Abstract
Adolescent cannabis use is associated with an increased risk of schizophrenia and with impairments in cognitive processes reliant on the circuitry of the dorsolateral prefrontal cortex (DLPFC). Additionally, maternal cannabis use is associated with cognitive dysfunction in offspring. The effects of cannabis are mediated by the cannabinoid 1 receptor (CB1R), which is present in high density in the primate DLPFC. In order to determine how developmental changes in CB1Rs might render DLPFC circuitry vulnerable to cannabis exposure, we examined the density and innervation patterns of CB1R-immunoreactive (IR) axons and the expression of CB1R mRNA in the DLPFC from 81 macaque monkeys, ranging in age from embryonic 82 days to 18 years. Overall CB1R immunoreactivity in the gray matter robustly increased during the perinatal period and achieved adult levels by 1 week postnatal. However, laminar analyses revealed that CB1R-IR axon density significantly decreased with age in layers 1–2 but significantly increased in layer 4, especially during adolescence. In contrast, CB1R mRNA levels were highest 1 week postnatal, declined over the next 2 months, and then remained unchanged into adulthood. These findings provide a potential substrate for discrete, age-dependent effects of cannabis exposure on the maturation of primate DLPFC circuitry.
Keywords: cholecystokinin, GABA, interneurons, parvalbumin, schizophrenia
Introduction
Cannabis use predominates in adolescents (Gruber and Pope 2002) and current data document a new trend for continuing cannabis use through the childbearing years (Sundram 2006). These patterns of use suggest that Δ9-tetrahydrocannabidiol (Δ9-THC), the principal psychoactive ingredient in cannabis, could interfere with the proper maturation of brain circuitry during adolescence in users and/or during the pre- and perinatal periods in their offspring (Viveros et al. 2005). Indeed, the children of cannabis-smoking mothers exhibit cognitive dysfunction (Fride 2004), and cannabis use during adolescence is associated with later impairments in certain cognitive functions (Viveros et al. 2005; Sundram 2006), such as working memory, that are mediated by the dorsolateral prefrontal cortex (DLPFC) (Egerton et al. 2006). Furthermore, cannabis use, especially before 15 years of age, is associated with an increased risk of schizophrenia (Moore et al. 2007; Murray et al. 2007), a disorder characterized by both dysfunction of the DLPFC and impaired working memory (Lewis et al. 2005).
These developmental consequences of cannabis use might reflect the effects of Δ9-THC on endocannabinoid signaling mediated by the cannabinoid 1 receptor (CB1R) (Freund et al. 2003), which is highly expressed in the mature primate DLPFC (Eggan and Lewis 2007). In the neocortex, CB1Rs are heavily localized to the terminals of a subset of γ-aminobutyric acidergic (GABAergic) basket interneurons that contain the neuropeptide cholecystokinin (CCK) and that target the perisomatic domain (i.e., cell body and proximal dendrites) of pyramidal cells (Marsicano and Lutz 1999; Bodor et al. 2005). In the rodent neocortex and hippocampus, CB1R-immunoreactive (IR) cells and axons undergo distinct developmental refinements in relative density and laminar distribution, and in the hippocampus, these refinements parallel those of CCK-IR cells and axons (Morozov and Freund 2003a, 2003b; Deshmukh et al. 2007). CCK-IR structures also undergo distinct changes in relative density and laminar distribution during perinatal and postnatal development in the macaque monkey DLPFC (Oeth and Lewis 1993). However, the developmental trajectories of the innervation density and laminar distribution pattern of CB1R-IR structures have not been determined in the primate DLPFC.
The maturation of primate DLPFC circuitry is associated with substantial changes in other sources of perisomatic GABAergic inputs to pyramidal neurons during both the perinatal and adolescent periods of development. For example, both pre- and postsynaptic markers of inputs from parvalbumin (PV)-IR basket and chandelier neurons to the cell bodies and axon initial segments, respectively, of pyramidal neurons exhibit marked developmental refinements (Anderson et al. 1995; Erickson and Lewis 2002; Cruz et al. 2003 2009). In addition, the maturation of the axon arbors and perisomatic synapses from PV-IR basket neurons during adolescence is shaped, at least in part, by GABA signaling (Chattopadhyaya et al. 2007). Interestingly, cannabinoid activation of CB1Rs suppresses the release of GABA from terminals and completely abolishes inhibitory postsynaptic currents elicited by stimulation of CCK-IR basket cells (Galarreta et al. 2004; Trettel et al. 2004; Bodor et al. 2005; Foldy et al. 2006). Together, these data converge on the idea that the exogenous activation of CB1Rs during pregnancy or adolescence may alter the balance of inhibitory inputs to the perisomatic region of DLPFC pyramidal neurons, disrupt the developmental trajectories of these GABAergic inputs, and produce persistent alterations in DLPFC circuitry that could impair cognitive function.
