Abstract
Background
Abnormalities of colonic motility were reported in relatively small studies of patients with lower functional gastrointestinal disorders (FGID) including irritable bowel syndrome (IBS). The influence of gender and body mass on the observed motor pathophysiology is unclear.
Aim
To compare colonic transit in patients with different lower FGID subgroups and healthy controls, controlling for gender and BMI, and to determine whether BMI independently influences colonic motility.
Methods
We evaluated a scintigraphic gastrointestinal and colonic transit database of 287 lower FGID patients associated with constipation (IBS-C, or functional constipation, n=118), diarrhea (IBS-D or functional diarrhea, n=139) or mixed bowel function (IBS-M, n=30) and 170 healthy controls. We measured colon filling at 6 hours (CF 6h), and overall colonic transit at 8, 24, and 48 hours.
Results
CF at 6h did not differentiate health from FGID. Colonic transit was abnormal at 24 hours (GC24 of <1.50 or >3.86) in 29.7% of all lower FGID patients. There was a significant overall association between colonic transit and subject group (healthy controls and FGID subgroups) at 8 (P=0.01), 24 (P<0.001) and 48 hours (P<0.001) in particular for those with diarrhea or constipation at 24 and 48 hours (P <0.05), even after adjusting for age, gender, and BMI. In addition, BMI was associated with colonic transit after adjusting for age, gender and subject group.
Conclusions
Abnormal transit is documented noninvasively with scintigraphy in 30% of lower FGID patients; transit measurement may help document pathophysiology and inform selection of therapy in lower FGID.
Keywords: motility, irritable bowel, diarrhea, constipation
INTRODUCTION
Lower functional gastrointestinal disorders (FGID), including irritable bowel syndrome (IBS), are commonly encountered in the community (prevalence 5% –25%) and account for 36% of all visits to gastroenterologists (1). Annually, there are 2.2 million drug prescriptions to treat IBS in the USA (2). Aggregate health care costs are 49% higher in IBS patients than in population controls [non-gastrointestinal patients (3)].
Understanding the pathophysiological mechanisms underlying these conditions may provide the basis for selection of more specific therapy. Altered bowel motility (4) as a pathophysiological mechanism in patients with IBS has been de-emphasized in the last decade, and there has been increased focus on hypersensitivity to explain gastrointestinal (GI) symptoms (5). Studies of the role of abnormal GI motor functions have involved relatively small numbers of IBS patients or used invasive (intubated small bowel or colonic) approaches that are not necessarily generalizable, since they may induce artifact as a result of stress or discomfort, and have been recorded in a very small number of patients.
The relationship between colonic motor dysfunction and predominant bowel function in IBS has been assessed in a few studies, chiefly measuring transit by radiopaque markers (6,7). However, this method to measure transit is best validated and sensitive for identifying slow colonic transit. In fact, there is evidence that it is less accurate than scintigraphy to detect accelerated colonic transit (8), and we have previously documented accelerated proximal and overall colonic emptying in detailed measurements of colonic transit in patients with IBS-D (9). Thus, we considered that the more refined, validated methods using scintigraphy and larger numbers of patients with IBS were needed to define the association between GI motor patterns and symptoms.
A confounding factor in assessing the role of motor dysfunction in IBS or lower FGID is the impact of obesity. According to recent U.S. national surveys, 19.5% of adult men and 25% of adult women are obese [body mass index (BMI) >30 kg/m2 (10)]. Population-based studies suggest there is gastrointestinal dysfunction, as well as increased frequency of abdominal pain and diarrhea in overweight and obese subjects (11-13). The potential role of obesity in IBS remains largely unexplored. An initial step is to assess whether the effect of IBS sub-phenotype on colonic transit is independent of the effect of BMI and to determine whether there is a statistically independent effect of BMI on colonic transit.
The validated noninvasive scintigraphic method that has been available for two decades provides a simple, yet comprehensive noninvasive approach by which transit of solid material through the entire, unprepared GI tract can be measured (14-16). In the research laboratory, we have established a data base consisting of patients and healthy volunteers who participated in research studies of gastrointestinal and colonic transit and were randomized to placebo treatment. The first aim of our study was to compare colonic transit between patients with subtypes of lower FGID based on predominant bowel function and healthy controls. The second aim was to assess whether there is an independent association of BMI with abnormal colonic transit in the same participants.
METHODS
Subjects
This study assessed 287 patients with IBS [Rome II positive, 30 IBS-mixed or alternating (IBS-M), 118 constipation-predominant IBS (IBS-C) or functional constipation (FC), 139 diarrhea-predominant IBS (IBS-D) or functional diarrhea (FD)] and 170 healthy volunteers. All participants were residing within 200 miles of Mayo Clinic, Rochester, MN. They had been recruited for previous original studies (see Appendix for references) by means of letters or public advertisements from 2000 to 2008 and had signed informed consent for the respective studies.
