Abstract
Background
mRNA expression signatures are frequently used as surrogate measures of cellular function and pathway changes. Few studies have directly compared results obtained using gene expression and multiplex protein assays for corresponding gene products.
Methods
We utilized data available from a clinical trial of an HPV-16 vaccine that tracked gene expression and cytokine/chemokine production by peripheral blood mononuclear cells (PBMCs) stimulated in culture with various antigens to evaluate the degree to which gene expression levels reflect observed levels of cytokines/chemokines. Twenty-six women enrolled in a phase II clinical trial of an HPV-16 vaccine were evaluated for gene expression (using the Affymetrix Human Genome Focus Array) and cytokine/chemokine levels (using a bead-based 22-plex cytokine assay developed by Linco Research, Inc.) before and after vaccination.
Results
Our results suggest the presence of a wide range of correlations between mRNA expression and secreted protein levels. The strongest correlation was observed for IFN-γ (R=0.90 overall levels; 0.69 when vaccine induced changes were evaluated). More modest overall correlations ranging from 0.40 to 0.80 were observed for MIP1A, IP10, TNF-α, MCP1, IL2, GM-CSF, IL5, RANTES, and IL-8. Weaker or no correlation was observed between gene expression and protein levels for the remaining cytokines/chemokines evaluated.
Conclusion
The degree of correlation between gene expression and protein levels varied among different cytokines/chemokines.
Impact
Researchers should be cautious when using mRNA expression array results as a proxy for protein levels using existing technologies.
Keywords: cytokines, mRNA expression, protein levels, affymetrix gene expression microarray, multiplex protein assays
Introduction
With the development of microarray technology for gene expression (1) and multiplexing technologies for protein measurements (2), scientists are able to study many molecular candidate markers including both mRNA and protein markers. Gene array technologies permit global evaluation of expression patterns in a way that is currently not yet possible at the protein level due to the proteome complexity. Since biological processes are typically driven by proteins, mRNA expression signature results are often thought of as a proxy for functional pathway changes, which involve changes in protein levels. This requires the assumption that global differences in mRNA levels reflect differences in protein levels. Few studies have attempted to directly compare mRNA expression and protein measures to determine how closely mRNA expression levels reflect levels of their corresponding proteins, and those that have revealed weak to moderate correlations between these markers (3–6).
As part of our effort to understand immunological responses following vaccination with the new virus-like particle (VLP) human papillomavirus (HPV) vaccine (7–9), we have measured gene expression profiles (using the Human Genome Focus Array from the Affymetrix GeneChip) and levels of cytokines/chemokines (using a bead-based multiplex cytokine assay developed by Linco Research, Inc.) in cultured peripheral blood mononuclear cells (PBMCs) obtained from individuals vaccinated with an HPV-16 VLP vaccine. This provided the opportunity for us to directly compare results obtained from an expression array with those obtained by directly measuring protein levels of cytokines in supernatants from the same biological specimens. Here we report results from this direct comparison and implications of our findings.
Methods
Study design
Details of our study design have been reported previously (9). In brief, we studied 26 (19 vaccine recipients and 7 placebo recipients) women 18 to 25 years of age who participated in a phase II clinical trial of an HPV16 L1 VLP vaccine without adjuvant (Novavax, Rockville, MD) conducted at the Johns Hopkins Center for Immunization Research (9). Blood specimens used for our evaluation were collected from participants before the first vaccine dose was administered (pre-vax) and at month 2 (one month after the second dose was administered; post-vax). Cryopreserved peripheral blood mononuclear cells (PBMCs) obtained from the blood specimens were used to perform the assays described below. The Johns Hopkins University Institutional Review Board approved the protocol for this study.
Laboratory methods
Microarray gene expression analysis and multiplex cytokine assay were performed on PBMC cultures after 72 hours of culturing as previously described (7–9). In brief we thawed and cultured cryopreserved PBMCs (10 ×106 cells total, cultured at 2.0×106 cells/ml in a volume of 5ml) for 72 hours at 37°C. Cells were cultured in single wells in vitro with: HPV-16 L1 VLP (2.5 µg/mL); influenza A virus (Flu, H3N2, 1:100; ATCC); Sf9/baculovirus insect cell lysate (BAC, 0.1 µg/mL, Novavax); or media. Media was used as a background measurement for untreated cells. Supernatants and cell pellets were obtained from the same well. Cell free supernatants were aliquoted, and frozen at −80°C then subsequently thawed for cytokine/chemokine testing in duplicate using the 22-plex cytokine assay developed by Linco Research, Inc. as previously described (8). Remaining supernatant and cell pellets were used for the total RNA extractions, frozen at −80C and subsequently used for microarray gene expression testing using the Affymetrix Human Genome Focus Array as previously described (7).
