Abstract
Dyskeratosis congenita (DC) is characterized by the triad of reticulate skin pigmentation, nail dystrophy, and leukoplakia. Epidermal atrophy, hair growth defects, bone marrow failure, and increased risk of cancer are also common in DC patients. DC is caused by mutations in genes encoding for telomerase complex factors. Although there is an association of epidermal abnormalities with DC, epidermal cells from DC donors have not been previously characterized. We have isolated skin keratinocytes from affected members of a family with an autosomal dominant form of DC that is due to a mutation in the RNA component of telomerase, TERC. Here we demonstrate that, similar to DC fibroblasts from these donors, DC keratinocytes have short telomeres and a short lifespan. DC keratinocytes also exhibited impaired colony forming efficiency and migration capacity. Exogenous expression of the reverse transcriptase component of telomerase, TERT, activated telomerase levels to half that of TERT expressing normal cells and maintained telomeres at a short length with concomitant extension of lifespan. Unlike fibroblasts, transduction of human papillomavirus type 16 E6/E7 genes into DC keratinocytes activated telomerase to half that of E6/E7 expressing normal cells, and robust proliferation was observed. While expression of TERC has no measurable effect on telomerase in fibroblasts, expression of TERC in keratinocytes upregulated telomerase activity and, rarely, allowed rescue of proliferative defects. Our results point to important differences between DC fibroblasts and keratinocytes and show, for the first time, that expression of TERC can increase the lifespan of primary human epithelial cells.
Keywords: Dyskeratosis, Telomerase, Telomeres, Keratinocytes, TERC
INTRODUCTION
Telomeres consist of hexameric tandem repeats (TTAGGG) of DNA located at the chromosome ends and are necessary for maintaining chromosome integrity, function, and replication (1, 2). In the absence of a mechanism to maintain them, telomeres normally shorten with each cell division and with aging (3). Telomeres can be maintained by the telomerase complex, which minimally consists of an RNA component called TERC (hTERC or mTERC for the human or mouse gene, respectively; also often referred to as TR or TER) that acts as a template for a reverse transcriptase component, TERT, that catalyzes the addition of telomere repeats to the telomere ends (4, 5). Other protein components such as dyskerin, a nucleolar ribonucleoprotein with pseudouridinylation properties, bind to the telomerase complex to stabilize and affect its localization and regulate its activity (6). Telomerase is active in human germline cells and most cancers (7, 8). Most normal human somatic cells express TERC but have low to undetectable levels of TERT and low levels of telomerase (8). Epidermal skin cells and hematopoietic cells have some telomerase activity (9–11).
Dyskeratosis congenita (DC) is a premature aging syndrome characterized by the triad of reticulated skin hyperpigmentation, nail dystrophy, and oral leukoplakia, and most patients die as a result of bone marrow failure (12–15). A variety of other somatic abnormalities normally seen in aged individuals have also been reported in DC, including hair loss, epidermal atrophy, gray hair, osteoporosis, and pulmonary and hepatic fibrosis (15–17). DC patients also display an increased risk for malignancy (18). DC is due to mutations in genes involved in telomerase or telomere function (16, 17). The DKC gene involved in the X-linked version of the disease encodes dyskerin, which interacts with TERC and is required for its accumulation (6, 19–22). Autosomal dominant forms of DC (AD DC), can be caused by mutations in TERC or TERT (23–25). In these families, one allele of TERC or TERT is generally made nonfunctional by point mutation or deletion and this leads to haploinsufficiency for telomerase and short telomeres. Other mutations in telomerase component genes and telomere binding proteins have also recently been detected in DC patients (17).
Previous studies from our laboratory have shown that fibroblasts from DC donors from a family with a deletion of a single alleles of TERC have very short telomeres, a short lifespan, and proliferative defects (26). Despite the clear association of epidermal and mucosal abnormalities with DC, no studies have been performed using epidermal cells from DC donors. Fibroblasts and epidermal cells are known to differ in telomerase regulation and proliferation. For example, keratinocytes have some active telomerase, due to expression of TERT, but telomerase in fibroblasts is not detectable (27). Exogenous expression of TERT has been shown to immortalize both fibroblasts and keratinocytes, when grown in the right growth conditions (28–30). Expression of TERC alone has no detectable effects on telomerase or proliferation in primary fibroblasts (26, 31) and there have been no reports on the effects of TERC in keratinocytes or other primary epithelial cells. Here, we have isolated and characterized skin keratinocytes from affected donors of a TERC deficient DC family. We hypothesized that the DC keratinocytes would differ from DC fibroblasts because of intrinsic differences in telomerase biology between keratinocytes and fibroblasts. We found that, like DC fibroblasts, DC keratinocytes exhibited both proliferative and functional defects. Exogenous expression of TERT alone activated telomerase and extended the lifespan of DC keratinocytes. Unlike DC fibroblasts, exogenous expression of HPV-16 E6/E7 or the RNA component, TERC, activated telomerase in DC keratinocytes and provided some measure of rescue of proliferative defects. To our knowledge, this is the first characterization of skin keratinocytes from DC patients and the first demonstration that TERC alone can increase telomerase activity and the lifespan of primary human cells.
METHODS
Cell Isolation and Culture
Keratinocytes were isolated as previously described from punch biopsies of the skin from the buttocks region of AD DC and normal donors (26, 32). The punch biopsies were from third and a second generation adult DC subjects (DC-HSK-1 and DC-HSK-4). The studies were approved by the University of Iowa Institutional Review Board for human studies and conformed to protocols outlined by the Declaration of Helsinki. Donors gave their written, informed consent for collection of tissue. Normal sex and age (within 1 year) matched biopsies were used as controls (referred to as N-HSK-1 and N-HSK-2). Briefly, the tissue was incubated overnight in dispase and on the following day, the epidermal layer was treated with trypsin to dissociate epithelial cells. The cells were plated on irradiated J2 3T3 feeders in E media as described (33, 34). When 80% confluent, the cells were passed 1:4 (approximately 2 population doublings per passage). Passaging of cells was done by differentially removing feeder fibroblasts with a low percent trypsin/EDTA followed by complete trypsinization of remaining epithelial cells. For certain procedures, such as isolation of DNA, epithelial cells were passaged into keratinocyte serum free media (KSFM) for two passages to remove feeders before collection of cells.