The inclusion criteria and characteristics of patients with each subtype of IBS appear in the original studies; all patients fulfilled Rome II criteria for diagnosis of IBS and its subtypes, FD or FC. For simplicity, these will be summarized as IBS-C/FC (mainly constipation), IBS-D/FD (mainly diarrhea), and IBS-M (alternating bowel habit). The healthy participants had less than 4 positive (only mild for severity or sometimes for frequency being permissible) out of 19 gastrointestinal symptoms on a screening questionnaire. During the transit studies, participants were allowed to continue low, stable doses of thyroid replacement, estrogen replacement, low-dose aspirin (81 mg per day), birth control pills or depot estrogen injection, and SSRI antidepressants, but not tricyclic agents.
Exclusion criteria included organic diseases that might explain the patients’ symptoms, use of any medication for IBS or bowel dysfunction (within 7 days before the study and throughout the course of study), and any structural or metabolic diseases that affect the gastrointestinal system including diabetes. Patients who had participated in another clinical study within the prior 30 days were ineligible.
All individual studies had been conducted with Mayo Clinic Institutional Review Board approval. Use of the data base from which this analysis was conducted was also reviewed and approved by Mayo Clinic Institutional Review Board, and all participants had authorized use of their medical records for research studies.
Orocecal and Colonic Transit Measurements
Orocecal transit was assessed by the colonic filling of 99mTc egg meal ingested at time 0 hours. To evaluate colonic transit (8,14,15), 111In adsorbed on activated charcoal particles was delivered to the colon by a methacrylate-coated, delayed-release capsule (15). All participants ingested the capsule containing 111In charcoal at 6:00 a.m. after an overnight fast (last meal prior to 9:00 p.m. on the previous day). When the capsule had been demonstrated to have emptied from the stomach (with images obtained every 30 minutes while the patient remained fasting), all participants received a standard breakfast (218 kcal egg meal) at time 0 and two standardized meals at 4 hours (530 kcal chicken meal) and 8 hours (750 kcal steak dinner).
A variable region of interest program was used to measure transit, as in previous studies (8, 14, 15). We obtained abdominal images every 30 minutes for the first 10 hours, with scans being increased to every 15 minutes for the first 2 hours after meals. A final scan was obtained at 48 hours. The performance characteristics of this test are summarized elsewhere (16).
Transit Data Analysis
Colonic filling at 6 hours, or the proportion of radiolabeled meal to have reached the colon at 6 hours, is an indirect measurement of small bowel transit time. Overall colonic transit was summarized as the colonic geometric center (GC) at specified times. The GC is the weighted average of counts in the colonic regions (ascending, transverse, descending, rectosigmoid) and stool, respectively, 1 to 5. Thus, at any time, the proportion of counts in each colonic region is multiplied by its weighting factor as follows: (% ascending * 1 + % transverse * 2 + % descending * 3 + % rectosigmoid * 4 + % stool * 5)/100 = GC. Thus, a higher GC reflects a faster colonic transit.
Statistical Analysis
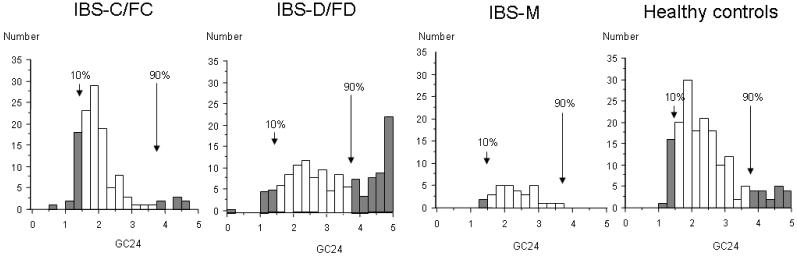
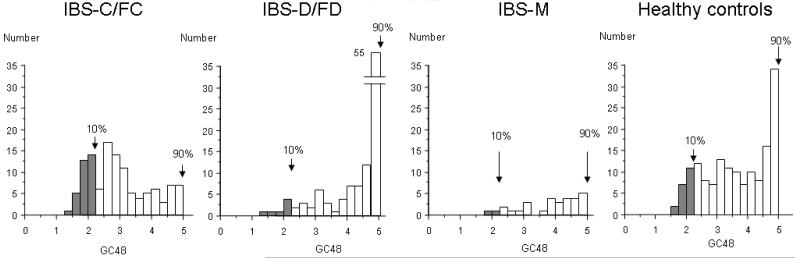
Data are expressed as the mean ± standard error of the mean (SEM), or as proportions (%). The association of gender with subgroup status was assessed using χ2 analysis. The association of age and BMI with subgroup status was assessed using the Kruskal-Wallis test. The proportion of patients with abnormal colonic transit was calculated based on the 10th and 90th percentiles for transit in the normal healthy volunteers studied concurrently in these studies (GC at 24h <1.50 or GC at 48h <2.13 for slow transit and >3.86 at 24h for accelerated transit, Figure 1a and b). We used the 10th and 90th percentiles as cutoffs for two reasons:
Figure 1a.