Statistical analysis
Analysis of the expression array data was limited to the probes that mapped to genes coding for the cytokines/chemokines in our multiplex panel (Listed in Table 1). IL-7 and IL-12 protein expression were below the detection limits for all specimens tested and these two cytokines were therefore not considered further for this analysis.
Table 1.
Correlations between mRNA and protein measures of cytokines
| All Conditions/ | All Conditions/ | |
|---|---|---|
| Gene, cytokine | All Time points | Post - Pre vaccination |
| 208200_at, IL1A | −0.01 | −0.39† |
| 39402_at, IL1B | 0.01 | 0.004 |
| 207849_at, IL2 | 0.55* | 0.18 |
| 207539_s_at, IL4 | 0.17 | 0.25 |
| 207952_at, IL5 | 0.41* | 0.39† |
| 205207_at, IL6 | 0.31* | 0.36† |
| 211506_s_at, IL8 | 0.24† | 0.49* |
| 207433_at, IL10 | 0.15 | 0.23 |
| 207844_at, IL13 | 0.29† | 0.26‡ |
| 205992_s_at, IL15 | 0.19‡ | 0.15 |
| 208402_at, IL17A | −0.02 | −0.17 |
| 220273_at, IL17B | 0.04 | −0.03 |
| 210354_at, IFN-γ | 0.90* | 0.69* |
| 207442_at, G-CSF | 0.21‡ | 0.17 |
| 210229_s_at, GM-CSF | 0.53* | 0.17 |
| 207113_s_at, TNF | 0.60* | 0.21 |
| 210133_at, EOTAXIN | 0.05 | −0.09 |
| 216598_s_at, MCP1 | 0.56* | 0.56* |
| 205114_s_at, MIP1A | 0.78* | 0.38† |
| 204533_at, IP10 | 0.62* | 0.28† |
| 204655 at, RANTES | 0.41* | −0.12 |
p <0.0001
p <0.001
p <0.01
p <0.05
Expression values in the media-alone culture were considered as background measurements. Expression values in treated cultures (VLP, Flu, and BAC) were corrected for background by subtracting the results of the media-alone culture. In a similar fashion, cytokine values were expressed as the levels observed after subtraction of background levels observed in the media-alone culture. The means of duplicate cytokine values were used for the analysis. The resultant values were used to address the question of whether individual assay results correlated with each other. In addition, because we were interested in determining whether changes in immune responses over time (i.e., post-vax responses relative to pre-vax responses) could be evaluated using mRNA and protein-based arrays, differences in expression and protein levels post-vax minus pre-vax were also calculated and compared.
We used the spearman correlation to determine the magnitude of the correlation between gene expression and its corresponding protein level results. We present results in table format that combined vaccine and placebo recipients, the various culture conditions used, and the two time points tested (unless otherwise noted). Notable findings from analyses that stratified on these factors are presented in the text.
Results
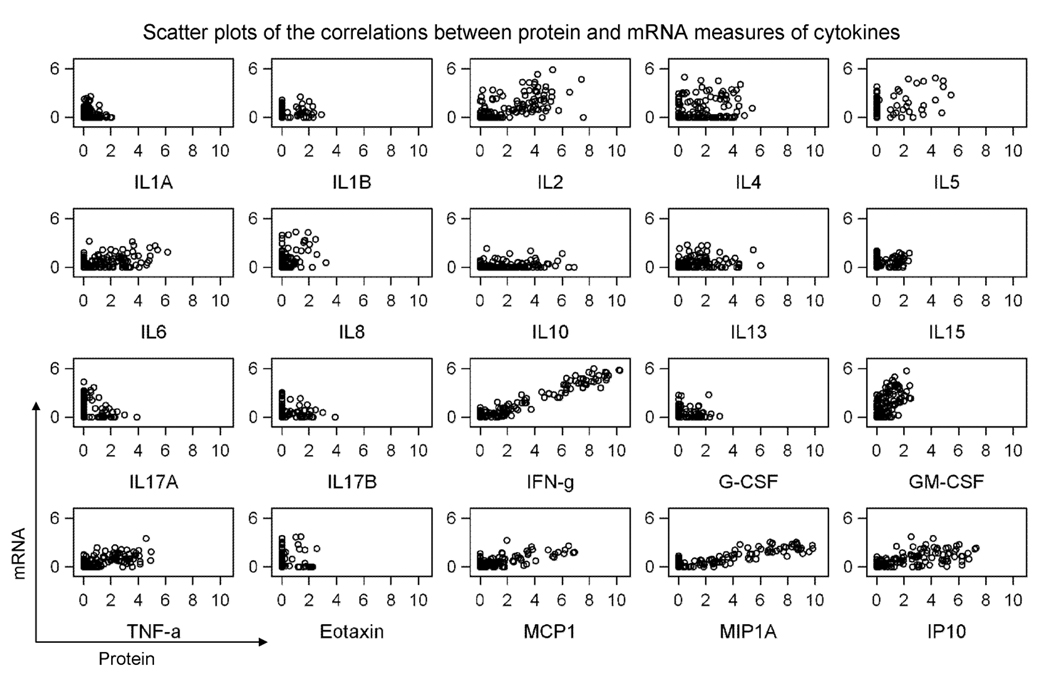
As shown in table 1 and figure 1, correlations between gene expression and secreted protein levels varied for the various markers evaluated. When we evaluated all results regardless of condition or time point, the strongest correlation was observed for IFN-γ (R=0.90, p< 0.0001). Correlations that were 0.60 or higher were observed for an additional three markers: MIP1A (R=0.78, p< 0.0001), IP10 (R=0.62, p< 0.0001), and TNF-α (R=0.60, p< 0.0001). Modest correlations on the order of .40 – 0.59 were observed for five markers: MCP1 (R=0.56, p< 0.0001), IL2 (R=0.55, p< 0.0001), GM-CSF (R=0.53, p< 0.0001), IL5 (R=0.41, p< 0.0001), and RANTES (R=0.41, p< 0.0001).
Figure 1.
Correlations between gene expression levels measured by microarray and protein levels of cytokines determined by Luminex bead arrays. All data are shown as log2 and refer to all conditions and time points tested. Protein levels (in pg/ml) are presented in X axis and gene expression levels are presented in y axis.
