 5 point decline 3MS; 2.02 (95% CI 1.00, 4.07) fold increased risk of 10 point decline on 3MS.
Sensitivity Analyses: RIA cohort
We also examined the association between cognitive performance and sex steroids as measured by RIA. As in the GC/MS cohort, there was no association between free-T and free-E and cognitive performance. However, the association between Trails B performance and SHBG was stronger in the larger RIA cohort. We found that higher SHBG was associated with worse performance on Trails B at both the baseline and follow-up visit (p=0.002 and p<0.001, respectively) and that those with higher SHBG had more decline on Trails B between visits (p=0.01). There was a borderline (p=0.05) association between SHBG and 3MS at baseline in the RIA cohort.
Men with lowest T levels
To examine whether there was a threshold effect, we divided men into hypogonadal [free T in lowest 2.5% (mean free-=0.1 ng/dl (0.0 nmol/L); N=430) and eugonadal (mean free-T=0.3 ng/dl (0.1 nmol/L); N=1321)] categories. Hypogonadal and eugonadal men had similar baseline and follow-up Trails B and 3MS performance and comparable change in cognitive performance over time.
Additional sensitivity analyses
We examined the relationship between free sex steroids and cognitive performance in the oldest group of participants (age ≥75 years; N=518). The findings in the oldest men were similar to the results in men overall. When only men who had cognitive testing performed at both visits (longitudinal cohort) were analyzed, the results were essentially unchanged. Adding physical activity to the models did not change the results.
DISCUSSION
We found no evidence of a relationship between testosterone or estradiol and cognition as measured by Trails B or 3MS. Higher baseline SHBG was associated with worse follow-up cognitive performance and more cognitive decline.
Despite cellular and animal data suggesting sex steroids act in brain regions important for cognition,8–11, 35–38 clinical studies are conflicting and thus inconclusive. Of seven 12–15, 17, 20, 21 previous studies, only the Baltimore Longitudinal Study of Aging (BLSA) found an association of free-T with Trails B.13 In Rancho Bernardo, a U-shaped relationship was reported between total estradiol and Trails B;20 however, other studies did not confirm this.12, 14, 15, 17 In both BLSA and Rancho Bernardo, sex steroids were measured using RIA, which is inaccurate at low values.39, 40
One study found that higher bioavailable T levels were less likely to be associated with low MMSE scores12 but others have not.13, 15, 17, 18, 20, 21 Although in Rancho Bernardo higher estradiol was associated with worse MMSE performance,20 other studies have not confirmed this.12, 14, 15, 17, 18 However, mean MMSE scores were close to the upper limit (27–29 out of 30), which could have precluded finding a significant association (ceiling effect). Only 4% of our participants were at ceiling; their mean score was 94 (maximum 100). 3MS significantly declined over time, suggesting adequate sensitivity to detect cognitive changes. Therefore, our null findings are unlikely from ceiling effects.
We did not find worse cognition in hypogonadal men. This contrasts with BLSA where men with the lowest 2.5% bioavailable-T, as measured by RIA, had lower Trails B scores.13 BLSA participants were approximately 10 years younger than MrOS participants. We were unable to evaluate whether each participant had their own critical level below which cognition is affected.
We examined the association of sex steroid levels with cognition in the oldest men, i.e. those >74 years of age, because sex steroids may affect aging brains differently. Although one study found a positive linear association between testosterone and Trails B in the oldest group,12 we did not find different effects in the oldest group compared to the entire cohort.
Previous studies have found no association between total or free testosterone and Trails B or MMSE performance assessed 1–8 years later.13, 15, 20 Estradiol was associated with prospective MMSE and Trails B performance in Rancho Bernardo.20 In our study, free-T and free-E levels did not predict cognition 4.5 years later or cognitive performance change over that time period. In BLSA, higher testosterone was associated with poorer visual memory but not executive function decline over 10 years.13 Together our data suggest that currently circulating free-T and free-E levels do not influence future cognition or change in cognition; however, we did not measure testosterone levels at follow-up and only MMSE, and not Trails B, performance declined significantly during the study.