Histograms showing distribution of GC24 for healthy controls and for patients with each lower FGID subtype. The 10th and 90th percentile cut off are defined using the distribution of the healthy control sample for GC24.
Figure 1b.
Histograms showing distribution of GC48 for healthy controls and for patients with each lower FGID subtype. The 10th and 90th percentile cut off are defined using the distribution of the healthy control sample for GC48.
First, it is generally recommended (O’Brien rule) to use the 5th and 95th percentiles as cutoff points for a sample with 200 or more subjects. When a group has less than 200 subjects, as in the subgroups of healthy control and IBS subtypes in our study, the 5th and 95th percentiles may be more susceptible to data outliers or to skewing (as in a non-Gaussian distribution) than the 10th and 90th percentiles. Using the 10th–90th percentiles gives an accurate representation of the most extreme data points within each subgroup.
Second, there is a ceiling effect when we use the 5th and 95th percentiles as cutoff, because the maximum for all GC measurements only go up to 5. This is a particular problem with the healthy control group at GC48, as well as with the IBS-D/FD group at GC24 and GC48 since the GC of 5 is achieved by >5% of the participants in each of these groups. Given these reasons, we have chosen to lean toward slightly more stringent cutoff values for the normal range.
The association of colonic filling at 6 hours and overall colonic transit (GC8,GC24,GC48) with subject subgroup [the three lower FGID subtypes (diarrhea, constipation or mixed) and controls] was assessed using an analysis of covariance (ANCOVA). The covariates included in the ANCOVA models were age, gender and BMI. Since BMI was associated overall with colonic transit, additional models to examine the potential “differential” association between colonic transit and BMI were examined by including a subgroup by BMI interaction term in these models. In addition, the association of BMI with overall colonic transit separately in each specific subgroup was examined (linear regression models). In all analyses, P<0.05 was considered significant.
RESULTS
Participants
The characteristics of the subjects are shown in Table 1. Healthy controls had a significantly lower proportion of females than the IBS-C/FC and IBS-D/FD groups (P<0.01). The patients with IBS-M were all female. An overall association of age with subgroup was observed (p<0.001) with the lower FGID subgroups being similar in age, but older than healthy controls (P<0.01). An overall association of BMI with subgroup was also observed (p<0.01) with IBS-D/FD and IBS-M patients having higher BMI values than healthy volunteers and IBS-C/FC subjects.
Table 1. Characteristics of 457 Participants.
| IBS-C/FC | IBS-D/FD | IBS-M | Healthy controls | |
|---|---|---|---|---|
| Number | 118 | 139 | 30 | 170 |
| Male :Female (% of female) |
5:113a, b (95.8%) |
24:115a (82.7%) |
0:30a, b (100%) |
52:118 (69.4%) |
| Age (years) | 38.7 ± 1.0a | 41.4 ± 1.2a | 37.1 ± 2.1 | 33.7 ± 0.9 |
| BMI (kg/m2)c | 25.3 ± 0.4 | 27.4 ± 0.5 | 27.8 ± 0.8 | 25.5 ± 0.3 |
Age and BMI are shown by mean ± SEM. Overall association of subject group with age (p<0.001), gender(p<0.001), and BMI (p<0.01).
p<0.01 vs. healthy controls
p<0.01 vs. IBS-D/FD
p<0.001 for overall association of BMI with subgroup
Effect of Gender on Orocecal and Colonic Transit
Male gender was associated with accelerated orocecal transit compared to female gender. Male gender was also associated with accelerated colonic transit based on GC24 and GC48, but not on GC8, compared to female gender. These effects of gender on orocecal and colonic transit were taken into account and adjusted for in our statistical analysis using ANCOVA, as described above in the Method section.
Comparison of Orocecal and Colonic Transit in Subtypes of IBS
There was no significant association of orocecal transit (CF 6h) between healthy controls and subtypes of IBS (p=0.38, Table 2).
