Abstract
Characterizing normal brain development in the rhesus macaque is a necessary prerequisite for establishing better nonhuman primate models of neuropathology. Structural magnetic resonance imaging scans were obtained on 37 rhesus monkeys (20 Male, 17 Female) between 10 and 64 months of age. Effects of age and sex were analyzed with a cross-sectional design. Gray matter (GM) and white matter (WM) volumes were determined for total brain and major cortical regions using an automatic segmentation and parcellation pipeline. Volumes of major subcortical structures were evaluated. Unlike neural maturation in humans, GM volumes did not show a postpubertal decline in most cortical regions, with the notable exception of the prefrontal cortex. Similar to humans, WM volumes increased through puberty with less change thereafter. Caudate, putamen, amygdala, and hippocampus increased linearly as did the corpus callosum. Males and females showed similar maturational patterns, although males had significantly larger brain volumes. Females had a proportionately larger caudate, putamen, and hippocampus, whereas males had both an absolute and relatively larger corpus callosum. The authors discuss the possible implications of these findings for research using the rhesus macaque as a model for neurodevelopmental disorders.
Keywords: adolescence, cerebral cortex, Macaca mulatta, puberty, rhesus
Introduction
A comprehensive understanding of normal brain development is essential to advancing our understanding of neurodevelopmental disorders including autism, schizophrenia, and mental retardation. The advent of quantitative magnetic resonance imaging (MRI) has significantly expanded our knowledge about human brain development during childhood and adolescence and several large-scale cross-sectional (Pfefferbaum et al. 1994; Reiss et al. 1996; Caviness et al. 1996; Courchesne et al. 2000; De Bellis et al. 2001) and longitudinal studies have now been published (Giedd et al. 1999; Sowell et al. 2004; Lenroot and Giedd 2006). Although this research has identified critical periods in human brain development, especially the reduction in gray matter during the peripubertal years which is thought to reflect dendritic pruning, animal models are also needed to enable controlled manipulations of genetic and environmental factors, and to test the efficacy of treatment interventions. Among the possible nonhuman primate models, the rhesus macaque (Macaca mulatta) has been the most widely used monkey to investigate the neural substrates of human behavior, due to its phylogenetic closeness and the potential to examine more complex cognitive functions and social behavior. For more than 40 years, this species has been used to evaluate how disturbances of the early rearing environment can induce behavioral abnormalities (Harlow and Harlow 1962; Harlow and McKinney 1971; Harlow et al. 1971), and it still holds great potential as a primate model of developmental neuropathology (Machado and Bachevalier 2003; Nelson and Winslow 2009). Although there are excellent neuroanatomical descriptions of early brain maturation in the monkey (Rakic and Goldman-Rakic 1982), information on the normal maturation of the monkey brain during puberty remains more limited, especially with regard to the analytic approaches used in MRI.
The prevailing view from studies in humans is that total brain volume undergoes a rapid nonlinear increase during childhood and reaches a maximum around puberty. Brain growth in the first 2 years of life is driven primarily by increases in gray matter (GM) (Gilmore et al. 2007; Knickmeyer et al. 2008). From age 4 to age 22, total GM as well as GM within the frontal, parietal, and temporal lobes follows an inverted U-shaped maturational trajectory, with the age of maximum volume being attained during the peripubertal period, although the age point for the actual peak varies by region. In contrast, GM volume in the occipital lobe increases linearly (Giedd et al. 1999). White matter (WM) generally increases in a linear fashion well into adulthood (Jernigan and Tallal 1990; Reiss et al. 1996; Giedd et al. 1999; Schaefer et al. 1990). The only MRI study of juvenile and adolescent brain development in the rhesus macaque reported that as in humans, they attain maximum total brain volume around sexual maturity and have an extended period of WM growth into adulthood (Malkova et al. 2006). However, this study did not measure GM volumes or examine variation between different cortical regions or in subcortical structures. Pierre et al. (2008) have reported that in a closely related species, juvenile bonnet macaques (Macaca radiata) had lower total brain volumes and GM/WM ratios than adolescent and adult monkeys, but did not provide data on regional variation. A neuroimaging study of capuchin monkeys, which are more phylogenetically distant from humans, also revealed rapid nonlinear growth in total brain size during the first few years of life, which was driven primarily by WM expansion (Phillips and Sherwood 2008). No growth was seen in total GM or GM in the frontal lobe. Although human studies suggest that postnatal cortical development is very heterochronous (i.e., with different timing in diverse cortical regions), postmortem studies suggest that cortical development in monkeys is more synchronous (Rakic et al. 1986; Bourgeois and Rakic 1993).
Studies in humans also indicate that there are marked sexual dimorphisms in the central nervous system during both childhood and adulthood. The most consistent findings are greater intracranial volume (ICV) and total brain volumes in males (Dekaban and Sadowsky 1978; Caviness et al. 1996; Giedd et al. 1996, 1997; Reiss et al. 1996; Nopoulos et al. 1997; Filipek 1999; De Bellis et al. 2001; Goldstein et al. 2001); higher proportion of GM-to-WM in females (Gur et al. 1999; Goldstein et al. 2001; Allen et al. 2003), relatively greater volume of the amygdala in males (Caviness et al. 1996; Good et al. 2001), and relatively greater volume of the caudate (Filipek et al. 1994; Caviness et al. 1996; Giedd et al. 1996, 1997) and hippocampus (Filipek et al. 1994; Caviness et al. 1996) in females. Males and females also differ in specific developmental growth trajectories. Total cerebral volume, caudate volume, and GM volume in the frontal and parietal lobes peak earlier in girls than in boys (though the exact ages vary depending on the region), a pattern which may relate to sex differences in timing of puberty. In adolescence, WM increases more rapidly in males than in females (Lenroot et al. 2007). These sex differences in neurobiology may be highly relevant to neurodevelopmental pathologies, which frequently show marked sex differences in risk, phenotypic expression, and treatment response (Szatmari et al. 1989; Moffitt 1990; Gur et al. 1996; Hafner et al. 1998; Chakrabarti and Fombonne 2001; Moffitt and Caspi 2001; Baird et al. 2006; Kulkarni et al. 2008). Research on the monkey may be particularly informative about the role of pubertal onset in determining these brain changes because females reach puberty 1–3 years before males. In addition, adult rhesus monkeys also display large sex differences in brain size (Falk et al. 1999), in part because the male brain continues to grow significantly after puberty (Franklin et al. 2000). Nevertheless, some subcortical areas, such as the amygdala, have been reported to not differ in size between male and female monkeys.
It is clear that a better characterization of normal brain development in the rhesus macaque is necessary to identify the similarities and differences between humans and the macaque as well as to better align the maturational changes associated with the critical transitional period of puberty. This is a necessary prerequisite for investigating the developmental impact of experimental manipulations that induce neuropathology. In this paper, we report the first structural MRI study to systematically measure GM and WM volume in different cortical regions in a large sample of male and female rhesus macaques, ranging in age from juveniles to young adults. Our initial hypotheses were 1) because the rhesus is born relatively more mature than humans, GM development at 1 year of age would be far more comparable to the mature adult in macaques than in humans, 2) WM would increase in a linear fashion throughout the juvenile and adolescent period, 3) growth patterns would be similar across cortical regions, and 4) development would be prolonged in male monkeys compared with females.