CFE and In Vitro Scratch Assay
For the colony forming efficiency (CFE) assay, feeder cells were differentially removed with low percent trypsin and washes with PBS. Epithelial cells were collected with further trypsinization and counted. The cells were plated at 200 cells per 60 mm plate in triplicate. Irradiated feeders (0.5 million/plate) were added to each plate and cells were media-changed every 3rd day. On the 12th day, colonies were stained by first fixing in 95% ethanol, followed by staining with 1% methylene blue in 50% ethanol, followed by a water rinse. Only those colonies that were 50 cells or more were counted. Percent CFE was calculated as the number of colonies divided by the total number of cells plated times 100. Average and standard error were calculated from triplicate samples.
For the scratch wound migration assay, cells were grown to 85–90% confluency in E-media and plates were marked so that a grid on the bottom of the plate could be used to keep track of location. The cultures were then scratched with 4 scrapes using a 200 ul yellow pipette tip. Images were captured for each cell type immediately after the scrapes were performed (0 hrs) followed by monitoring of scrape closure by taking digital photographs of the same locations over a time course. The width of each scrape was measured for each of the 4 images at different time points and the average percent closure (as compared to 0 hours) was calculated along with standard error of the mean.
Retroviral Constructs, Virus Generation, and Transduction
For expression of HPV-16 E6E7, cells were transduced with HPV-16 E6/E7 LXSN replication defective retrovirus and selected in G418. The HPV-16 E6/E7 LXSN construct has been previously described (35). The TERT-cherry construct was generated by cloning full-length hTERT into a pHIV7 vector (36). Expression of TERT was driven by a spleen focus forming virus promoter (SF promoter). Expression of mCherry was accomplished through an IRES. The TERC-Cherry vector was generated by cloning TERC gene, plus the 3′ genomic region, driven by a U3 promoter followed by Cherry driven by an SF promoter. Virus was produced in 293 packaging lines by the University of Iowa Gene Vector Core using established protocols (36). Cells were infected at 5 MOI. For neomycin vectors, cells were selected in G418. For the mCherry vectors, cells were sorted by FACS by gating for cherry fluorescence.
Southern Blotting, Telomerase Assay, and Quantitative Reverse Transcriptase PCR
Southern blotting was performed as previously described using 3 ug of genomic DNA digested with RsaI/HinfI (37). Digested DNA was separated on a 0.7% agarose gel, transferred to Hybond N membrane (Amersham), and fixed with UV by a Stratalinker (Stratagene). Telomere signal was detected using a DIG labeled telomere sequence probe followed by chemiluminescence detection methodology from Roche according to the manufacturer’s protocol (Roche). Telomere length was calculated by finding the midpoint of the telomere signal and comparing to DIG labelled size standards run on the same gel.
The telomerase assays were performed as previously described using a quantitative PCR methodology (26). One thousand cells were used per assay and each assay was performed in quadruplicate and average values were calculated along with standard error. Results were calibrated to a standard curve and made relative to the telomerase activity detected in normal skin keratinocytes for comparison.
Quantitative reverse transcriptase PCR (q-RT-PCR) was performed as described (26). Briefly, total RNA was extracted from cells grown for one passage without feeders using Trizol Reagent (Invitrogen) according to the manufacturer’s instructions and purified with RNeasy Mini Columns (Qiagen). RNA was reverse transcribed using a Retroscript kit (Ambion) with random decamers as recommended by the manufacturer. Quantitative PCR was performed in triplicate using the ABI PRISM 7900 Sequence Detection System with standard cycling in SYBR-Green PCR Master Mix (Applied Biosystems) with the following primers:
TERC-F, 5′ TCTAACCCTAACTGAGAAGGGCGTAG-3′;
TERC-R, 5′ GTTTGCTCTAGAATGAACGGTGGAAG-3′;
TERT-v1-F, 5′-TGTACTTTGTCAAGGTGGATGTGA-3′;
TERT-v1-R, 5′-GCTGGAGGTCTGTCAAGGTAGAG-3′;
GAPDH-F, 5′-AAGGTCATCCATGACAACTTTG-3′;
GAPDH-R, 5′-GTAGAGGCAGGGATGATGTTCT-3′
Expression levels were normalized to GAPDH and made relative to normal untransduced N-HSK-1 cells.