Table 2. Key Gastrointestinal Physiologic Data in Subjects Based on Bowel Function.
| Subgroup | ||||
|---|---|---|---|---|
| IBS-C/FC | IBS-D/FD | IBS-M | Healthy Controls | |
|
Colonic filling at 6 h, %
(n=378) |
52.0 ± 2.9 (n=89) |
55.2 ± 3.2 (n=93) |
44.1 ± 5.1 (n=29) |
50.0 ± 2.4 (n=167) |
|
Colonic GC 8
*
(n=346) |
1.3 ± 0.1 (n=79) |
1.7 ± 0.1 (n=93) |
1.3 ± 0.1 (n=29) |
1.3 ± 0.1 (n=145) |
|
Colonic GC 24
**
(n=453) |
2.0 ± 0.1 (n=118) |
3.2 ± 0.1 (n=138) |
2.3 ± 0.1 (n=30) |
2.4 ± 0.1 (n=167) |
|
Colonic GC 48
**
(n=411) |
3.0 ± 0.1 (n=118) |
4.3 ± 0.1 (n=112) |
3.9 ± 0.2 (n=30) |
3.6 ± 0.1 (n=151) |
Overall ANOVA
P<0.01
P<0.001
Overall colonic transit at 24 hours
Data on colonic transit at 24 hours (GC24) were available in 286 lower FGID patients. GC24 was abnormal (GC24 of <1.50 or >3.86) in 29.7% (85/286) of all lower FGID patients. Delayed transit was detected in 17.8% (21/118) of IBS-C/FC patients, 8.0% (11/138) of IBS-D/FD patients, and 6.7% (2/30) of IBS-M patients. In contrast, accelerated transit at 24 hours was found in 4.2% (5/118) of IBS-C/FC patients and 33.3% (46/138) of IBS-D/FD patients (Table 2).
Overall colonic transit at 48 hours
Data on colonic transit at 48 hours (GC48) were available in 260 lower FGID patients. GC48 was delayed (GC48 of <2.13) in 13.1% (34/260) of all lower FGID patients. Delayed transit was detected in 22.9% (27/118) of IBS-C/FC patients, and 4.5% (5/112) of IBS-D/FD patients, and 6.7% (2/30) of IBS-M patients.
There were no patients who had accelerated GC48. It is worth noting that at 48 hours, a large proportion of the healthy controls (11%) and the IBS-D/FD (37%) group had reached the maximum value of GC48 of 5.0.
Among the 260 lower FGID patients with GC24 and GC48 available, 73 patients had abnormal GC24 and, of these, 21 had abnormal GC48: 15 IBS-C/FC, 4 IBS-D/FD, and 2 IBS-M patients. Out of 187 lower FGID patients with normal GC24, 13 had abnormal GC48: 12 IBS-C/FC and 1 IBS-D/FD patient.
IBS subgroups and colonic transit
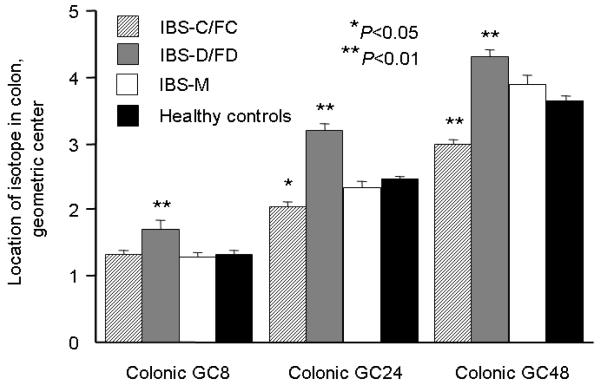
There was a significant association of colonic transit at 8, 24 and 48 hours with subgroup status (ANCOVA P <0.01 for GC8, P<0.001 for GC24 and GC48, Table 2). Significant differences were found in the colonic transit of individual lower FGID subtypes versus healthy controls, adjusted for gender and BMI: in IBS-C/FC at 24 and 48 hours (P <0.05), and in lower IBS-D/FD at 8, 24 and 48 hours [P <0.01 (Figure 2)]. The numerical difference suggesting faster transit at 48 hours in the IBS-M group was not significant.
Figure 2.