When changes over time (post-vax minus pre-vax) were evaluated, we typically observed weaker correlations (−0.39 to 0.69) than those observed when all results were evaluated (−0.01 to 0.90). The strongest correlation between gene expression and protein levels was again observed for IFN-γ (R=0.69, p< 0.0001). The only other markers where correlations of 0.40 or greater were observed were MCP1 (R=0.56, p< 0.0001), and IL8 (R=0.49, p< 0.0001).
When the individual culture conditions were examined separately (VLP, FLU, BAC), the correlations observed ranked similarly to the ranking observed using all conditions combined (spearman correlation of the ranking of the correlations varied from 0.66 for FLU, all time points to 0.90 for VLP, post-vax minus pre-vax), with the exception of BAC (spearman correlation of the ranking of the correlations was 0.34). It is interesting to note that correlations were weakest for BAC (as expected, since BAC was used as a negative control in our assay) and strongest for VLP.
Discussion
In this study we directly compared gene expression and secreted protein levels for a set of cytokines/chemokines using gene microarray and protein multiplexing technologies. Our results suggest that while for some cytokines/chemokines expression levels closely mirror protein levels (IFN-γ, MIP1A, IP10, and TNF-α) or moderately parallel protein levels (IL-2, GM-CSF, IL5, RANTES, and MCP1), for other markers this is not the case (IL-1A, IL-1B, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17A, IL-17B, G-CSF, and EOTAXIN). The imperfect and variable correlation between mRNA and protein levels is in agreement with previous reports (4, 10–13) and can be explained by post-transcriptional and post-translational regulation and by misclassification due to measurement errors (14–17). The different levels of inaccuracy, noise, sensitivity and dynamic ranges of the methods used for transcript and protein analysis likely contribute to the lack of correlation observed for several of the markers examined (18–23). In addition, the measurement of levels of secreted cytokines instead of total intracellular levels may also have contributed in part for differences in between the two measures.
Presence of misclassification error is supported by the lower correlations observed when the post-vax minus pre-vax measures were examined, since it involves the difference between two measures, each with error, making the error larger and therefore biasing the results towards the null. In addition, the lower correlations observed in post-vax minus pre-vax measures might arise from differences in dynamic range between the two assays. Alternative methods to measure gene expression levels, such as RT-PCR methods, might have a greater dynamic range and therefore theoretically allow for more precise quantitation of expression levels than the microarray method (24). This, in turn, could increase agreement between RNA expression and protein measures. Having said this, our previous attempt to confirm microarray findings using RT-PCR methods have suggested that the expression pattern of the genes selected for confirmation concurred with the microarray data for a small subset of markers evaluated (7).
Regardless of the exact reason for the lack of correlation observed (i.e., whether technical and/or true biological differences), individuals who utilize expression array tools should be mindful of the fact that levels or changes in expression detected by the Affymetrix microarrays do not always reflect the levels of changes in secreted protein detected using currently available protein arrays. Thus, it is possible that different profiles/signatures may result from these two technologies.