RESULTS
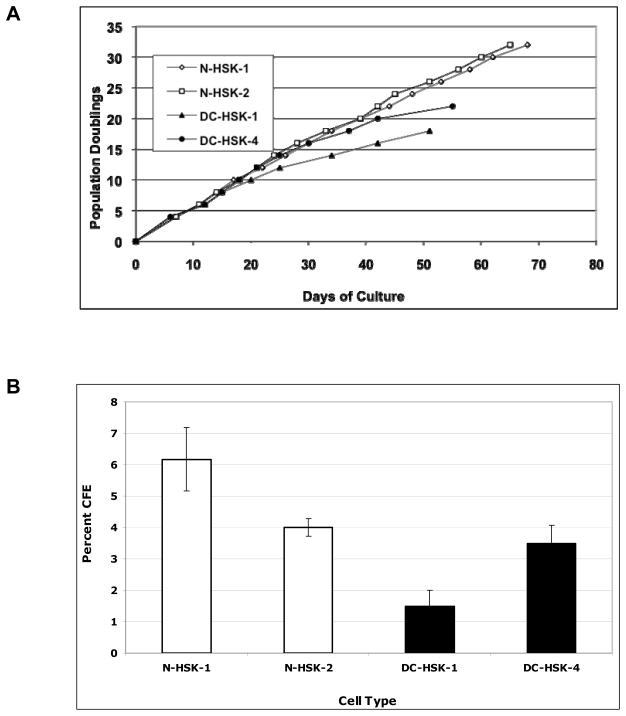
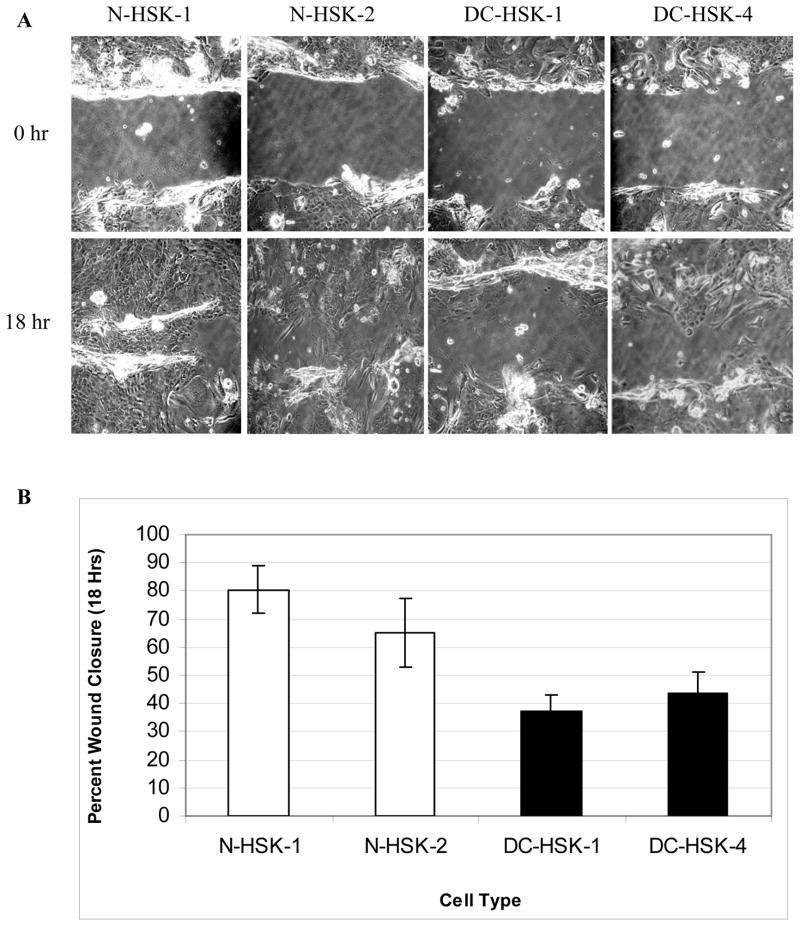
We isolated and cultured skin keratinocytes from skin punch biopsies of third and second generation DC individuals (DC-HSK-1 and DC-HSK-4, respectively) and age/sex matched controls (N-HSK-1 and N-HSK-2) for our analysis (Table I). Affected members of the DC family have a mutation in the TERC gene that results in deletion of the last 74 bases of the TERC RNA (23). Initially, DC-HSK proliferated at rates similar to their normal counterparts, but the lifespan of the DC-HSK cells was shorter than that of the normal controls (Figure 1a and Table I). Using a colony forming efficiency (CFE) assay, we found that keratinocytes from both the 2nd and 3rd generation donors had significantly less CFE than normal counterparts (Figure 1b and Table I). Not only were there fewer colonies in the DC plates but also the colonies were smaller, overall (data not shown). The least CFE was noted for cells from the 3rd generation DC-HSK-1 cells while the best CFE was noted for N-HSK-1 (2 percent and 7 percent, respectively). We also assessed migration ability using a scratch wound assay. For this assay, we chose a time point of 18 hours for comparison since normal keratinocytes exhibited ~60–80% closure at this time but were closed completely at 24 hours (example photographs of the scratch assay at 0 and 18 hrs. are shown in figure 2a). DC-HSKs exhibited defects in migration and were only 30–50% closed at 18 hours (figure 2b and Table I). As with CFE, the cells from the 3rd generation donor (DC-HSK-1) exhibited the greatest defect and the normal N-HSK-1 cells had the most rapid closure. Flow cytometric analysis of cell cycle profiles of subconfluent cells indicated that the differences in wound closure capacity between DC and normal keratinocytes were not due to an overall block in cell cycle or proliferation (data not shown). Overall, our results indicate that DC-HSKs are defective in their ability to proliferate and in their ability to migrate in a scratch wound assay.
Table I.
Characteristics of primary skin keratinocytes from dyskeratosis congenita donors compared to normal donors
| Primary Keratinocyte Strain | Donor Characteristicsa | Maximum Lifespan in Cultureb | Percent Colony Forming Efficiencyc | Percent Wound Closure (18 Hours)d | Average Telomere Lengthe |
|---|---|---|---|---|---|
| N-HSK-1 | Normal/M/40 yrs | 32 pd | 6.2 +/− 1.0 | 80.4 +/− 8.2 | 10.1 kb |
| N-HSK-2 | Normal/F/22 yrs | 32 pd | 4.0 +/− 0.3 | 65.0 +/− 12.1 | 9.6 kb |
| DC-HSK-1 | DC/F/21 yrs/3rd gen. | 18 pd | 1.5 +/− 0.5 | 37.1 +/− 5.9 | 5.9 kb |
| DC-HSK-4 | DC/M/40 yrs/2nd gen. | 22 pd | 3.5 +/− 0.6 | 43.4 +/− 7.6 | 6.2 kb |
Keratinocytes were obtained from either normal or affected dyskeratosis congenita (DC) donors. The sex (M or F), age of the donor, and generation (if applicable) are shown.
Maximum in vitro lifespan in population doublings (pd)(see figure 1a).
Colony forming efficiency on irradiated feeders (see figure 1b)
Percent scratch wound closure 18 hours compared to 0 hours (see figure 2).
Average telomere length estimated from Southern blot analysis (see figure 3a).
Figure 1. Keratinocytes from dyskeratosis congenita (DC) donors have proliferative defects as compared to keratinocytes from age and sex matched normal donors.
A. Population doublings of skin keratinocytes grown with irradiated feeder fibroblasts. Cells were passaged as described in Materials and Methods. DC-HSK-1 and DC-HSK-4 are keratinocytes from 3rd and 2nd generation affect DC subjects, respectively. N-HSK-1 and N-HSK-2 are sex and age matched keratinocytes from a normal donors. DC-HSK-1 is age and sex matched with N-HSK-2 and DC-HSK-4 is age and sex matched with N-HSK-1.