Comparison of colonic GC at 8, 24 and 48 hours in healthy controls and lower FGID patients with different types of bowel dysfunction. *P <0.05; **P <0.01
Colonic Transit and BMI
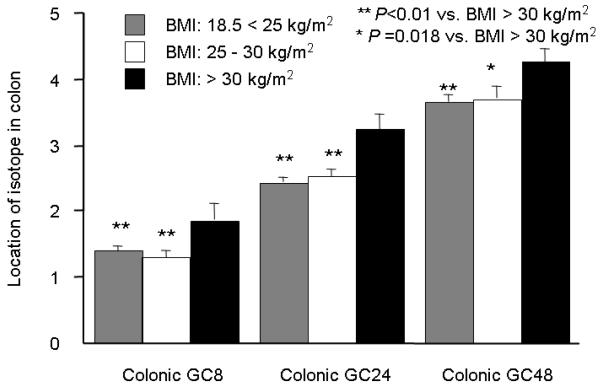
When participants were divided into three groups according to WHO criteria for BMI values (18.5 < 25 kg/m2, 25–30 kg/m2, and >30 kg/m2), colonic transit at 8, 24, and 48 hours were significantly accelerated in participants who weighed >30 kg/m2 (Dunnett-Hsu test with BMI >30 as the “control”, Figure 3, adjusted for age, gender, and subject subtype).
Figure 3.
Comparison of least square means for colonic GC at 8, 24 and 48 hours among 3 groups according to the BMI values. **P <0.01 versus BMI >30 kg/m2; P=0.018 versus BMI >30 kg/m2
DISCUSSION
Our study demonstrates that colonic transit is significantly different among subtypes of lower FGID compared to healthy controls. This finding is important for clinical assessment of patients with lower FGID, and confirms that motor dysfunction contributes to the pathophysiology in lower FGID (17). We also show that BMI independently influences colonic transit, regardless of the presence of subtype of lower FGID. In particular, BMI >30 kg/m2 is associated with significantly accelerated colonic transit. Scintigraphic colonic transit measurement may identify subgroups more likely to respond to treatment directed at dysmotility.
In this study, age was significantly associated with subject subgroup (lower FGID subtypes and healthy controls). However, our group had previously (16) shown that colonic transit was not significantly influenced by age in the range that is relevant in the current cohort (<65). Therefore, we do not consider it likely that group age differences influenced our results, and in fact, although age was included in the ANCOVA models as a covariate, it was not significantly associated with colonic transit in this large series of subjects as well.
Previously, disturbances of large bowel motility have been postulated to be an important feature of IBS. Given that one of the key features of IBS is abnormal stool form or frequency, it seems likely that abnormal colonic motility plays a major role in IBS. However, proof of abnormal colonic transit has not been forthcoming in many studies conducted in IBS. For example, in a factor analysis, factor-based scores for IBS correlated significantly with aberrant viscerosomatic referral patterns and correlated inversely (r=−0.207, p<0.05) with right colon transit (7). Bouchoucha et al. noted no association between segmental colonic transit values and signs or symptoms comprising the Rome II criteria (18). In contrast, our study shows that patients with IBS-C/FC may present with delayed colonic transit, whereas those with IBS-D/FD often have accelerated colonic transit. This study also suggests that measurement of colonic transit may impact patient care, identifying optimized treatment such as the use of colonic prokinetics in patients with IBS-C/FC associated with delayed colonic transit.
Many studies have examined the role of abnormal GI motor patterns and functions in patients with IBS. GI and colonic transit measurements are simple to perform and may have direct implications on the conduct of further diagnostic tests and on the choice of therapy (19). However, the sample sizes in prior studies were small, and the majority of studies assessed transit in patients with predominant constipation and usually with radiopaque maker transit measurements that are not typically conducted with multiple radiographs during the first two days after marker ingestion, which would appear necessary to identify accelerated colonic transit. The current observations on colonic transit among subtypes of patients with lower FGID are consistent with our previous studies using scintigraphy in patients with IBS-D, IBS-C, or chronic constipation (9,20,21). However, these were all smaller studies. Bouchoucha et al. (18) reported a marked difference in transit times between IBS patients and normal subjects in a large study (134 controls and 752 patients with IBS) using radiopaque markers, although there was no association between colonic transit and bowel function. These observations are not consistent with our study. Possible reasons for the discrepancy between these studies may be differences in criteria defining the study groups. We classified patients with lower FGID into the three subtypes as defined in the Rome II criteria, whereas Bouchoucha et al. divided patients into four subgroups based on radiopaque marker colonic transit time. Another reason for the discrepant results is the difference in the method to evaluate colonic transit by radiopaque markers or scintigraphy. As mentioned above, radiopaque marker techniques, which are widely used for assessment of delayed colonic transit by imaging 4 or 5 days after marker ingestion, would require earlier radiographs to detect accelerated transit. Sadik et al. demonstrated that this approach with multiple imaging times can identify accelerated transit in patients with diarrhea, but permissible radiation exposures would have to be checked at each center (19). Furthermore, larger non-digestible radiopaque markers may transit through the colon at a different rate when compared with the 1–2 mm or smaller markers used in scintigraphy (22).