Materials and Methods
Subjects
Thirty-seven rhesus monkeys (20 Male, 17 Female) between the ages of 10 and 64 months (see Supplementary Table S1). All subjects were generated from a large 500+ monkey-breeding colony at the Harlow Primate Laboratory (University of Wisconsin, Madison, WI), with known pedigree and clinical history extending back 8 generations and over 50 years. The cohort of monkeys used for these MR analyses were generated from 35 different adult females bred with 22 different adult males. There were no full siblings included. There were 2 sets of maternal half-siblings, and 9 sets of paternal half-siblings (7 sets had 2 members; 3 sets had 3 members and 1 set had 5 members). 12 monkeys had no half-sibs in the study. The number of half-siblings is not sufficient for a statistical test of the contribution of genetics to interindividual variability and is unlikely to compromise the independence of the data points.
Rearing Conditions
All monkeys were reared and housed in standardized conditions. Infants were reared by the mother until 6–8 months of age, and then weaned into small social groups, each comprised of 4–8 animals. Subadult animals were housed in mixed-age groups or as social pairs with another animal of the same age and sex. Thus, all animals scanned for this project had been socially reared, maintained in a controlled manner, and were free of any experimental manipulations that would alter brain development. Animals were fed a standardized diet of commercial biscuits (Teklad, Harlan Laboratories, Indianapolis, IN) and provided fruit supplements and foraging devices for enrichment. Water was available ad libitum, temperature controlled at 23 °C, and the light:dark cycle maintained at 2:10 PM with lights on at 06:00 AM. The housing conditions were in accordance with regulatory guidelines and the experimental procedures were approved by the institutional Animal Care and Use Committee of the University of Wisconsin-Madison.
MRI Acquisition
Imaging was performed on a GE SIGNA 3T scanner (General Electric Medical Systems, Milwaukee, WI) with a high-resolution coronal 3D, Inversion-Recovery -Prepped, fast SPGR sequence for the T1-weighted scan (inversion time [TI] = 600 ms; time repetition [TR] = 8.6 ms, time echo [TE] = 2.0 ms, field of view [FOV] = 14 cm, flip angle = 10°, matrix = 256 × 256 [interpolated to 512 × 512], slice thickness = 1 mm [interpolated to 0.5 mm], bandwidth = ±15.63 kHz) which yielded a resulting continuous voxel size of 0.273 × 0.273 × 0.5 mm across the entire cranium. A T2-weighted scan (TR = 12 000 ms, TE = 90 ms, FOV = 14 cm, flip angle = 90°, 256 × 256 [interpolated to 512 × 512], voxel size = 0.2734 mm, slice thickness = 1.5 mm, slice gap = 0 mm, bandwidth = 31.25 kHz) provided a resulting continuous voxel size of 0.2734 × 0.2734 × 1.5 mm across the entire cranium. Immobilization was maintained throughout the 1-h scanning procedures by an initial administration of ketamine hydrochloride (10 mg/kg im) followed by medatomidine (50 μg/kg im). The heads of all of the younger monkeys were oriented identically within a stereotaxic platform; the larger 4- to 5-year-old subadults were oriented in the same plane on a pillow; all within an 18-cm-diameter quadrature extremity coil (IGC Medical Advances, Milwaukee, WI). Similar orientation and the absence of marked head tilt or yaw were verified when visualizing the initial brain images in the sagittal and coronal planes.
MRI Analysis
A framework was developed for fully automatic atlas-based segmentation of brain tissue, lobar parcellation and delineation of subcortical structures in the rhesus macaque (Styner et al. 2007). A structural atlas was built from 18 training images (approximately uniformly distributed across age range of 16–34 months) by iterative, joint deformable registration into an unbiased average image. All monkeys in the training data were also employed in the study. On this atlas, probabilistic tissue maps, a lobar parcellation, and subcortical structures were determined. This information was then applied to each subject. First, the probabilistic tissue maps were affinely registered to each subject's T1-and T2-weighted images for an atlas-based Expectation Maximization tissue classification (see Fig. 1). This step provided intensity inhomogeneity correction, intensity normalization, and skull stripping, all of which are necessary for the second step which is a deformable registration of the lobar and subcortical parcellations. The deformed parcellation regions mask the tissue segmentations to define the parcellation for WM and GM separately. Regional volumes are the sum of the bilateral regions in both hemispheres. See Supplementary Material for full details of anatomical boundaries.
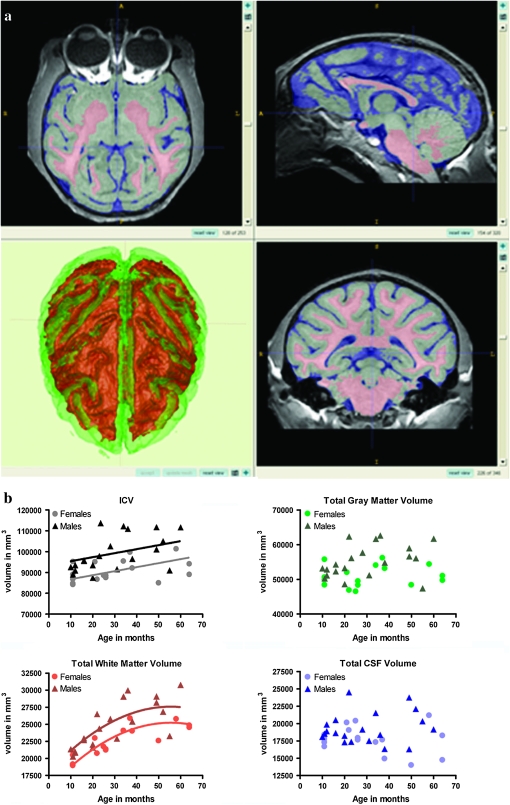
Figure 1.
Results of the tissue classification. (a) Tissue segmentation overlaid on the smoothed, intensity corrected image of a representative case. GM in green; WM in red; cerebrospinal fluid in blue. (b) Development of ICV and major tissue classes shown as scatter plots of volume in mm3 by age in months. When there was no evidence of a significant effect of age on estimation of brain volume, no regression line was fitted.