B. Colony forming efficiency (CFE) of cells grown on irradiated feeder fibroblasts. CFE assays were performed as described in Materials and Methods. Error bars represent standard error based on three replicates.
Figure 2. Primary dyskeratosis congenita keratinocytes exhibit slower closure of scratch wounds in vitro.
A. Confluent cultures were scraped with a pipette tip and followed over time to assess closure as described in Materials and Methods. Representative photos of 0- and 18-hour time point are shown.
B. Average percent scratch wound closure at 18 hours as compared to 0 hour time point. Error bars represent standard error of four replicate scratches.
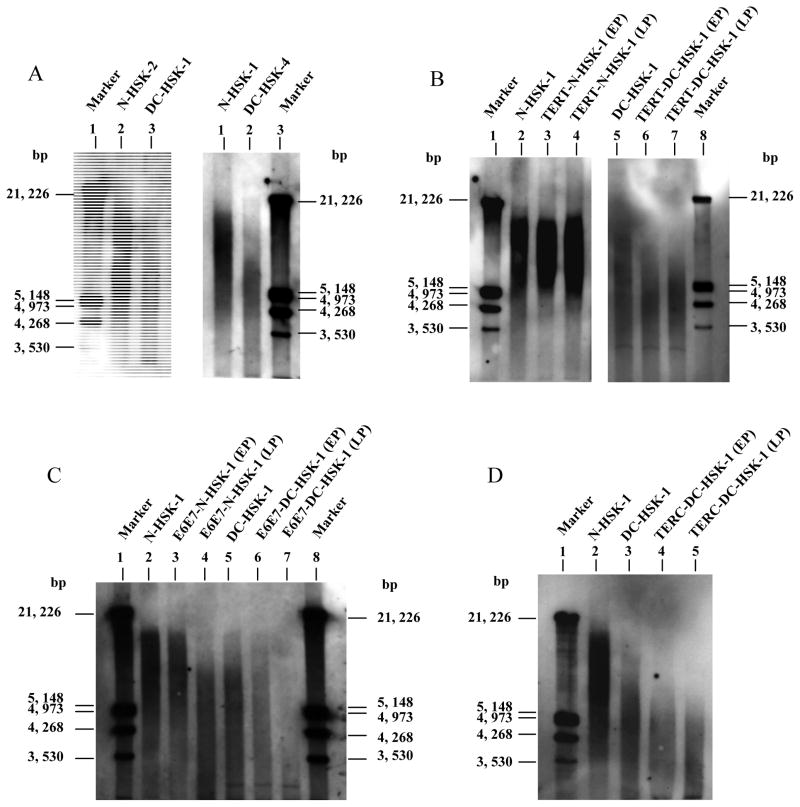
Telomere length in the primary DC and normal keratinocytes was assessed by Southern blot analysis using a telomere specific probe on digested genomic DNA. This analysis revealed that DC keratinocytes had significantly shorter telomeres (~6 kb) than normal keratinocytes from age and sex matched normal donors (range of 9–11 kb) (figure 3a and Table I). Telomeres in keratinocytes from the two DC donors were of similar length, although it should be noted that the third generation donor was younger (21 years old) than the second generation donor (41 years old).
Figure 3. Telomere length analysis of primary and transduced keratinocytes from dyskeratosis congenita (DC) and normal donors. Southern analysis was performed as described in the Materials and Methods. For transduced cells, EP=early passage (with 10 pd after transduction), LP=later passage (~30–40 pd post transduction). Calculated average telomere lengths are summarized in Table II.
A. Primary DC and normal keratinocytes. Sex and age-matched controls are shown in pairs. Lane 1, size marker; Lane 2, N-HSK-2; Lane 3, DC-HSK-1; Lane 4, N-HSK-1; Lane 5, DC-HSK-4; Lane 6, size marker.
B. Telomere length in TERT transduced keratinocytes with time in culture. Lane 1, size marker; Lane 2, primary N-HSK-1; Lane 3, TERT transduced N-HSK-1; Lane 4, TERT transduced N-HSK-1 at later passage; Lane 5, primary DC-HSK-1; Lane 6, TERT transduced DC-HSK-1; Lane 7, TERT transduced DC-HSK-1 at later passage.
C. Telomere length analysis of E6/E7 transduced keratinocytes. Lanes 1, size marker; Lane 2, primary N-HSK-1; Lane 3, E6/E7 transduced N-HSK-1; Lane 4, E6/E7 transduced N-HSK-1 at later passage; Lane 5, primary DC-HSK-1; E6/E7 transduced DC-HSK-1; Lane 7, E6/E7 transduced DC-HSK-1 at later passage; Lane 8, size marker.
D. Telomere length in TERC transduced DC keratinocytes. Lane 1, size marker; Lane 2, primary N-HSK-1; Lane 3, primary DC-HSK-1; Lane 4, TERC transduced DC-HSK-1; TERC transduced DC-HSK-1 at later passage.
Because DC-HSK-1 and N-HSK-1 represented the two extremes for CFE and scratch wound closure, we decided to further characterize these cells for telomerase activity and immortalization studies. Telomerase activity, as measured by a quantitative telomere repeat amplification protocol (TRAP) assay, was barely detectable in DC keratinocytes and was approximately half the level of that observed in primary untransduced normal cells (Table II). Similar findings have been reported in DC lymphocytes compared to age-matched controls and would be expected since the DC cells have one wild type and one defective copy of TERC (38). As measured by quantitative reverse transcriptase PCR (q-RT-PCR), levels of TERC transcript in the DC keratinocytes were lower than in normal keratinocytes and, in addition, levels of TERT transcript were lower in DC cells, both of which could account for the lower levels of telomerase in untransduced DC cells.
Table II.