In our study overall colonic transit in IBS-M was not significantly different from that in healthy controls. Until now, there have been no reports regarding colonic motility in a large number of patients with IBS-M. In our prior study, we did identify acceleration of transit at 48 hours in IBS-M; in the current study, the GC48 in the group with IBS-M did not reach statistical significance, though there is a numerically higher GC48 relative to healthy controls (P=0.68). In this relatively large sample of lower FGID patients, 26.3% of 118 patients with IBS-C/FC had delayed colonic transit (at either GC24 or GC48), and 33.3% of 138 patients with IBS-D/FD had accelerated colonic transit (at either GC24 or GC48). Transit measurements may be applicable in patients who report significant alteration of bowel function (IBS-M), as they may verify the reported symptoms and provide further assessment of the severity of the physiological abnormalities. Sadik et al. (19) reported 34% of 70 patients with predominant constipation had delayed colonic transit; whereas 28% and 7% of 54 patients with predominant diarrhea had accelerated and delayed colonic transit, respectively. These findings are consistent with our current study.
There was a relatively low prevalence of slow colonic transit in 16.4% (47/286) of lower FGID patients, and no significant small bowel transit delay in our patients, reflecting in part the large coefficient of variation in small bowel transit (23). Small intestinal motor function has been investigated in smaller studies in the literature from 2 to 4 decades ago using laboratory based or ambulatory methods (9,21). A larger study of 74 patients reported by Posserud et al. in 2007 (24) revealed quantitative data that were similar to those published in healthy controls. Hence, our findings on small bowel transit generally are consistent with the previous studies showing no significant changes in small bowel motor function in IBS.
In this study, we observed positive correlation between overall colonic transit and BMI, with higher BMI showing an independent and significant relationship to faster colonic transit. Our results are also supported by previous epidemiological studies (11,25). Delgado-Aros et al. noted a positive association between BMI and diarrhea in a random population sample from Olmsted County, Minnesota (11). BMI >25 kg/m2 was associated with decreased colonic compliance and pain sensation, but there was no significant association between obesity and sensation for gas, colonic tone, and contraction after meal ingestion (26). Although the reason why obesity is associated with acceleration of colonic transit is unclear, we consider two potential explanations. First, a high fat and carbohydrate load can overwhelm normal jejunal absorption, propelling the osmotic load distally and resulting in delivery of greater content of water distally (27,28). Second, a positive correlation between body weight and fecal excretion of bile acids has been shown (29), and increased delivery of bile acids into the colon can lead to cholerrheic diarrhea (30). Sadik et al. showed a significantly higher BMI in idiopathic bile acid malabsorption patients compared with healthy subjects (31). The clinical impact of diarrhea in obesity is unknown, so further studies are required to explore the mechanisms of accelerated transit in lower FGID.
Until now, there have been few studies regarding differences of BMI among IBS subtypes. Recently, Simren et al. reported a large study showing that there was no significant difference between IBS and healthy controls, but they did not show the differences of BMI among IBS subtypes (32). Our prior study, which included 44 patients with IBS-D who had higher BMI (33) is confirmed in our current study which includes 94 more patients with IBS-D/FD. These findings also confirm the previous observations in the literature that demonstrated increased number of postprandial high amplitude propagated contractions (34,35) in IBS-D/FD, which are generally associated with propulsion or mass movements in the colon (36).
There are some limitations to this study. First, we used the Rome II criteria (23) to sub-classify IBS, based on patient symptoms. The Rome III criteria simplified this classification by using only stool consistency (37). Most patients with IBS have a stool frequency within the normal range regardless of bowel pattern (38), but stool consistency reflects intestinal transit time (39). If we had used the Rome III criteria, we may have shown a greater difference in gastrointestinal transit patterns among the IBS subtypes. Second, it is possible that symptoms of some patients with IBS changed during the time period between classification of bowel habit subtype and the testing, because previous study indicated IBS symptoms are unstable overtime and variable in intensity (40). We do not perceive this is likely to be a pitfall in this study, since the time lag between classification of bowel habit subtype and the transit testing was within 14 days. Third, we did not pursue detailed analysis of colonic segmental transit time. A frequently reported motor abnormality in patients with IBS is an exaggerated sigmoid colonic motility response to meal ingestion (35). It may be helpful to evaluate the differences in colonic segmental transit time, such as ascending colon emptying time, in greater detail to further characterize the motility patterns seen among the subtypes of IBS. Fourth, we did not investigate weight-related behaviors as covariates in studying the relationship between colonic transit and BMI. Levy et al. showed that a diet lower in fat and high in fruit/fiber, as well as higher physical activity were associated with fewer GI symptoms (41). Thus, further studies are required to explore the effect of these weight-related behaviors on colonic transit.