Previous data on the association between SHBG and cognition are scant.14, 15, 17 Higher SHBG levels were recently shown to be associated with increased dementia risk.41 SHBG likely has effects beyond its carrier protein role by amplifying sex steroid sufficiency or deficiency42 or having independent effects.43 SHBG has been shown to stimulate a membrane-associated receptor in the brain43 and could thereby directly influence intracellular signaling. Such signaling may require SHBG-sex steroid interactions42, 44 or use a megalin-mediated endocytic pathway involving unbound SHBG.42, 45 However, SHBG could be a marker for other factors affecting cognition. Lower insulin, lower IGF-1, and aging increase SHBG, while obesity, frailty, and nutritional status decrease SHBG.46, 47 In our study, we examined physical health, self-rated health, BMI, age, and insulin levels as confounders and did not find they altered the association. A possible association between SHBG and cognitive function is intriguing and should be explored in future studies.
To our knowledge, this is one of the largest studies to address associations between cognition and sex steroids in elderly men and the first to use GC/MS to measure sex steroids. GC/MS is reasonably accurate at the low concentrations seen in elderly men.39, 40 Cognitive testing was carefully performed and potentially important confounding variables, including age, education, medications, medical history, mental functioning, and physical health, were evaluated. Many men were over age 80 years, a segment of the population that is increasing steadily but has not been well studied.
There are several limitations. The primary outcome of MrOS was fractures, not cognition. For Trails B, we did not utilize a control for speed (Trails A). We could not have distinguished effects on executive function from speed. Because there was no decline on Trails B, detecting associations between cognitive change and sex steroids was difficult. 3MS was not normally distributed and 3MS change was narrowly distributed. Sex steroids could be related to cognitive domains not tested in this study (visuo-spatial function or memory).48–51 For longitudinal analyses, we excluded men without follow-up cognitive measures. Participants who were not studied may have had less cognitive decline, biasing our results to the null. We did not measure inflammatory markers, which can influence cognition. Free sex steroid levels were calculated and not directly measured;30 however, calculation using mass action equations is comparable to direct measurement by equilibrium dialysis.52, 53 Finally, we did not measure hormone level changes.
In conclusion, testosterone and estradiol were not related to cognitive performance in a large cohort of elderly men. The large sample size and accurate sex steroid levels make it unlikely we missed important associations between native, endogenous sex steroid levels and cognitive performance on Trails B or MMSE in elderly men. High SHBG deserves further examination as a risk factor for cognitive decline.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Footnotes
Competing interests/financial disclosure:
ESL: Nothing to disclose, PYW: Nothing to disclose, JSJ: Nothing to disclose, MBN: Nothing to disclose, HAF: Nothing to disclose, KY: Served on the Women’s Health Initiative OSMB, LMM: Nothing to disclose, JAL: Nothing to disclose, MLS: Nothing to disclose, ESO: Nothing to disclose
References
- 1.Vermeulen A. Clinical review 24: Androgens in the aging male. J Clin Endocrinol Metab. 1991;73:221–224. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- 2.Davidson JM, Chen JJ, Crapo L, et al. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab. 1983;57:71–77. doi: 10.1210/jcem-57-1-71. [DOI] [PubMed] [Google Scholar]
- 3.Bardin CW, Swerdloff RS, Santen RJ. Androgens: risks and benefits. J Clin Endocrinol Metab. 1991;73:4–7. doi: 10.1210/jcem-73-1-4. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Daselaar SM, Dolcos F, et al. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 6.Gabrieli JD. Memory systems analyses of mnemonic disorders in aging and age-related diseases. Proc Natl Acad Sci USA. 1996;93:13534–13540. doi: 10.1073/pnas.93.24.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wecker NS, Kramer JH, Wisniewski A, et al. Age effects on executive ability. Neuropsychology. 2000;14:409–414. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 8.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leranth C, Prange-Kiel J, Frick KM, et al. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- 10.Melcangi RC, Celotti F, Ballabio M, et al. Testosterone 5 alpha-reductase activity in the rat brain is highly concentrated in white matter structures and in purified myelin sheaths of axons. J Steroid Biochem. 1988;31:173–179. doi: 10.1016/0022-4731(88)90051-9. [DOI] [PubMed] [Google Scholar]