Telomerase and telomere length in primary and transduced normal and dyskeratosis congenita keratinocytes
| Cell Name | Relative Telomerase Activitya | Relative TERT Transcript Levelsb | Relative TERC Transcript Levelsc | Average Telomere Lengthd | Average Telomere Length (later passage)e |
|---|---|---|---|---|---|
| N-HSK-1 | 1.0 +/− 0.4 | 1.0 +/− 0.2 | 1.0 +/− 0.2 | 10.1 | ND |
| DC-HSK-1 | 0.5 +/− 0.1 | 0.5 +/− 0.4 | 0.3 +/− 0.1 | 5.9 kb | ND |
| TERT-N-HSK-1 | 444.0 +/− 115.0 | 3.2×105 +/− 2.0×104 | 0.7 +/− 0.1 | 10.0 kb | 10.7 kb |
| TERT-DC-HSK-1 | 290.0 +/− 31.0 | 6.5×104 +/− 3.0×103 | 0.6 +/− 0.1 | 4.4 kb | 4.3 kb |
| E6/E7-N-HSK-1 | 17.8 +/− 7.5 | 5.8 +/− 1.1 | 2.8 +/− 0.4 | 8.6 kb | 6.6 kb |
| E6/E7-DC-HSK-1 | 6.7 +/− 0.5 | 4.1 +/− 0.5 | 1.4 +/− 0.3 | 6.0 kb | 3.2 kb |
| TERC-DC-HSK-1 | 4.2 +/− 0.2 | 0.3 +/− 0.2 | 34.9 +/− 3.3 | 3.8 kb | 3.6 kb |
Relative telomerase activity as measured by a quantitative real-time TRAP assay. The shown activity is relative to normal untransduced keratinocytes (N-HSK-1).
Relative TERT transcript as measured by quantitative reverse transcriptase PCR (q-RT-PCR). The shown levels were normalized to GAPDH and are relative to untransduced N-HSK-1.
Relative levels of the RNA component, TERC, as measured by q-RT-PCR. Shown levels were normalized to GAPDH and are relative to untransduced N-HSK-1.
Average telomere length of various primary or transduced cells within 5 to 10 pd after transduction as measured by Southern blotting (summarized from blots shown in figure 3).
Average telomere length of various transduced cells approximately 30 to 40 pd after transduction (i.e. latter passage) as measured by Southern blotting (summarized from blots shown in figure 3).
Because the DC keratinocytes had lower levels of TERC, we were interested in determining whether they were defective in their ability to be immortalized by exogenous expression the reverse transcriptase component of telomerase, TERT, which has been shown to robustly activate telomerase and immortalize various cell types. Exogenous expression of TERT using a retroviral vector greatly increased telomerase (more than 200 fold) in both DC and normal keratinocytes (Table II). Like the untransduced cells, the TERT transduced DC cells exhibited approximately half the telomerase activity of TERT transduced normal cells (Table II). As expected, TERT transcript was highly increased in the TERT transduced normal and DC cells, although, for unknown reasons, the TERT transduced DC cells had much lower levels of TERT transcript than TERT transduced normal cells. Exogenous expression of TERT slightly increased levels of TERC transcript in DC cells but not in normal cells, indicating that the greatly increased levels of telomerase activity in both cell types, mostly due to high levels of TERT expression. Both TERT transduced DC and normal keratinocytes had an extended lifespan of more than 5 times that of untransduced cells and no apparent crisis was observed (Table III). The cells continue to proliferate and are apparently immortal. Although telomere length was not significantly elongated in TERT expressing DC or normal cells, it was maintained at a stable length with passaging in culture (Figure 2b and Table II). These results indicate that TERT expression in DC keratinocytes upregulates telomerase to a level that is sufficient for maintenance but not necessarily enough to allow for significant lengthening of telomeres. A similar finding was previously observed for TERT expressing DC skin fibroblasts (26). Expression of exogenous TERT increased CFE in both normal and DC keratinocytes, although the TERT transduced DC keratinocytes exhibited consistently lower CFE than TERT transduced normal keratinocytes, perhaps because of lower telomerase activity or shorter telomere length (Tables II and III). Both TERT transduced DC and normal keratinocytes had a similar rate of scratch wound closure compared to that of untransduced normal cells, indicating rescue of this defect by exogenous expression of TERT in DC cells (Table III).
Table III.
Characteristics of transduced normal and dyskeratosis congenita keratinocytes
| Cell Name | Maximum Lifespana | Percent Colony Forming Efficiencyb | Percent Wound Closure (18 Hours)c | Karyotyped |
|---|---|---|---|---|
| N-HSK-1 | 32 pd | 7.0 +/− 0.4 | 87.2 +/− 8.6 | 46, XY[20] (normal) |
| DC-HSK-1 | 18 pd | 2.1 +/− 0.3 | 21.3 +/− 12.7 | 46, XX[20] (normal) |
| TERT-N-HSK-1 | >90 pd | 20.8 +/− 1.6 | 100.0 +/− 0 | 46, XY[20] (normal) |
| TERT-DC-HSK-1 | >90 pd | 11.7 +/− 2.1 | 90.3 +/− 7.9 | 46, XX[20] (normal) |
| E6/E7-N-HSK-1 | >90 pd | 39.3 +/− 4.0 | 79.4 +/− 7.9 | 46, XY, i(8)(q10)[18]/47, XY,+6[2] |
| E6/E7-DC-HSK-1 | >90 pd | 45.2 +/− 6.3 | 44.0 +/− 1.7 | 48, XX, i(8)(q10),+11,+20[20] |
| TERC-DC-HSK-1 | >60 pd | 16.3 +/− 0.3 | 51.7 +/− 1.6 | 46, XX[20] (normal) |
Lifespan in cell culture in population doublings (pd). Maximum lifespan shown for untransduced cells. For the transduced cells, a “>” symbol signifies continuous growth.
Percent colony forming efficiency on irradiated feeders.
Percent scratch wound closure at 18 hours as compared to 0 hours.
Cytogenetic analyses were performed within 20 to 30 pd post transduction. The number in bracket represents the number of metaphases out of 20 that exhibited the noted karyotype.