Colonic transit time has been shown to correlate with stool form, as measured by the Bristol Stool Form Scale (42). The effect of various kinds of medications that affect GI transit time has been studied in patients with IBS and correlations between effects of medications on total colonic transit and stool number or stool form have been shown for a number of drugs, such as alosetron (43), tegaserod (44), or prucalopride (45, 46). In addition, these improvements in colonic transit and stool number or stool form are also correlated with clinical end points such as improved quality of life and decreased IBS-related symptoms (47).
The observations in this study support the hypothesis that scintigraphic colonic transit fulfills many qualifications as a biomarker in lower FGID. There are other important characteristics in support of this hypothesis: First, we recently reported on the stability of colonic transit measurements in patients over a period of up to 6 years with a relatively low coefficient of variation [around 0.2 geometric center (Deiteren et al. submitted)]; second, responsiveness to medications that accelerate or retard transit; third, the predictive value of such pharmacodynamic measurements of transit to clinical responsiveness in phase IIB or III trials (47).
In conclusion, lower FGID including IBS presents as a constellation of symptoms, as well as a definable phenotype that reflects an underlying motor pathophysiology in about 30% of patients. Optimal management may require identification and characterization of this pathophysiology in selecting patients for pharmacologically-directed therapy for each subtype of lower FGID.
Acknowledgements
Dr. Camilleri is supported by RO1 grant DK-54681 from the National Institutes of Health. We thank Mrs. Cindy Stanislav for secretarial support.
Abbreviations used in this paper
- FGID
functional gastrointestinal disorders
- IBS
irritable bowel syndrome
APPENDIX
Publications of previously performed gastrointestinal transit studies conducted in patients with IBS and healthy controls from which data were derived in a retrospective manner for the current study
- Bharucha AE, Camilleri M, Haydock S, et al. Effects of a serotonin 5-HT(4) receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut. 2000;47:667–74. doi: 10.1136/gut.47.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather CM, Camilleri M, Zinsmeister AR, et al. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–8. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- Viramontes BE, Camilleri M, McKinzie S, et al. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2671–6. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- Viramontes BE, Malcolm A, Camilleri M, et al. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1468–76. doi: 10.1152/ajpgi.2001.281.6.G1468. [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S, Chial HJ, Camilleri M, et al. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G558–66. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:784–91. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- Gonenne J, Esfandyari T, Camilleri M, et al. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Nuerogastroenterol Motil. 2006;18:911–8. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- Park MI, Ferber I, Camilleri M, et al. Effect of atilmotin on gastrointestinal transit in healthy subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:28–36. doi: 10.1111/j.1365-2982.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- Esfandyari T, Camilleti M, Ferber I, et al. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–8. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Vazquez-Roque MI, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–8. doi: 10.1111/j.1365-2982.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–8. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Grudell AB, Camilleri M, Jensen KL, et al. Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1114–9. doi: 10.1152/ajpgi.00051.2008. [DOI] [PubMed] [Google Scholar]
- Sweetser S, Camilleri M, Linker Nord SJ, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Disclosures: The authors have no conflicts of interest.