We validated the cortical GM and subcortical parcellations on 10 separate scans. All 10 scans were used to compare the automatic segmentation results to a manual expert's segmentations. Three of the 10 scans appeared 3 times in the validation dataset. These 3 scans, segmented in triplicate, were used to calculate intrarater variability. Therefore, the manual rater segmented a total of 16 validation data sets, randomized and blinded. Validation segmentations were performed by a single manual rater. The assessment was performed via our standard segmentation evaluation battery (Gerig et al. 2001; van Ginneken et al. 2007) with the resulting errors combining metrics of volumetric difference, volumetric overlap and surface distance. The intrarater coefficient of variation (COV) of the volumetric measurements was between 1% and 4% for all regions. These COV values are comparable with those in other manual validation studies (Styner et al. 2002). The automatic GM segmentations for all cortical regions showed an error, as compared with the expert segmentation, that was within 1.14 times the average intrarater error (average error between segmentations of the same dataset by the same rater), with the exception of the temporal auditory lobe (1.68 times) and cerebellum (4.04 times). The relatively high difference to the manual rater for the cerebellum is due to 2 observations: 1) a slightly higher absolute error of the automatic method as compared with other lobes and 2) a significantly smaller error for intrarater analysis (volume difference < 0.6%, volumetric overlap error < 1.2%) as compared with other lobes. Thus our analysis indicates that the volumetric measurements of the cerebellum as well as of the temporal auditory lobe are not as accurate as those of the other lobes when compared with human raters and results for this structure should clearly be treated with caution. Volumetric differences between the automatic subcortical structure segmentation and the manual expert segmentation are within 1.1 times the intrarater differences. Although we did not perform an inter-rater variability assessment, we have observed in our own human studies that inter-rater differences are commonly 2–13% higher than intrarater differences (Yushkevich et al. 2006; Styner et al. 2007). Thus, we expect our automatic method to be within the range of inter-rater differences. In contrast to human rater segmentations, our methods are deterministic and 100% reproducible.
Statistical Analysis
For comparison of demographic characteristics between males and females, 2-sample t-tests and Wilcoxon rank-sum tests were used for continuous variables and Fisher Exact tests were used for categorical variables. ANCOVA was used to compare the growth pattern of brain volumes between males and females. Also, cubic, quadratic, or linear polynomial regression models were fitted by age to find the growth pattern of brain volumes. Regression models were fitted both with and without centering to improve estimation, but the pattern of results was similar for both methods and thus only the results of regression without centering are presented here.
A backward elimination strategy, based on F-statistics was used to select the best polynomial regression model. Starting at the largest model (cubic polynomial regression with different intercept, linear, quadratic, and cubic terms of age by gender), a polynomial of degree k was compared with polynomial of degree k − 1, eliminating the highest order term based on a 1 degree of freedom F-test. If the eliminated term was significant, then the polynomial model of degree k was considered the best model. Otherwise, we continued backward elimination until we achieved a significant model. When male and female groups had different curves using the best-fitting model, 2 polynomial regression models were fitted separately by gender so that each gender group has its own best model. Males and females were considered to have different curves if the highest nonintercept term was significantly different between male and female at a modeling step. When we were not able to find a significant difference in both curve and intercept by gender, a common regression model was fitted to both genders. All polynomial regression models and hypotheses examined to find the best-fitting model are listed in the supplemental methods. A flowchart of the order of testing is also available (Supplemental Fig. S1).
We did not use likelihood functions themselves, Akaike information criterion, finite-population corrected Akaike information criterion, or Schwarz's Bayesian information criterion to compare the models preferring to use the comparison of 2 nested models. All statistical hypothesis tests were conducted at a significance level of 0.05. For raw brain volumes (uncorrected for ICV), the cubic model was never the best fit model. As there was no missing data, degree of freedom were [1,34] for all linear models (Test 12—Supplemental Methods) and [1,33] for all quadratic models (Test 7—Supplemental Methods). F and p values reported in the results text refer to the indicated effect (linear or quadratic) rather than the overall model. In order to estimate the percent change across the age range studied, the regression equations from the best-fitting model were used to calculate mean brain volume at 10 months of age and mean brain volume at 64 months of age. % Change = ((brain volume at 64 months − brain volume at 10 months)/brain volume at 10 months) × 100. Percent change was calculated separately for males and females, and then averaged.
Results
Global Brain and Tissue Volumes
ICV increased significantly across this age range, which corresponds to juvenile stage through young adulthood in the rhesus macaque (F = 8.97, linear effect P = 0.005). The percent increase based on regression equations was 11%. Male brains were approximately 10% larger than female brains with no evidence for sex differences in the slope of the maturational trajectory. Total GM did not show significant age-related changes, although there was a general statistical trend for GM to increase across this period (F = 2.96, linear effect P = 0.09). Age-related changes in WM volumes were best described by a quadratic curve (F = 7.20, quadratic effect P = 0.01), with faster growth in early life and into the peripubertal transition period with little change thereafter. Overall, WM volume increased by 31%. There was not a significant age-related change in the total amount of cerebrospinal fluid (CSF) in the ventricles and external space surrounding the brain (F is very close to 0, linear effect P = 0.98). Males had approximately 9% greater GM volumes, 10% greater WM volumes, and 9% greater CSF volumes with no evidence for sex differences in developmental trajectories (see Fig. 1 and Supplementary Table S2).
Gray Matter
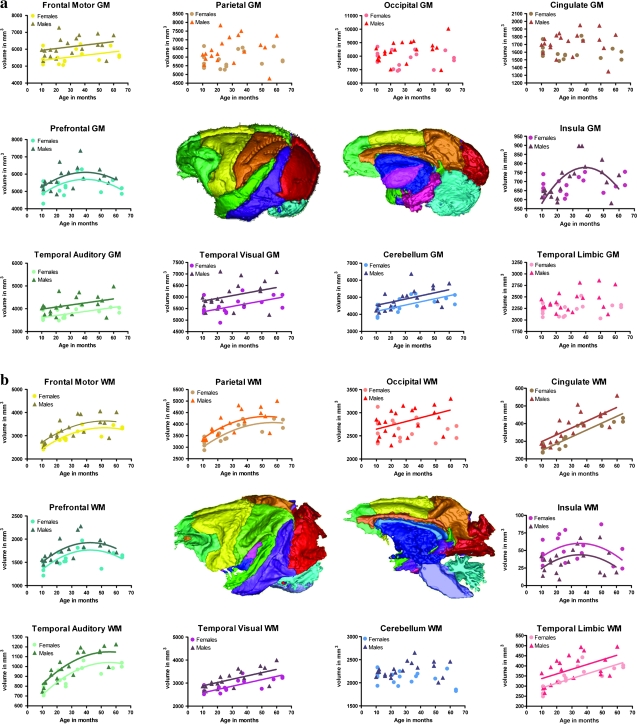
The developmental trajectories of cortical GM showed regional specificity. GM in the occipital, parietal, and cingulate cortex and the limbic portion of the temporal lobe showed no significant age-related changes. In contrast, GM increased in a linear fashion in the frontal motor cortex (F = 4.83, P = 0.03, % change = 10%), visual temporal cortex (F = 7.07, P = 0.01, % change = 12%), auditory temporal cortex (F = 10.01, P = 0.003, % change = 13%), and the cerebellum (F = 17.94, P = 0.0002, % change = 23%). GM in the prefrontal cortex followed a biphasic developmental course with peak volume reached around 40 months (F = 9.78, quadratic effect P = 0.0037). For the majority of cortical regions, males and females did not differ significantly in their developmental trajectories, but in all regions the males had larger GM volumes than did the females. The growth of GM in the insular cortex followed a biphasic pattern in males and declined after puberty, but showed no age-related change in females (see Fig. 2 and Supplemental Table S2).