We next wanted to assess whether the DC keratinocytes could be immortalized by exogenous expression of human papillomavirus type 16 (HPV-16) E6 and E7 which, together, are known to effectively immortalize many human epithelial cell types, including human keratinocytes (39). The E6 and E7 proteins from high risk HPV types such as HPV-16 inactivate p53 and pRb pathways, respectively, but are also known to activate endogenous telomerase through upregulation of TERT (40, 41, 42). Transduction of DC and normal keratinocytes with HPV-16 E6/E7 retrovirus resulted in upregulation of telomerase, as expected, and the E6/E7 transduced DC keratinocytes exhibited approximately half that of E6/E7 transduced normal cells, (Table II). Not surprisingly, TERT transcript was higher in E6/E7 transduced cells but was nowhere near the levels observed for TERT transduced cells (Table II). Interestingly, levels of TERC transcript were also up in E6/E7 transduced DC and normal keratinocytes, indicating that immortalization of cells by E6/E7 may result in activation of telomerase through upregulation of both endogenous TERT and TERC. Expression of E6/E7 resulted in a greatly extended lifespan in both DC and normal keratinocytes (greater than 5 times the lifespan of untransduced cells) with no apparent crisis or slow down in growth (Table III). At early passage, the E6/E7 transduced DC and normal cells had telomere lengths that were comparable to untransduced cells. Despite upregulation of telomerase, both E6/E7 transduced DC and normal keratinocytes exhibited significant telomere shortening with passaging in culture (figure 2c and Table II). E6/E7 expression greatly increased CFE in both DC and normal keratinocytes (Table III). Although E6/E7 increased scratch wound closure rate of DC keratinocytes, it did not approach the rate of closure observed for either non-transduced or E6/E7 expressing normal keratinocytes, suggesting that E6/E7 in DC cells could not completely correct the scratch wound defect (Table III).
Our previous studies demonstrated that exogenous expression of the RNA component of telomerase, TERC, had no effect on proliferation or telomerase activity in DC fibroblasts (26). Modest 2 to 3 fold increases in telomerase activity were observed upon transduction of primary DC and normal keratinocytes with TERC (data not shown). While we did not observe robust telomerase activation by TERC in DC keratinocytes, a subpopulation emerged from TERC transduced DC keratinocytes with telomerase activity that was 4 to 5 fold greater than untransduced DC keratinocytes (Table II). As expected, a concomitant increase in TERC transcript levels was observed in the TERC transduced cells. However, there was no detectable increase in TERT levels in these cells, indicating that the increase in telomerase was due to exogenously expressed TERC (Table II). The TERC transduced DC cells did not exhibit any apparent extension of telomere length beyond that of untransduced cells (in fact they were shorter) but maintained telomeres at a very short length of approximately 3.6 kb (Table II and figure 2d). Even with telomere shortening, the TERC transduced cells had a greatly extended lifespan of greater than 50 pd beyond that observed for untransduced DC cells (Table III). As mentioned above, exogenous expression of TERC slightly increased telomerase in normal keratinocytes, but we did not observe significant extension of lifespan in these cells (data not shown). However, in an independent study, we have noted upregulation of telomerase and slight extension of lifespan by TERC transduction in neonatal foreskin keratinocytes (unpublished data). Further characterization of the TERC transduced DC keratinocytes demonstrated increased CFE and some measure of amelioration of the scratch wound closure defect as compared to untransduced DC cells (Table III).
Finally, to assess whether the short telomeres in DC cells were associated with genetic instability upon immortalizaton, we examined the various transduced cells by Giemsa banding. Karyotypes were performed between 15 and 25 population doublings post transduction (Table III). Both the TERT transduced DC and normal keratinocytes exhibited completely normal karyotypes. The E6/E7 transduced DC and normal cells, on the other hand, exhibited some cytogenetic abnormalities, but the E6/E7 transduced DC cells did not have extensive gross genetic alterations as might be expected to result from having such short telomere length. However, we cannot rule out the possibility that genetically stable subclones emerged after a period of genetic instability at earlier passage. Interestingly, the TERC transduced DC keratinocytes with an extended lifespan had no apparent cytogenetic abnormalities, indicating the expression of TERC alone was sufficient to maintain telomeres and genetic stability in this cell population.
DISCUSSION
The studies presented here demonstrate that keratinocytes from DC subjects have short telomeres, and in addition, shortened lifespan and measurable defects in colony forming ability and migration capacity. Similar to our studies with DC fibroblasts, immortalization of DC keratinocytes by exogenous expression of TERT caused significant upregulation of telomerase, and maintenance of telomeres. Unlike fibroblasts, expression of HPV E6/E7 could upregulate telomerase in keratinocytes. While expression of TERC had no observable effects in fibroblasts, our studies indicate that TERC expression upregulated telomerase in keratinocytes and allowed extension of lifespan in a subpopulation of DC keratinocytes. This is, to our knowledge, the first report of TERC having any effect on the proliferative capacity of primary human cells. Overall, our results provide further understanding of the potential mechanisms that may be behind the epidermal defects associated with shortened telomeres in DC. They also corroborate significant differences between fibroblasts and keratinocytes with regard to telomerase biology.
How telomerase and telomere shortening affect the aging of the epidermis is not clear. It has been argued that epidermal stem cells do not age and that levels of telomerase in keratinocytes are sufficient to maintain telomeres throughout life (27, 43). The epidermis is one of the few regenerative tissues that expresses telomerase activity; telomerase has been detected in basal epithelial cells and in the bulb-containing region of hair follicles (10, 44, 45). Studies have indicated, however, that telomere length decreases in epidermal cells with age (46, 47). Significant telomere shortening has been observed in cultured skin autografts of patients with burns (48). Chronic ischemia in skin injuries and delayed wound healing are a feature of aging, and it has been speculated that this might be related to telomere shortening or downregulation of telomerase (49). While telomerase knockout mice have provided insight into the role of telomere shortening in aging, mice and humans are known to be different with regard to telomere length and telomerase regulation (16). DC provides a unique model system for studying how telomerase dysfunction and/or telomere shortening may be involved in the aging of human epidermis. The observation that DC individuals exhibit numerous skin defects, including poor wound healing (17, 50) and epidermal atrophy (15) indicates that telomere length does play a role in skin biology and our in vitro studies using DC keratinocytes support this hypothesis. It is noteworthy that keratinocytes isolated from the third generation DC subject (DC-HSK-1) had the most profound proliferative and migratory defects, and similarly had the most severe phenotype and lowest CD34+ hematopoietic stem cell counts (51). One possibility is that keratinocyte stem cells in DC patients have insufficient telomerase, and telomere associated senescence contributes to epidermal problems in DC patients. Similar mechanisms could be involved in normal aging of skin. However, other factors, including mesenchymal support cells in the skin, are also likely to play a role and these need to be taken into account when discussing models of skin aging (27). It is unknown whether the low levels of constitutive telomerase observed in keratinocyte stem cells contributes significantly to telomere maintenance and homeostasis in the adult epidermis. This possibility might need to be considered as a potential cause for side effects in treatment of cancers with telomerase inhibitors (52, 53).