- 11.Almeida OP, Waterreus A, Spry N, et al. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Muller M, Aleman A, Grobbee DE, et al. Endogenous sex hormone levels and cognitive function in aging men: is there an optimal level? Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- 13.Moffat SD, Zonderman AB, Metter EJ, et al. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- 14.Martin DM, Wittert G, Burns NR, et al. Testosterone and cognitive function in ageing men: data from the Florey Adelaide Male Ageing Study (FAMAS) Maturitas. 2007;57:182–194. doi: 10.1016/j.maturitas.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Lessov-Schlaggar CN, Reed T, Swan GE, et al. Association of sex steroid hormones with brain morphology and cognition in healthy elderly men. Neurology. 2005;65:1591–1596. doi: 10.1212/01.wnl.0000184512.08249.48. [DOI] [PubMed] [Google Scholar]
- 16.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Lui LY, Zmuda J, et al. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Fonda SJ, Bertrand R, O’Donnell A, et al. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 21.Perry PJ, Lund BC, Arndt S, et al. Bioavailable testosterone as a correlate of cognition, psychological status, quality of life, and sexual function in aging males: implications for testosterone replacement therapy. Ann Clin Psychiatry. 2001;13:75–80. doi: 10.1023/a:1016663523579. [DOI] [PubMed] [Google Scholar]
- 22.Committee on Assessing the Need for Clinical Trials of Testosterone, T. Testosterone and Aging. The National Academies Press; 2003. [Google Scholar]
- 23.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 27.Kortte KB, Horner MD, Windham WK. The trail making test, part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9:106–109. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- 28.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 29.Bassuk SS, Murphy JM. Characteristics of the Modified Mini-Mental State Exam among elderly persons. J Clin Epidemiol. 2003;56:622–628. doi: 10.1016/s0895-4356(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 30.Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 31.Pardridge WM, Mietus LJ, Frumar AM, et al. Effects of human serum on transport of testosterone and estradiol into rat brain. Am J Physiol. 1980;239:E103–E108. doi: 10.1152/ajpendo.1980.239.1.E103. [DOI] [PubMed] [Google Scholar]
- 32.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20:485–503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48:M152–M161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 34.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 35.Beyenburg S, Watzka M, Clusmann H, et al. Androgen receptor mRNA expression in the human hippocampus. Neurosci Lett. 2000;294:25–28. doi: 10.1016/s0304-3940(00)01542-1. [DOI] [PubMed] [Google Scholar]
- 36.Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- 37.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 38.Ramsden M, Nyborg AC, Murphy MP, et al. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003;87:1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 39.Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 40.Stanczyk FZ, Cho MM, Endres DB, et al. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68:1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Muller M, Schupf N, Manly JJ, et al. Sex hormone binding globulin and incident Alzheimer’s disease in elderly men and women. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175:113–120. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell JD, Suleman F, Chou SH, et al. Emerging roles of steroid-binding globulins. Horm Metab Res. 2006;38:206–218. doi: 10.1055/s-2006-925328. [DOI] [PubMed] [Google Scholar]
- 44.Rosner W, Hryb DJ, Khan MS, et al. Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids. 1999;64:100–106. doi: 10.1016/s0039-128x(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 45.Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 46.Legrand E, Hedde C, Gallois Y, et al. Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone. 2001;29:90–95. doi: 10.1016/s8756-3282(01)00478-1. [DOI] [PubMed] [Google Scholar]
- 47.Evans SF, Davie MW. Low body size and elevated sex-hormone binding globulin distinguish men with idiopathic vertebral fracture. Calcif Tissue Int. 2002;70:9–15. doi: 10.1007/s00223-001-2018-6. [DOI] [PubMed] [Google Scholar]
- 48.Cherrier MM, Matsumoto AM, Amory JK, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 49.Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 50.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 51.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 52.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 53.Morley JE, Patrick P, Perry HM., III Evaluation of assays available to measure free testosterone. Metabolism. 2002;51:554–559. doi: 10.1053/meta.2002.31975. [DOI] [PubMed] [Google Scholar]