Figure 2.
Results of the cortical parcellation. (a) Regional GM development shown as scatter plots of volume in mm3 by age in months. At the center are 3D meshes of the GM cortical parcellation (the one on the right has had the outer lobes removed to reveal interior structures). Light green (prefrontal), yellow (frontal motor), orange (parietal), red (occipital), brown (cingulate), fuschia (insula), bright pink (temporal limbic), aqua (cerebellum), purple (temporal visual), bright green (temporal auditory). (b) Regional WM development shown as scatter plots of volume in mm3 by age in months. At the center are 3D meshes of the WM cortical parcellation (the one on the right has had the outer lobes removed to reveal interior structures). Colors as above. Regional volumes are the sum of the bilateral regions in both hemispheres. When there was no evidence of a significant effect of age on estimation of brain volume, no regression line was fitted.
White Matter
WM development also showed regional specificity. WM increased linearly across this age range in the cingulate cortex (F = 87.28, P < 0.0001, % change = 78%), visual temporal cortex (F = 36.55, P < 0.0001, % change = 29%), limbic temporal cortex (F = 23.92, P < 0.0001, % change = 40%), and the corpus callosum (F = 51.32, P < 0.0001, % change = 70%). WM development was best described by a quadratic curve in the frontal motor cortex (F = 8.12, P = 0.0075, % change = 34%), parietal (F = 5.31, P = 0.0276, % change = 34%), and auditory temporal cortex (F = 11.77, P = 0.0016, % change = 40%), with accelerated growth in early life and into young adulthood with little change thereafter. WM in the prefrontal and insular cortex followed a biphasic pattern, first enlarging and then declining after puberty (F = 14.85, P = 0.0005 and F = 6.87, P = 0.0132, respectively). No age-related changes in WM were observed in the cerebellum. For the majority of cortical regions, the WM in males and females did not follow different developmental trajectories, but males had larger WM volumes than females in all regions except the insula. In the occipital lobe, WM volume increased linearly in males, but showed no age-related change in females. Although neither males nor females showed a significant linear effect of age on cerebellar WM, the sexes did have significantly different slopes, with an opposite sign in the estimated regression coefficient of slope. Male volumes increased slightly in this period, whereas female volumes declined slightly (see Fig. 2 and Supplemental Table S2).
Subcortical Structures
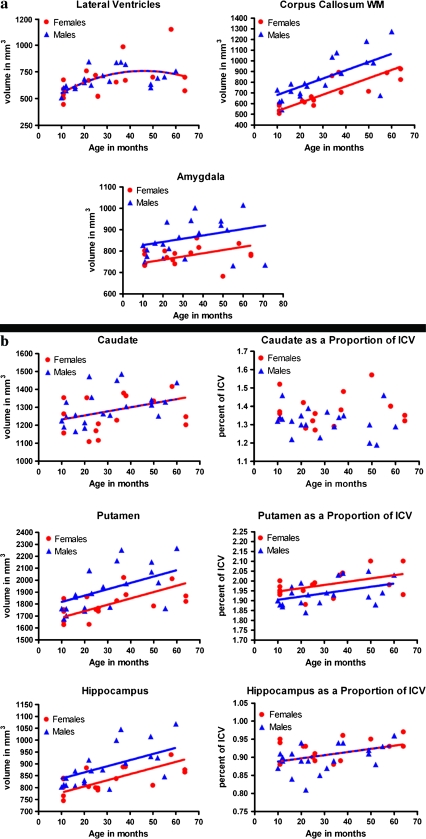
Volumes of the putamen, caudate, hippocampus, and amygdala all increased in a linear manner during this period (F = 18.02, P = 0.0002, % change = 16%; F = 6.09, P = 0.0187, % change = 10%; F = 22.49, P < 0.0001, % change = 18%; and F = 6.02, P = 0.02, % change = 11%, respectively). Males had larger absolute volumes than females, but the shape and slope of the trajectory did not differ between the sexes. Change in the volume of the lateral ventricles was best described by a quadratic curve (F = 4.20, P = 0.0482, % change = 29%), with accelerated growth in early life and into young adolescence with little change thereafter. Lateral ventricle volume did not differ between males in females and the shape of the developmental trajectory was the same in each sex (see Fig. 3 and Supplementary Table S2).
Figure 3.
Development of specific structures. (a) Development of the ventricles, corpus callosum and amygdala shown as scatter plots of volume in mm3 by age in months. (b) Development of the caudate, putamen, and hippocampus. Scatter plots in the left column represent absolute volume. Scatter plots in the right column represent volume as a percentage of ICV. Note that for the lateral ventricles, caudate, and hippocampus as a proportion of ICV; there was no significant difference in curve and intercept by gender, so a common regression model was fitted to both genders (represented by a red and blue striped line). Regional volumes are the sum of the bilateral regions in both hemispheres.
Sex Differences
Because males had a significantly larger ICV than females across the entire age range, we were interested in testing whether the significant sex differences we observed in specific lobar regions and subcortical structures were simply a consequence of the greater brain volume in males. Volumes can be normalized by ICV to calculate the ratio of tissue volumes and anatomical regions relative to the full brain. This allows one to identify regions that are proportionally larger in one sex. For GM volumes, most sex differences disappeared when controlling for ICV. However, females had proportionally more GM in the insular cortex than did males. In addition, GM in the frontal motor, parietal, and insular cortex showed different developmental trajectories when controlling for ICV. For WM volumes, no regions were significantly greater in males than females when controlling for ICV, although there was a trend for males to have proportionately greater total brain WM and relatively more WM in the cingulate. After ICV correction, females continued to have relatively more WM in the insular cortex. Similarly, females had proportionately larger caudate and putamen volumes than males, and there was a trend for females to have proportionately greater hippocampal volumes. There were no sex differences in amygdala volume after controlling for ICV, and males continued to have greater volume of the corpus callosum both before and after ICV correction (see Table 1).
Table 1.