Our studies suggest that exogenous expression of TERT or TERC may provide a means to correct certain proliferative problems in the epidermis. Studies using rabbits and mice have indicated that overexpression of TERT can increase wound healing capacity (54, 55). The published finding that exogenous expression of TERT, even in TERC knockout mice, can lead to mobilization of epidermal stem cells would indicate that TERT has an important role in keratinocyte biology independent of telomere elongation (56–58). In this regard, TERT has been implicated in being involved in inhibition of apoptosis and resistance to DNA damaging agents through widespread changes in gene expression (56, 57, 59–64). Some of these observed effects may be due to gross overexpression of TERT, which could impact other cell processes. That exogenous expression of TERC can upregulate endogenous telomerase, albeit slightly, in primary keratinocytes and extend their lifespan is of significant interest. Recently, it was reported that exogenous expression of TERC could increase telomerase in primary T cells and EBV-transformed lymphoblastoid cell lines (LCL) derived from DC patients (65). In EBV transformed LCLs, TERC expression was shown to increase proliferative capacity, as well. Extension of lifespan by TERC alone has not been previously demonstrated for primary human cells, likely because most studies have been done with cells such as fibroblasts, which have detectable levels of TERC but nearly undetectable levels of TERT. Keratinocytes have low levels of TERT, and our results would suggest that a subpopulation of keratinocytes can survive by expression TERC alone such that they have increased telomerase and an extended lifespan. It is unknown whether the few immortal cells that emerged from transduced DC keratinocytes were progenitor-like cells that already had sufficient levels of TERT to maintain telomeres. Our results would suggest that both TERT and TERC are rate limiting for telomerase activity in human keratinocytes. Such a mechanism has been proposed previously for other cell types (66). While exogenous expression of either or both of these genes might upregulate telomerase and maintain telomeres in keratinocytes, any attempts to introduce TERT or TERC into tissues for treatment of disease would need to take into account the fact that telomerase activation could result in malignant transformation.
Short telomeres have been implicated in the development of genetic instability and concomitant malignant transformation, which has been suggested as a potential mechanism to explain the increased risk of malignancy in DC patients (17, 67). Interestingly, we did not observe a large number of chromosome alterations in E6/E7 transduced DC cells even though they had very short telomeres. The E6/E7 cells continued to exhibit telomere shortening and continued to proliferate, most likely due to bypass of the normal DNA damage response through abrogation of p53 and pRb pathways by E6 and E7. With further telomere shortening, these cells may eventually exhibit more profound genetic instability or genetic catastrophe and cell death. However, we and others have shown that E6/E7 expressing keratinocytes eventually stabilize and sometimes even elongate telomeres with time in culture (37, 68). It is interesting to note that no large-scale cytogenetic alterations were observed in the TERC-expressing DC HSK-1 clones that emerged in culture. Although other unapparent transforming events may have occurred in these cells, this result would suggest that exogenous expression of TERC maintains telomeres at sufficient length to prevent end-to-end fusions and gross genetic instability.
In summary, our studies implicate a causal role of telomere shortening in the aging of skin keratinocytes and the epidermal pathologies observed in dyskeratosis congenita. While similar to DC fibroblasts in their proliferative defects and ability to be immortalized by TERT, DC keratinocytes differ from fibroblasts in their ability to mobilize telomerase upon expression of E6/E7 or TERC. Our studies suggest that exogenous expression of TERC can increase telomerase and lifespan in primary cells such as keratinocytes that naturally express low levels of endogenous telomerase. Overall, our results may have implications for understanding the role of telomere shortening in the aging of human skin and for developing novel strategies for treatment of epidermal problems associated with aging.
Acknowledgments
The authors wish to thank associates of the Gene Transfer Vector Core Facility of the University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases, supported by NIH/NIDDK P30 DK 54759 for their assistance with viral vector production. We are also grateful to Kathy Collins for the hTERC construct, Robert Weinberg for the hTERT construct, and Jiing-Kuan Yee for the pHIV7 vector. We thank Kimberly Lee for technical assistance. Cytogenetic analysis was performed at the University of Iowa Cytogenetics Facility. The work in the laboratory of PML was supported by a grant from the Canadian Institutes of Health Research (MOP38075). The work in the laboratories of FDG and AJK was supported by a grant from the National Institutes of Health (R01 AG0227388).