REFERENCES
- 1.Lee KT, Rhee PL, Kim J, et al. Assessment of autonomic tone over a 24-hour period in patients with irritable bowel syndrome (abstr) Gastroenterology. 1997;112:A773. [Google Scholar]
- 2.Everhart JE, Renault PF. Irritable bowel syndrome in office based practice in the United States. Gastroenterology. 1991;100:988–1005. doi: 10.1016/0016-5085(91)90275-p. [DOI] [PubMed] [Google Scholar]
- 3.Levy RL, Stang P, von Korff M, et al. Longitudinal study of the comparative costs of IBS in an HMO (abstr) Am J Gastroenterol. 2000;95:A2540. [Google Scholar]
- 4.Camilleri M. Motor function in irritable bowel syndrome. Can J Gastroenterol. 1999;13(Suppl A):8A–11A. doi: 10.1155/1999/240329. [DOI] [PubMed] [Google Scholar]
- 5.Harraf F, Schmulson M, Saba L, et al. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. 1998;43:388–394. doi: 10.1136/gut.43.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao SS, Ozturk R, De Ocampo S, et al. Clinical utility of diagnostic tests for constipation in adults: a systemic review. Am J Gastroenterol. 2005;100:1605–1615. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 7.Mertz H, Naliboff B, Mayer EA, et al. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol. 1999;94:131–138. doi: 10.1111/j.1572-0241.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 9.Vassallo M, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Odgen CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Aros S, Locke GR, Camilleri M, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–1806. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Howel S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. 2004;99:1807–1814. doi: 10.1111/j.1572-0241.2004.30388.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Quan C, Jones MP, et al. Association of upper and lower gastrointestinal symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16:413–419. doi: 10.1111/j.1365-2982.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Proano M, Camilleri M, Phillips SF, et al. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–G862. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 15.Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 16.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 17.Bennett EJ, Evans P, Scott AM, et al. Psychological and sex features of delayed gut transit in functional gastrointestinal disorders. Gut. 2000;46:83–87. doi: 10.1136/gut.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchoucha M, Devroede G, Dorval E, et al. Different segmental transit times in patients with irritable bowel syndrome and “normal” colonic transit time: is there a correlation with symptoms? Tech Coloproctol. 2006;10:287–296. doi: 10.1007/s10151-006-0295-9. [DOI] [PubMed] [Google Scholar]
- 19.Sadik R, Stotzer PO, Simren M, et al. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary center. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 20.Cann PA, Read NW, Brown C, et al. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom pattern. Gut. 1983;24:405–411. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stivland T, Camilleri M, O’Brien MD, et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–115. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 22.Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74:698–703. [PubMed] [Google Scholar]
- 23.Thompson WG, Longstreth G, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Corazziari E, Talley NJ, et al., editors. Rome II: The functional gastrointestinal disorders. 2nd Ed. Degnon Associates; McLean, VA: 2000. pp. 351–432. [Google Scholar]
- 24.Posserud I, Stotzer PO, Bjomsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulpitt CJ, Palmer AJ, Battersby C, et al. Association of symptoms of type II diabetic patients with severity of disease, obesity, and blood pressure. Diabetes Care. 1998;21:111–115. doi: 10.2337/diacare.21.1.111. [DOI] [PubMed] [Google Scholar]
- 26.Delgado-Aros S, Camilleri M, Garcia MA, et al. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol. 2008;295:G382–G388. doi: 10.1152/ajpgi.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab. 2001;27:357–364. [PubMed] [Google Scholar]
- 28.Verdich C, Madsen JL, Toubro S, et al. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord. 2000;24:899–905. doi: 10.1038/sj.ijo.0801250. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen TA. Cholesterol production in obesity. Circulation. 1971;44:842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- 30.Reuben A, Qureshi Y, Murphy GM, et al. Effect of obesity and weight reduction on biliary cholesterol saturation and the response to chenodeoxycholic acid. Eur J Clin Invest. 1986;16:133–42. doi: 10.1111/j.1365-2362.1986.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 31.Sadik R, Abrahamsson H, Ung KA, et al. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 32.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;163:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 33.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi MG, Camilleri M, O’Brien MD, Kammer PP, Hanson RB. A pilot study of motility and tone of the left colon in patients with diarrhea due to functional disorders and dysautonomia. Am J Gastroenterol. 1997;92:297–302. [PubMed] [Google Scholar]
- 35.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 36.Cook IJ, Furukawa Y, Panagopoulos V, Collins PJ, Dent J. Relationships between spatial patterns of colonic pressure and individual movements of content. Am J Physiol. 2000;278:G329–G341. doi: 10.1152/ajpgi.2000.278.2.G329. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth GF, Thompson WG, Houghton LA, et al. Functional bowel disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., editors. Rome III: The functional gastrointestinal disorders. 3rd Ed. Degnon Associates; McLean, VA: 2006. pp. 487–555. [Google Scholar]
- 38.Mearin F, Balboa A, Badia X, et al. Irritable bowel syndrome subtypes according to bowel habit: Revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15:165–172. doi: 10.1097/00042737-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 40.Mearin F, Baró E, Roset M, et al. Clinical pattern over time in irritable bowel syndrome: Symptom instability and severity variability. Am J Gastroenterol. 2004;99:113–21. doi: 10.1046/j.1572-0241.2003.04023.x. [DOI] [PubMed] [Google Scholar]
- 41.Levy R, Linde JA, Feld KA, et al. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol. 2005;3:992–996. doi: 10.1016/s1542-3565(05)00696-8. [DOI] [PubMed] [Google Scholar]
- 42.Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viramontes BE, Camilleri M, McKinzie S, et al. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2671–2676. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 44.Prather CM, Camilleri M, Zinsmeister AR, et al. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 45.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 46.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;29:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 47.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]