In summary, our comparison of expression and protein levels for a set of 20 cytokines/chemokines suggests that expression results obtained from microarrays do not necessarily correlate with secreted protein levels detected by a Luminex-based technology. Investigators who utilize currently available expression array tools should be careful not to assume that mRNA expression changes identified by expression studies would necessarily reflect similar changes in corresponding protein levels. Improvement of gene probe sets definition, method accuracy for absolute concentrations of DNA and protein, and use of proteomic techniques might help improve our understanding of the relationship between mRNA expression and protein production.
Footnotes
This project was funded with federal funds from the National Cancer Institute, National Institute of Health (N01-CO-12400).
References
- 1.Hardiman G. Microarrays Technologies 2006: an overview. Pharmacogenomics. 2006;7:1153–1158. doi: 10.2217/14622416.7.8.1153. [DOI] [PubMed] [Google Scholar]
- 2.Melton L. Protein arrays: proteomics in multiplex. Nature. 2004;429:101–107. doi: 10.1038/429101a. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 4.Tian Q, Stepaniants SB, Mao M, et al. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Cox B, Kislinger T, Emili A. Integrating gene and protein expression data: pattern analysis and profile mining. Methods. 2005;35:303–314. doi: 10.1016/j.ymeth.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 6.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Pineres AJ, Hildesheim A, Dodd L, et al. Gene expression patterns induced by HPV-16 L1 virus-like particles in leukocytes from vaccine recipients. J Immunol. 2009;182:1706–1729. doi: 10.4049/jimmunol.182.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Pineres A, Hildesheim A, Dodd L, et al. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16 L1 Virus-like particles. Clin Vaccine Immunol. 2007;14:984–989. doi: 10.1128/CVI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto LA, Edwards J, Castle PE, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188:327–338. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 10.Waghray A, Feroze F, Schober MS, et al. Identification of androgen-regulated genes in the prostate cancer cell line LNCaP by serial analysis of gene expression and proteomic analysis. Proteomics. 2001;1:1327–1338. doi: 10.1002/1615-9861(200110)1:10<1327::AID-PROT1327>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Gharib TG, Huang CC, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- 12.Griffin TJ, Gygi SP, Ideker T, et al. Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol Cell Proteomics. 2002;1:323–333. doi: 10.1074/mcp.m200001-mcp200. [DOI] [PubMed] [Google Scholar]
- 13.Lichtinghagen R, Musholt PB, Lein M, et al. Different mRNA and protein of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. Eur Urol. 2002;42:398–406. doi: 10.1016/s0302-2838(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 14.Mehra A, Lee KH, Hatzimanikatis V. Insights into the relation between mRNA and protein expression patterns: I. Theoretical considerations. Biotechnol Bioeng. 2003;84:822–833. doi: 10.1002/bit.10860. [DOI] [PubMed] [Google Scholar]
- 15.Nie L, Wu G, Zhang W. Correlation of mRNA expression and protein abundance affected by multiple sequence features related to translational efficiency in Desulfovibrio vulgaris: a quantitative analysis. Genetics. 2006;174:2229–2243. doi: 10.1534/genetics.106.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen L, Pili R. Posttranscription regulation of prostate cancer growth. Cancer J. 2008;14:46–53. doi: 10.1097/PPO.0b013e318162108a. [DOI] [PubMed] [Google Scholar]
- 17.Kozak M. Some thoughts about translational regulation: forward and backward glances. J Cell Biochem. 2007;102:280–290. doi: 10.1002/jcb.21464. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Bebu I, Li X. Microarray probes and probe sets. Front Biosci (Elite Ed) 2:325–338. doi: 10.2741/e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan PK, Downey TJ, Spitznagel EL, Jr, et al. Evaluation of gene expression measurements from commercial microarray platforms. Nucleic Acids Res. 2003;31:5676–5684. doi: 10.1093/nar/gkg763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hack CJ. Integrated transcriptome and proteome data: the challenges ahead. Brief Funct Genomic Proteomic. 2004;3:212–219. doi: 10.1093/bfgp/3.3.212. [DOI] [PubMed] [Google Scholar]
- 21.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 22.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djoba Siawaya JF, Roberts T, Babb C, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One. 2008;3:e2535. doi: 10.1371/journal.pone.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallas PB, Gottardo NG, Firth MJ, et al. Gene expression levels assessed by oligonucleotide microarray analysis and quantitative real-time RT-PCR -- how well do they correlate? BMC Genomics. 2005;6:59. doi: 10.1186/1471-2164-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]