Thus, knowing how CB1R protein and mRNA normally develop in the monkey DLPFC is essential to understand how cannabis exposure during sensitive periods may underlie the associated increased liability for cognitive impairments and schizophrenia later in life. Consequently, to understand the involvement of CB1R-mediated signaling in the maturation of DLPFC circuitry, we used immunocytochemical and in situ hybridization techniques to quantify changes across development in the density and laminar distribution of CB1R-IR structures and of CB1R mRNA levels in DLPFC area 46 of macaque monkeys.
Materials and Methods
Animals and Tissue Preparation
For immunocytochemical studies, 2 cohorts of macaque monkeys were utilized. The first cohort of animals consisted of 11 pigtail macaque monkeys (Macaca nemestrina) of both sexes, ranging in age from embryonic (E)82 days to postnatal (PN)11 months (Supplementary Table S1) (Oeth and Lewis 1993; Condé et al. 1996). The second cohort consisted of 40 rhesus monkeys (Macaca mulatta) of both sexes, ranging in age from PN2 days to 18 years (Supplementary Table S2) (Erickson and Lewis 2002; Cruz et al. 2003). Animal perfusion, brain removal and blocking, tissue fixation and tissue storage procedures have been described in previous studies that utilized tissue from both monkey cohorts (Oeth and Lewis 1993; Condé et al. 1996; Erickson and Lewis 2002; Cruz et al. 2003) (see Supplementary Materials and Methods for details). We have previously shown that our storage procedure does not affect cortical immunoreactivity for a number of antigens (Rosenberg and Lewis 1995; Erickson et al. 1998; Pierri et al. 1999; Lewis et al. 2001; Erickson and Lewis 2002; Cruz et al. 2003). Blocks from the left hemisphere were sectioned coronally (40 μm) and every 10th section was stained for Nissl substance to identify both the location of area 46 within the principal sulcus and laminar boundaries using cytoarchitectonic criteria (Barbas and Pandya 1989).
For in situ hybridization experiments, a third cohort of 30 female rhesus macaque monkeys ranging in age from PN1 week to 11.5 years was used (Supplementary Table S3). Sixteen monkeys were deeply anesthetized and perfused transcardially with ice-cold modified artificial cerebrospinal fluid (aCSF) (Gonzalez-Burgos et al. 2008). In 4 animals, 2–4 weeks prior to perfusion with aCSF, a small block of tissue containing dorsolateral area 9 and the medial bank of the principal sulcus was surgically excised from the rostral third of the principal sulcus in the left hemisphere for electrophysiology studies. The remaining 14 animals were unperfused and euthanized with an overdose of sodium pentobarbital (Supplementary Table S3). Following extraction, brains were blocked coronally and the right hemisphere was flash frozen in isopentane on dry ice and stored at −80 °C. Serial coronal sections (16 μm) containing DLPFC area 46 were cut from the caudal third of the principal sulcus in the right hemisphere of each monkey on a cryostat, thaw mounted on Supra-frost slides (VWR Scientific, West Chester, PA), and stored at −80 °C until processed for in situ hybridization.
For all 3 cohorts of monkeys, animals were housed as described previously (Cruz et al. 2003) and experimentally naive at the time of perfusion except as noted above. All procedures were conducted in accordance with federal guidelines and with the approval of the University of Pittsburgh's Institutional Animal Care and Use Committee.
Immunocytochemistry
Free-floating coronal tissue sections containing DLPFC area 46 were processed for CB1R immunoreactivity as previously described (see Eggan and Lewis 2007 and Supplementary Materials and Methods) with an affinity-purified rabbit anti-CB1R antibody raised against the last 15 amino acid residues of the C-terminus of the rat CB1R (anti-CB1R-L15; diluted 1:7500; generously provided by Dr Ken Mackie, Indiana University, Bloomington, IN) and CB1R immunoreactivity was visualized using 3,3′-diaminobenzidine (Sigma, St Louis, MO). The specificity of the primary antibody has been verified by several lines of evidence (see Eggan and Lewis 2007 and Supplementary Materials and Methods). One section from every animal was processed in a single immunocytochemistry experiment. Three immunocytochemistry experiments were performed in order to process a total of three sections for each animal, and so that any interexperimental differences were perfectly controlled for across animals.
In Situ Hybridization
Sense and antisense riboprobes against human CB1R mRNA were synthesized as previously described (Eggan et al. 2008) from a 714-bp fragment corresponding to bases 435–1148 of the human CNR1 gene (Genbank accession number NM_033181) and reduced in length to approximately 100 bp by alkaline hydrolysis to increase the effectiveness of tissue penetration (Supplementary Materials and Methods). The specificity of the riboprobe for CB1R mRNA was confirmed as previously described (see Eggan et al. 2008 and Supplementary Materials and Methods). One section from each monkey was used to analyze the expression of CB1R mRNA levels in area 46. Animals were divided into 2 groups, each containing an equivalent number of animals from each age group and a total of 2 in situ hybridization runs were performed (Supplementary Materials Methods).