Sex differences in developmental trajectories and overall brain volumes
| Structure | Male–female comparisons |
Comparisons adjusted for ICV (ratio) |
||||||
| Shape of curve difference |
Height (intercept) difference |
Shape of curve difference |
Height (intercept) difference |
|||||
| P value | F[df], (Test)a, polynomial | P value | F[df], (Test) | P value | F[df], (Test), polynomial | P value | F[df], (Test) | |
| ICV | 0.18 | 1.91[1,33], (Test 10), linear | <0.001b | 15.62[1,34], (Test 11) | n.a. | n.a. | n.a. | n.a. |
| Total gray | 0.33 | 0.98[1,33], (Test 10), linear | <0.01b | 12.35[1,34], (Test 11) | 0.03 | 5.51[1,29], (Test 1), cubic | n.a. | n.a. |
| Total white | 0.45 | 0.58[1,31], (Test 6), quadratic | <0.001b | 16.09[1,33], (Test 9) | 0.61 | 0.26[1,31], (Test 6), quadratic | 0.09 | 2.98[1,34], (Test 9) |
| Total CSF | 0.29 | 1.17[1,33], (Test 10), linear | 0.03b | 5.17[1,34], (Test 11) | 0.61 | 0.27[1,33], (Test 10), linear | 0.92 | 0.01[1,34], (Test 11) |
| Prefrontal gray | 0.56 | 0.34[1,31], (Test 6), quadratic | 0.02b | 5.94[1,33], (Test 9) | 0.89 | 0.02[1,31], (Test 6), quadratic | 0.56 | 0.35[1,33], (Test 9) |
| Frontal motor gray | 0.54 | 0.39[1,33], (Test 10), linear | <0.001b | 14.76[1,34], (Test 11) | 0.02 | 6.12[1,29], (Test 1), cubic | n.a. | n.a. |
| Parietal gray | 0.88 | 0.02[1,33], (Test 10), linear | 0.01b | 8.06[1,34], (Test 11) | 0.01 | 9.03[1,29], (Test 1), cubic | n.a. | n.a. |
| Occipital gray | 0.23 | 1.48[1.33], (Test 10), linear | 0.01b | 7.23[1,34], (Test 11) | 0.68 | 0.17[1,31], (Test 6), quadratic | 0.57 | 0.33[1,33], (Test 9) |
| Temporal visual gray | 0.58 | 0.31[1,33], (Test 10), linear | <0.01b | 9.56[1,34], (Test 11) | 0.23 | 1.48[1,33], (Test 10), linear | 0.45 | 0.59[1,34], (Test 11) |
| Temporal auditory gray | 0.62 | 0.25[1,33], (Test 10), linear | <0.001b | 14.98[1,34], (Test 11) | 0.13 | 2.40[1,33], (Test 10), linear | 0.85 | 0.04[1,34], (Test 11) |
| Temporal limbic gray | 0.33 | 0.99[1,33], (Test 10), linear | <0.01b | 9.91[1,34], (Test 11) | 0.65 | 0.21[1,33], (Test 10), linear | 0.50 | 0.45[1,34], (Test 11) |
| Cingulate gray | 0.71 | 0.14[1,33], (Test 10), linear | 0.01 | 8.12[1,34], (Test 11) | 0.26 | 1.33[1,33], (Test 10), linear | 0.29 | 1.15[1,34], (Test 11) |
| Insula gray | 0.01 | 8.12[1,31], (Test 6), quadratic | n.a. | n.a. | 0.01 | 8.15[1,31], (Test 10), linear | n.a. | n.a. |
| Cerebellum gray | 0.28 | 1.22[1,33], (Test 10), linear | 0.02b | 5.70[1,34], (Test 11) | 0.96 | 0.00[1,33], (Test 10), linear | 0.49 | 0.50[1,34], (Test 11) |
| Prefrontal white | 0.3 | 0.95[1,31], (Test 6), quadratic | 0.01b | 8.73[1,33], (Test 9) | 0.01 | 6.79[1,29], (Test 1), cubic | n.a. | n.a. |
| Frontal motor white | 0.42 | 0.67[1,31], (Test 6), quadratic | <0.01b | 10.18[1,33], (Test 9) | 0.84 | 0.04[1,29], (Test 1), cubic | 0.39 | 0.77[1,32], (Test 4) |
| Parietal white | 0.50 | 0.46[1,31], (Test 6), quadratic | <0.001b | 13.58[1,33], (Test 9) | 0.68 | 0.17[1,31], (Test 6), quadratic | 0.25 | 1.39[1,33], (Test 9) |
| Occipital white | 0.03 | 5.26[1,33], (Test 10), linear | n.a. | n.a. | 0.14 | 2.34[1,33], (Test 10), linear | 0.22 | 1.53[1,34], (Test 11) |
| Temporal visual white | 0.13 | 2.36[1,33], (Test 10), linear | <0.001b | 14.37[1,34], (Test 11) | 0.87 | 0.03[1,33], (Test 10), linear | 0.50 | 0.46[1,34], (Test 11) |
| Temporal auditory white | 0.48 | 0.51[1,31], (Test 6), quadratic | <0.001b | 20.27[1,33], (Test 9) | 0.86 | 0.03[1,31], (Test 6), quadratic | 0.15 | 2.21[1,33], (Test 9) |
| Temporal limbic white | 0.94 | 0.00[1,33], (Test 10), linear | 0.01b | 8.01[1,34], (Test 11) | 0.80 | 0.07[1,31], (Test 6), quadratic | 0.55 | 0.36[1,33], (Test 9) |
| Cingulate white | 0.14 | 2.34[1,33], (Test 10), linear | <0.01b | 12.65[1,34], (Test 11) | 0.62 | 0.24[1,33], (Test 10), linear | 0.09 | 3.00[1,34], (Test 11) |
| Insula white | 0.68 | 0.17[1,31], (Test 6), quadratic | 0.01c | 7.98[1,33], (Test 9) | 0.89 | 0.02[1,31], (Test 6), quadratic | <0.01c | 10.98[1,33], (Test 9) |
| Cerebellum white | 0.04 | 4.58[1,33], (Test 10), linear | n.a. | n.a. | 0.37 | 0.82[1,33], (Test 10), linear | 0.69 | 0.17[1,34], (Test 11) |
| Lateral ventricles | 0.59 | 0.30[1,31], (Test 6), quadratic | 0.94 | 0.01[1,33], (Test 9) | 0.80 | 0.06[1,31], (Test 6), quadratic | 0.16 | 2.05[1,33], (Test 9) |
| Corpus callosum | 0.15 | 2.18[1,33], (Test 10), linear | <0.001b | 17.04[1,34], (Test 11) | 0.60 | 0.28[1,33], (Test 10), linear | <0.01b | 10.36[1,34], (Test 11) |
| Caudate | 0.66 | 0.19[1,33], (Test 10), linear | 0.06 | 3.83[1,34], (Test 11) | 0.36 | 0.87[1,33], (Test 10), linear | 0.04c | 4.75[1,34], (Test 11) |
| Hippocampus | 0.07 | 3.33[1,33], (Test 10), linear | <0.01b | 10.31[1,34], (Test 11) | 0.70 | 0.15[1,33], (Test 10), linear | 0.06 | 3.83[1,34], (Test 11) |
| Putamen | 0.12 | 2.54[1,33], (Test 10), Linear | <0.01b | 9.29[1,34], (Test 11) | 0.75 | 0.10[1,33], (Test 10), Linear | 0.02c | 5.59[1,34], (Test 11) |
| Amygdala | 0.08 | 3.21[1,33], (Test 10), linear | <0.001b | 15.84[1,34], (Test 11) | 0.31 | 1.08[1,33], (Test 10), linear | 0.36 | 0.86[1,34], (Test 11) |
n.a., not applicable
See Supplemental Methods for detailed descriptions of each statistical test. F and P values for “shape of curve” are based on male–female contrast for the single term of the indicated polynomial (e.g., linear for ICV).