References
- 1.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3:444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 3.Allsopp RC, Chang E, Kashefiaazam M, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 4.Autexier C, Pruzan R, Funk WD, Greider CW. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 1996;15:5928–5935. [PMC free article] [PubMed] [Google Scholar]
- 5.Weinrich SL, Pruzan R, Ma L, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 7.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 8.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Chiu CP, Dragowska W, Kim NW, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 10.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 13.Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 14.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 15.Bessler M, Du HY, Gu B, Mason PJ. Dysfunctional telomeres and dyskeratosis congenita. Haematologica. 2007;92:1009–1012. doi: 10.3324/haematol.11221. [DOI] [PubMed] [Google Scholar]
- 16.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet. 2008;73:103–112. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 18.Mason PJ, Wilson DB, Bessler M. Dyskeratosis congenita -- a disease of dysfunctional telomere maintenance. Curr Mol Med. 2005;5:159–170. doi: 10.2174/1566524053586581. [DOI] [PubMed] [Google Scholar]
- 19.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 20.Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol Cell. 2003;11:1361–1372. doi: 10.1016/s1097-2765(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 21.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 25.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westin ER, Chavez E, Lee KM, et al. Telomere restoration and extension of proliferative lifespan in dyskeratosis congenita fibroblasts. Aging Cell. 2007;6:383–394. doi: 10.1111/j.1474-9726.2007.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boukamp P. Skin aging: a role for telomerase and telomere dynamics? Curr Mol Med. 2005;5:171–177. doi: 10.2174/1566524053586644. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez RD, Morales CP, Herbert BS, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darbro BW, Lee KM, Nguyen NK, Domann FE, Klingelhutz AJ. Methylation of the p16(INK4a) promoter region in telomerase immortalized human keratinocytes co-cultured with feeder cells. Oncogene. 2006:7421–7433. doi: 10.1038/sj.onc.1209729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darbro BW, Schneider GB, Klingelhutz AJ. Co-regulation of p16INK4a and migratory genes in culture conditions that lead to premature senescence in human keratinocytes. J Invest Dermatol. 2005;125:499–509. doi: 10.1111/j.0022-202X.2005.23844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague D, Phillips S, Mitchell C, et al. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology. 2002;301:247–254. doi: 10.1006/viro.2002.1542. [DOI] [PubMed] [Google Scholar]
- 34.Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 35.Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yam PY, Li S, Wu J, Hu J, Zaia JA, Yee JK. Design of HIV vectors for efficient gene delivery into human hematopoietic cells. Mol Ther. 2002;5:479–484. doi: 10.1006/mthe.2002.0558. [DOI] [PubMed] [Google Scholar]
- 37.Klingelhutz AJ, Barber SA, Smith PP, Dyer K, McDougall JK. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994;14:961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ly H, Schertzer M, Jastaniah W, et al. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- 41.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 42.James MA, Lee JH, Klingelhutz AJ. HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer. 2006:1878–1885. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stern MM, Bickenbach JR. Epidermal stem cells are resistant to cellular aging. Aging Cell. 2007;6:439–452. doi: 10.1111/j.1474-9726.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez RD, Wright WE, Shay JW, Taylor RS. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J Invest Dermatol. 1997;108:113–117. doi: 10.1111/1523-1747.ep12285654. [DOI] [PubMed] [Google Scholar]
- 45.Boukamp P, Mirancea N. Telomeres rather than telomerase a key target for anti-cancer therapy? Exp Dermatol. 2007;16:71–79. doi: 10.1111/j.1600-0625.2006.00517.x. [DOI] [PubMed] [Google Scholar]
- 46.DiPaolo JA, Woodworth CD, Popescu NC, Notario V, Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989;4:395–399. [PubMed] [Google Scholar]
- 47.Kosmadaki MG, Gilchrest BA. The role of telomeres in skin aging/photoaging. Micron. 2004;35:155–159. doi: 10.1016/j.micron.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Counter CM, Press W, Compton CC. Telomere shortening in cultured autografts of patients with burns. Lancet. 2003;361:1345–1346. doi: 10.1016/S0140-6736(03)13042-5. [DOI] [PubMed] [Google Scholar]
- 49.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 50.Reimann C, Kloeckener-Gruissem B, Niemeyer CM, Vanscheidt W. Late manifestation of dyskeratosis congenita presenting as chronic dermal ulcer in a 37-year-old man. J Eur Acad Dermatol Venereol. 2007:897–898. doi: 10.1111/j.1468-3083.2007.02530.x. [DOI] [PubMed] [Google Scholar]
- 51.Goldman FD, Aubert G, Klingelhutz AJ, et al. Characterization of primitive hematopoietic cells from patients with dyskeratosis congenita. Blood. 2008;111:4523–4531. doi: 10.1182/blood-2007-10-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shariftabrizi A, Eller MS. Telomere homolog oligonucleotides and the skin: current status and future perspectives. Exp Dermatol. 2007;16:627–633. doi: 10.1111/j.1600-0625.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 53.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Suarez E, Samper E, Ramirez A, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mogford JE, Liu WR, Reid R, et al. Adenoviral Human Telomerase Reverse Transcriptase Dramatically Improves Ischemic Wound Healing Without Detrimental Immune Response in an Aged Rabbit Model. Hum Gene Ther. 2006:651–660. doi: 10.1089/hum.2006.17.651. [DOI] [PubMed] [Google Scholar]
- 56.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 57.Sarin KY, Cheung P, Gilison D, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegl-Cachedenier I, Flores I, Klatt P, Blasco MA. Telomerase reverses epidermal hair follicle stem cell defects and loss of long-term survival associated with critically short telomeres. J Cell Biol. 2007;179:277–290. doi: 10.1083/jcb.200704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell. 2004;3:399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 60.Oh H, Taffet GE, Youker KA, et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci U S A. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young JI, Sedivy JM, Smith JR. Telomerase expression in normal human fibroblasts stabilizes DNA 5-methylcytosine transferase I. J Biol Chem. 2003;278:19904–19908. doi: 10.1074/jbc.M301685200. [DOI] [PubMed] [Google Scholar]
- 62.Masutomi K, Possemato R, Wong JM, et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci U S A. 2005;102:8222–8227. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi J, Southworth LK, Sarin KY, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart SA, Hahn WC, O’Connor BF, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci U S A. 2002;99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirwan M, Beswick R, Vulliamy T, et al. Exogenous TERC alone can enhance proliferative potential, telomerase activity and telomere length in lymphocytes from dyskeratosis congenita patients. Br J Haematol. 2008;144:771–781. doi: 10.1111/j.1365-2141.2008.07516.x. [DOI] [PubMed] [Google Scholar]
- 66.Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 68.Baege AC, Berger A, Schlegel R, Veldman T. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am J Pathol. 2002;160:1251–1257. doi: 10.1016/S0002-9440(10)62552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]