Measurement and Analysis of CB1R Immunoreactivity and mRNA Expression
Levels of CB1R immunoreactivity were measured without knowledge of animal number or age by random coding of slides. Slide-mounted sections were illuminated under a microscope (Leitz Diaplan, Wild Leitz GmbH, Wetzlar, Germany) and images of the ventral bank of the principal sulcus in DLPFC area 46 from each of the 3 sections for each rhesus monkey were captured at a final magnification of ×74 by a video camera, digitized, and quantified at a final resolution of 4.0 μm/pixel using a Microcomputer Imaging Device (MCID) system (Imaging Research Inc, London, ON, Canada). Within each image, relative optical density (ROD) levels of CB1R immunoreactivity were measured in the total cortical gray matter by drawing contours of the full cortical thickness that included areas as large as possible (Eggan and Lewis 2007; Eggan et al. 2008). The mean (±standard deviation, SD) total area sampled per animal was 11.9 ± 2.6 mm2.
In order to assess laminar patterns of CB1R immunoreactivity, 2–3 cortical traverses ∼1 mm wide extending from pial surface to white matter were arbitrarily placed over the ventral bank of the principal sulcus where the tissue section was cut perpendicular to the pial surface and the ROD within each traverse was measured in 2 sections from each animal (Eggan et al. 2008). Laminar boundaries were determined by calculating the percent depth of each layer in each traverse in adjacent Nissl-stained sections using the Neurolucida program (MicroBrightfield, Inc., Colchester, VT); the mean ROD levels of CB1R immunoreactivity within each layer were then determined for each section.
Expression levels of CB1R mRNA were quantified using the MCID system without knowledge of animal number or age by random coding of film autoradiographs. Film autoradiographs were transilluminated on a lightbox, and images were captured by a video camera and digitized (final resolution 18.0 μm/pixel). Images of slide-mounted hybridized tissue sections were captured and superimposed on corresponding autoradiographic film images in order to delineate the pial surface and the gray and white matter borders (Eggan et al. 2008). Contours were drawn to include the full cortical thickness in the ventral and dorsal banks of the principal sulcus in DLPFC area 46. Optical density (OD) was measured in those contours and expressed as nanoCuries/gram (nCi/g) tissue by reference to radioactive carbon-14 standards (American Radiolabeled Chemicals, St Louis, MO) exposed on the same film. The mean (±SD) total area sampled per animal was 21.0 ± 6.7 mm2.
To determine differences in CB1R mRNA expression across lamina, OD was measured in ∼1-mm-wide cortical traverses as described above with the exception that a traverse was placed over the dorsal bank of the principal sulcus if quantifiable area was limited in the ventral bank. Because RNase A treatment (Supplementary Materials and Methods) destroys Nissl-stainable substances within the cytoplasm precluding the identification of laminar borders based on cytoarchitectonic criteria, the OD in each layer within each traverse was determined by dividing the total cortical thickness into zones of 1–10%, 10–20%, 20–50%, 50–60%, 60–80%, and 80–100% from the pial surface to white matter; these zones approximate the locations of cortical layers 1–6, respectively, in the monkey (Hashimoto et al. 2009). All cortical and laminar gray matter OD values were corrected by subtracting background OD values obtained from the white matter of each animal.
All images of slides or autoradiograms processed in an experimental run were acquired in the same session under identical microscope or lightbox and room illumination and with the same gain and black levels and flatfield correction.
Statistical Analysis
For cortical density measures, univariate analyses of covariance (ANCOVA) were conducted to examine differences in the developmental expression levels of CB1R immunoreactivity and CB1R mRNA. OD was entered as the dependent variable, age group as the main effect, and freezer storage time and sex (CB1R immunoreactivity measures only) as covariates. For measures of CB1R mRNA expression, perfusion and prior biopsy were included as blocking factors. Tissue storage time did not have a significant effect for either CB1R immunoreactivity (F1,39 = 0.0; P = 0.996) or CB1R mRNA expression (F1,20 = 1.3; P = 0.275). Sex did not have a significant effect for CB1R immunoreactivity (F1,39 = 0.2; P = 0.621). For laminar density measures, multivariate analyses of variance (MANOVA) were performed, with OD levels for each layer entered as the dependent variable and age group as the main effect (perfusion and prior biopsy were included as blocking factors for measures of CB1R mRNA expression). For all significant ANCOVAs and MANOVAs, the least significant difference post hoc test (with α = 0.05) was used to assess the differences between age groups.