Indicates that males had significantly greater volumes than females.
Indicates that females had greater volumes than males. When males and females had significantly different curves, differences in the height of the curve are not reported (listed as n.a.)—as these would vary across different ages.
Discussion
Our results significantly add to the extant literature on brain development in the juvenile and adolescent rhesus macaque (Falk et al. 1999; Franklin et al. 2000). They also revealed important similarities and differences in the neural growth trajectories of humans and macaques, which must be taken into account when using the monkey as a model for neurodevelopmental disorders. We observed a linear increase in ICV of approximately 11% from 10 to 64 months of age in both sexes, which concurs with the changes reported by Malkova et al. (2006) in a smaller longitudinal study. This age increment in size is broadly comparable to that for humans from age 2 to 20 years, where males and females are predicted to show a percent increase of 20% and 7%, respectively (percent increase calculated from the regression models presented by Lenroot et al. (2007)). However, the shape of the trajectory differs from humans, in which total cerebral volume peaks at 14.5 years of age in males and 11.5 years of age in females and then declines in the second decade, with a steeper decrease in females (Lenroot et al. 2007). For comparison purposes, the developmental rate in monkeys appears to be 3–4 times faster than in humans, depending on the specific physiological or neural system measured (Gibson 1991) so that 10–64 months in the monkey corresponds to the toddler stage through the young adult human in their third decade of life. Female macaques reach puberty around 3 years of age, whereas males are sexually mature by 4 years of age (Rawlins and Kessler 1986). Lifespan is approximately 30 years (Rowe 1996).
The developmental trajectory of cerebral volume in humans at least partially corresponds to the development of GM, which shows a clear biphasic pattern with declines in adolescence, both when the cortex is examined as a whole or more specifically in the frontal, parietal, and temporal lobes (Giedd et al. 1999). In contrast, in our study there was not an overt maturational change in total GM evident after 1–2 years of age in the rhesus macaque. This supports our initial hypothesis that GM development at 1 year of age would be more comparable to the mature adult in macaques than in humans. However, the development of GM showed marked regional specificity. No age-related effects were observed in the occipital, parietal, and temporal limbic areas, suggesting that these are early maturing areas in the macaque. In contrast, a postpubertal decline in GM, similar to that seen in humans, was observed in the prefrontal cortex (both sexes) and in the insular cortex (just in males). Although MRI is not currently capable of identifying the cellular processes which underlie these observed changes in volume, it has been suggested that the biphasic pattern in humans reflects overproduction of synapses followed by selective synaptic pruning during adolescence (Giedd et al. 1999). Postmortem histological studies in the macaque indicate that synaptic density increases rapidly in the first several months after birth throughout the cortex. In the visual cortex, the number of synapses begins to decline immediately after the peak is reached. This reduction accelerates sharply around puberty (Rakic et al. 1986). In the motor cortex, synapse reduction occurs until 10 years of age (Zecevic et al. 1989). In the prefrontal cortex there is an extended plateau in synaptic density with a gradual decrease beginning around puberty (Bourgeois et al. 1994). The changes we observed in GM volume in the prefrontal cortex mirror the changes in synaptic density as revealed in postmortem studies. This similarity is encouraging for the use of monkeys as models of schizophrenia given that it has been proposed that overpruning of the prefrontal cortex during puberty may be central to the etiology of this disorder (Feinberg 1983; Lewis and Gonzalez-Burgos 2008). Researchers using the macaque to investigate the impact of environmental exposures which increase the risk of developing schizophrenia, such as marijuana use, should consider timing their manipulations to coincide with this period of prefrontal GM loss.
In contrast to the prefrontal cortex, GM volumes as measured by MRI did not correspond with the reports of decreasing synaptic density in visual and motor cortex. In fact, GM volumes actually increased throughout this age range in frontal motor, visual temporal and auditory temporal cortex as well as in the cerebellum. Although postnatal neurogenesis has been demonstrated in certain discrete areas of the primate brain including the hippocampus (Gould et al. 1998; Kornack and Rakic 1999), this process is unlikely to account for the GM growth we observed in specific cortical regions. Postnatal neurogenesis of Purkinje cells does take place in the cerebellum, but is complete after 2–3 postnatal months (Rakic 1972). Presuming that synaptic density is actually decreasing in these regions, the GM volume increase may reflect changes in neuronal size or in the size and number of glial cells. A study of the striate cortex in rhesus monkeys showed a 10-fold increase in oligodendrocytes from birth to maturity. In contrast, the number of astrocytes and microglia appeared constant in early postnatal development (Okusky and Colonnier 1982).
WM development in the monkey was more comparable to that seen in humans. As seen in the human brain, total WM increased throughout the prepubertal period and then appeared to plateau in both females and males around 4 years of age (young adulthood). There is some controversy in the human literature as to when the increase in WM volume terminates, with some researchers arguing that WM volumes peak in young adulthood (Woo and Crowell 2005; Watson et al. 2006) whereas others report increases well into middle-age (Bartzokis et al. 2001). Regarding regional specificity, a quadratic pattern was recapitulated in frontal motor, parietal, and auditory temporal cortex, whereas the cingulate, visual temporal, and limbic temporal cortex as well as the corpus callosum increased linearly across the entire age range of our study and would presumably peak later in adulthood. Interestingly, we observed a biphasic curve with a postpubertal decline in WM in the prefrontal cortex. Giedd et al. (1999) reported no lobar differences in WM development in humans; however, they did not separate prefrontal and frontal motor cortex. Gogtay et al. (2008) did not report separate data on the prefrontal cortex and frontal motor cortex either, but they did report WM loss in the cingulate cortex during adolescence. Loss of WM may reflect axonal loss and is more likely to reflect refinement of intrinsic circuitry as opposed to associational circuitry (Woo et al. 1997). As we observed a decline of WM in the prefrontal cortex, it is unlikely that the loss in GM we observed reflects the encroachment of WM, which is sometimes given as the possible explanation for postpubertal declines in GM observed in human MRI studies.
The maturational trajectories of subcortical structures in monkey also showed both similarities and differences with that observed in humans. Lenroot and Giedd (2006) report that the caudate follows a biphasic pattern of growth before and after puberty similar to cortical GM. They have not yet reported longitudinal data on other subcortical structures, but cross-sectional analysis suggested that the hippocampus increased in volume in females only and the amygdala increased in volume only in males. In contrast, a study by Caviness et al. (1996) suggested that the volume of the hippocampus decreased in both males and females from age 11 to adulthood and that the putamen and amygdala decreased across this period for males only. We saw linear increases in the volume of all these structures in the macaque with no evidence for sex differences in trajectory, although there were differences between males and females in absolute and proportional volumes. Lenroot and Giedd (2006) also reported a linear increase in ventricle volume in adolescence; we observed a similar increase in the lateral ventricles in the macaque, which leveled off by young adulthood.
We also examined whether there were sex differences in brain volumes or developmental trajectories in order to compare our findings to the sexual dimorphisms in brain structure observed in humans. Like human males, male macaques have significantly larger brain volumes than do females throughout this age range. There was also a trend for males to have proportionately larger WM volumes than females; this pattern is also seen in humans, but emerges during puberty as a consequence of greater WM growth in males (Allen et al. 2003; Lenroot et al. 2007). After correcting for their smaller ICV, female macaques showed proportionately greater volumes of the putamen, caudate, and hippocampus. The caudate, hippocampus, and total basal ganglia have also been reported to be proportionately larger in human females, especially during childhood and adolescence (Filipek et al. 1994; Caviness et al. 1996; Giedd et al. 1997; Xu et al. 2000). Some sex differences observed in humans were not observed in the macaque. In humans, peak GM volumes are reached earlier in females than males in most cortical regions (Lenroot et al. 2007). We did not see this pattern in macaques, despite earlier reports that cranial capacity reaches its adult volume earlier in female macaques than in males (Konigsberg et al. 1990). It has been suggested that the human gender difference reflects an earlier puberty in females. However, levels of luteinizing hormone (a hormonal marker of pubertal status) are not associated with GM volumes (Peper et al. 2008). It would be extremely useful to perform MRI assessments of GM volume in even younger macaques during early infancy, to determine if the initial burst of synaptogenesis occurs earlier in females than males. If so, this would argue against a role for gonadal hormones as the mediator of the human sex differences in the age at which peak GM volumes are reached. WM volume increases far more steeply in adolescent boys than in adolescent girls and this change does appear to be related to pubertal staging as marked by both luteinizing hormone (Peper et al. 2008) and testosterone levels (Perrin et al. 2008). We did not observe this sex difference in macaques for total WM volume, but we did observe a similar pattern in the occipital lobe and to some extent in the cerebellum. Experimental manipulations of gonadal steroid exposure in adolescence and the neonatal period would clarify this issue. Lenroot et al. (2007) also reported that human males have larger lateral ventricles than do human females; we did not observe this structural difference in the macaque. We did not observe a greater relative amygdala volume in male macaques, though absolute amygdala volume was greater in males, in contrast to an earlier report of similar amygdala area in male and female monkeys (Franklin et al. 2000). Finally, we observed some sex differences in the macaque that have not been reported in humans. GM in the insular cortex followed a biphasic pattern before and after puberty in males, but showed no age-related change in females. Data on the development of the insular cortex has not been reported in humans. In addition, the total volume of the corpus callosum was proportionately larger in males throughout development. This sex difference concurs with an earlier report on callosal area (Franklin et al. 2000) although this sex difference was not observed in bonnet macaques (Pierre et al. 2008) or capuchin monkeys (Phillips and Sherwood 2008). Holloway and Heilbroner (1992) reported that the dorsoventral width of the splenium was larger in male rhesus macaques than in females, but this difference was eliminated when correcting for brain volume. Whether there is a sex difference in callosal size is a contentious issue in humans, in part because differences are sometimes found in only the genu or splenium. Meta-analysis shows that the midsagittal area of the corpus callosum is larger in males than females and a review of most studies suggests that there is no sex difference after correcting for total brain size (Bishop and Wahlsten 1997). However, there are some notable exceptions (Holloway et al. 1993). Recently, Lenroot et al. (2007) reported that girls and female adolescents had relatively larger callosal area compared with males. One possible parsimonious explanation for the discrepancies is that early life events seem to differentially impact the size and shape of the corpus callosum in males and females. That type of effect was found in a study of 1-year-old monkeys that had been subjected to an extended period of stress during the prenatal period (Coe et al. 2002).
When interpreting our results, it is important to keep the limitations of the study in mind. First, although this is the largest MRI study of the rhesus macaque yet reported, a larger sample size may have revealed other differences in the developmental trajectories, especially between males and females. Second, our study began at 1 year of age, and the infant monkey is precocious with many significant brain changes occurring during the first year of life. We began our MRI at 10 months of age, which is after the initial burst in synaptogenesis, especially in precocious regions such as the visual cortex which undergoes major development even prior to birth. Studies in younger infant monkeys are certainly needed, but would have to be carried out with care given the potential effects of even acute separations on both behavioral and brain development. It is already known that primate brain development can be markedly altered by disturbances of the early rearing environment, especially if they are reared away from the mother by humans or raised in a stressful manner including multiple separations from social companions (Martin et al. 1991; Sanchez et al. 1998). Our monkeys were all mother reared, housed with peers, and provided with social and environmental stimulation, and thus not deprived. However, MRI studies of brain development in macaque populations raised in different environments would complement our research and address whether the cross-species differences we observed are partly due to rearing conditions. Finally, longitudinal studies are also necessary to confirm growth patterns in the context of interindividual variation.
In conclusion, brain development in rhesus monkeys shows marked change from the juvenile period through young adulthood with a clear regional specificity in the slope and pattern of the maturational trajectories. In a number of regions and tissue types, the developmental profiles were similar to that observed in humans, but there are also important species differences that must be taken into account when using the macaque as a model of developmental neuropathology. Our study highlights the need for further longitudinal study of rhesus brain development from birth into adulthood using image acquisition and analysis tools that can also be applied to human populations. This information is particularly germane now that there is so much interest in discerning the influence of genetic factors as well as the impact of prenatal insults such as maternal infection, teratogenic drugs, and environmental toxicants on early brain development.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Allergy and Infectious Diseases (grant number AI067518) to C.L.C.; National Institute of Mental Health (grant numbers Conte Center Grant MH064065 to J.H.G., T32 MH019111-13 to R.C.K., 1 K01 MH083045-01 to R.C.K.); National Institute of Child Health and Development (grant number T32 HD40127 to R.C.K., HD039386 to C.L.C.); and UNC Neurodevelopmental Disorders Research Center (HD03110).
Supplementary Material
Acknowledgments
We would like to thank Garrett Gilmore and Elizabeth Redpath for their assistance in the validation study and Auriel Willette for valuable discussions. Conflict of Interest: None declared.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men—a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldmanrakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus-monkeys. Cereb Cortex. 1994;4:78. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual-cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. 2002;41:178–185. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Falk D, Froese N, Sade DS, Dudek BG. Sex differences in brain/body relationships of Rhesus monkeys and humans. J Hum Evol. 1999;36:233–238. doi: 10.1006/jhev.1998.0273. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia—caused by a fault in programmed synaptic elimination during adolescence. J Psychiatr Res. 1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Filipek PA. Neuroimaging in the developmental disorders: the state of the science. J Child Psychol Psychiatry. 1999;40:113–128. [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. Young-adult human brain—an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Kraemer GW, Shelton SE, Baker E, Kalin NH, Uno H. Gender differences in brain volume and size of corpus callosum and amygdala of rhesus monkey measured from MRI images. Brain Res. 2000;852:263–267. doi: 10.1016/s0006-8993(99)02093-4. [DOI] [PubMed] [Google Scholar]
- Gerig G, Jomier M, Chakos MH. VALMET: a new validation tool for assessing and improving 3D object segmentation. Proc MICCAI 2001 Lecture Notes Computer Sci. 2001;2208:516–523. [Google Scholar]
- Gibson K. Myelination and behavioral development. A comparative perspective on questions of neoteny, altriciality and intelligence. In: Gibson K, Petersen AC, editors. Brain maturation and cognitive development: comparative and cross-cultural perspectives. New York: Aldine de Gruyter; 1991. pp. 29–63. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilmore GH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans D, Smith JK, Hamer RM, Lieberman JA, Gerig G. Cerebral asymmetry, sexual dimorphism, and regional gray matter growth in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, et al. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia vizualized by using tensor-based morphometry. Proc Natl Acad Sci USA. 2008;105:15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness Jnr VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Petty RG, Turetsky BI, Gur RC. Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res. 1996;21:1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- Hafner H, Maurer K, Loffler W, an der Heiden W, Munk-Jorgensen P, Hambrecht M, Richer-Rossler A. The ABC schizophrenia study: a preliminary overview of the results. Soc Psychiatry Psychiatric Epidemiol. 1998;33:380–386. doi: 10.1007/s001270050069. [DOI] [PubMed] [Google Scholar]
- Harlow H, Harlow M. Social deprivation in monkeys. Sci Am. 1962;207:136. doi: 10.1038/scientificamerican1162-136. [DOI] [PubMed] [Google Scholar]
- Harlow H, McKinney W. Nonhuman primates and psychoses. J Autism Child Schizophr. 1971;1:368–375. doi: 10.1007/BF01540529. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK, Suomi SJ. From thought to therapy—lessons from a primate laboratory. Am Sci. 1971;59:538. [PubMed] [Google Scholar]
- Holloway RL, Anderson PJ, Defendini R, Harper C. Sexual dimorphism of the human corpus callosum from three independent samples: relative size of the corpus callosum. Am J Phys Anthropol. 1993;92:481–498. doi: 10.1002/ajpa.1330920407. [DOI] [PubMed] [Google Scholar]
- Holloway RL, Heilbroner P. Corpus-callosum in sexually dimorphic and nondimorphic primates. Am J Phys Anthropol. 1992;87:349–357. doi: 10.1002/ajpa.1330870309. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Dev Med Child Neurol. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Lin W, Evans D, Wilber K, Smith KJ, Kang C, Hamer RM, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg L, Falk D, Hildebolt C, Vannier M, Cheverud J, Helmkamp RC. External brain morphology in rhesus macaques (Macaca mulatta) J Hum Evol. 1990;19:269–284. [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, Gurvich C, Gilbert H, Mehmedbegovic F, Mu L, Marston N, Gavrilidis E, de Castella A. Hormone modulation: a novel therapeutic approach for women with severe mental illness. Aust N Z J Psychiatry. 2008;42:83–88. doi: 10.1080/00048670701732715. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006;24:3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus-monkeys alters the chemoarchitecture of the brain .1. Subcortical regions. J Neurosci. 1991;11:3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Juvenile-delinquency and attention deficit disorder—boys developmental trajectories from age 3 to age 15. Child Dev. 1990;61:893–910. doi: 10.1111/j.1467-8624.1990.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Winslow JT. Non-human primates: Model animals for developmental psychopathology. Neuropsychopharmacology. 2009;34:90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, Andreasen NC. Sex differences in brain morphology in schizophrenia. Am J Psychiatry. 1997;154:1648–1654. doi: 10.1176/ajp.154.12.1648. [DOI] [PubMed] [Google Scholar]
- Okusky J, Colonnier M. Postnatal changes in the number of astrocytes, oligodendrocytes, and microglia in the visual-cortex (area-17) of the macaque monkey—a stereological analysis in normal and monocularly deprived animals. J Comp Neurol. 1982;210:307–315. doi: 10.1002/cne.902100309. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GCM, van Leeuwen M, van den Berg SM, de Waal HADV, Janke AL, Collins DL, Evans AC, et al. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic-resonance-imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Cortical development in brown capuchin monkeys: a structural MRI study. Neuroimage. 2008;43:657–664. doi: 10.1016/j.neuroimage.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Hopkins WD, Taylialatela JP, Lees CJ, Bennett AJ. Age-related neuroanatomical differences from the juvenile period to adulthood in mother-reared macaques (Macaca radiata) Brain Res. 2008;1226:56–60. doi: 10.1016/j.brainres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rakic P. Extrinsic cytological determinants of basket and stellate cell dendritic pattern in cerebellar molecular layer. J Comp Neurol. 1972;146:335–354. doi: 10.1002/cne.901460304. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldmanrakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral-cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P, Goldman-Rakic PS. Development and modifiability of the cerebral-cortex. Neurosci Res Program Bull. 1982;20:429–611. [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior, and biology. Albany (NY): State University of New York Press; 1986. [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children—a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rowe N. The pictorial guide to the living primates. East Hampton (NY): Pogonias Press; 1996. [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Thompson JN, Bodensteiner JB, Hamza M, Tucker RR, Marks W, Gay C, Wilson D. Quantitative morphometric analysis of brain growth using magnetic-resonance-imaging. J Child Neurol. 1990;5:127–130. doi: 10.1177/088307389000500211. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Charles C, Park J, Gerig G. Multisite validation of image analysis methods: assessing intra and inter site variability. In: Sonka M, Fitzpatrick JM, editors. Medical imaging 2002: image processing. Bellingham, WA: SPIE. 2002. pp. 278–286. [Google Scholar]
- Styner M, Knickmeyer R, Joshi S, Coe C, Short SJ, Gilmore J. Automatic brain segmentation in rhesus monkeys. In: Pluim JPW, Reinhardt JM, editors. Medical imaging 2007: image processing. Bellingham, WA: SPIE. 2007. pp. L65121–L65128. [Google Scholar]
- Szatmari P, Offord DR, Boyle MH. Ontario Child health study—prevalence of attention deficit disorder with hyperactivity. J Child Psychol Psychiatry. 1989;30:219–230. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- van Ginneken B, Heimann T, Styner M. Segmentation in the clinic: a grand challenge. In: van Ginneken B, Heimann T, Styner M, editors. Workshop at Medical Image Computing and Computer Assisted Intervention. MICCAI. 2007. pp. 7–15. [Google Scholar]
- Watson RE, DeSesso JM, Hurtt ME, Cappon GD. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B. 2006;77:471–484. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Crowell AL. Targeting synapses and myelin in the prevention of schizophrenia. Schizophr Res. 2005;73:193–207. doi: 10.1016/j.schres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. Am J Neuroradiol. 2000;21:112–118. [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus-monkey during fetal and postnatal life. Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.