Results
Development of CB1R-IR Structures in Pigtail Macaque Monkeys
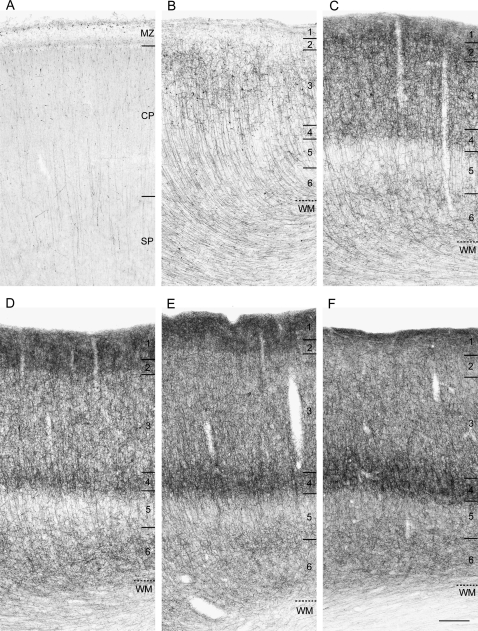
In DLPFC area 46 of pigtail macaque monkeys (Supplementary Table S1), the relative density and laminar distribution of CB1R-IR structures changed markedly through embryonic and perinatal development (Fig. 1). The overall level of CB1R-IR structures was lowest at E82, the earliest age examined (Fig. 1A), robustly increased from E132 to PN41 days of age (Fig. 1B–E), and then remained relatively unchanged, except for certain layer-specific changes (see below), through PN9 months (Fig. 1F).
Figure 1.
Brightfield photomicrographs of CB1R immunoreactivity in DLPFC area 46 of pigtail macaque monkeys at E82 days (A), E138 (B), E157 (C), PN4 days (D), PN41 days (E), and PN9 months (F) of age. In each panel, numbers and hash marks to the right indicate the boundaries of the cortical layers and the layer 6-white matter (WM) border. Marginal zone (MZ), cortical plate (CP), and subplate (SP). Note the overall increase and the shift in laminar distribution of CB1R immunoreactivity with age. Scale bar = 200 μm in F (applies to A–F).
At E82, thin, vertical CB1R-IR axons were already present in both the cortical plate and subplate (Fig. 1A). These axons had few varicosities and gave rise to occasional oblique or horizontally oriented branches (Fig. 2A). From E132 to E157 days, the number of CB1R-IR axon branches and varicosities markedly increased in layers 1–4 and 6, whereas the density of CB1R-IR axons in layer 5 did not appear to change (Fig. 1B,C). At E157 days, the density of CB1R-IR axons was highest in layers 1 and 2, slightly lower in layers 3 and 4, intermediate in layer 6, and lowest in layer 5 (Fig. 1C). From E157 to PN4 days, the relative density of CB1R-IR axons appeared to decrease in layer 3 but to increase in layer 4 producing a prominent dense band (compare Fig. 1C,D). From PN4 days to PN41 days to PN9 months, the density of CB1R-IR axons increased further in layer 4, whereas the density of CB1R-IR axons decreased in layers 1 and 2 (Fig. 1D–F). Thus, during late embryonic and early postnatal development the predominant laminar location of CB1R-IR axons shifted from the superficial layers to layer 4 of area 46. These developmental differences in laminar patterns of labeled axon distribution were confirmed by ROD measures (Supplementary Results and Fig. S1).
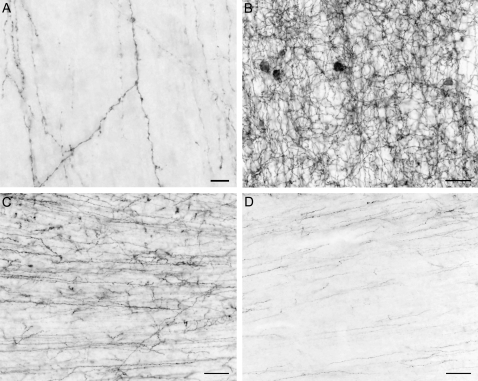
Figure 2.
High-power brightfield photomicrographs in the pigtail macaque monkey illustrating CB1R-IR axons in the cortical plate at E82 days (A), CB1R-IR neurons and axons in layer 3 at E157 days (B), and CB1R-IR axons at the layer 6-white matter (WM) border at E157 days (C), and at PN9 months (D) of age. Scale bar = 30 μm in A and 100 μm in B–D.
At E132, many CB1R-IR cells were detectable in layers 2-superficial 3 and the highest density of CB1R-IR cells was observed at E157 (Fig. 2B). By PN4 days of age, CB1R-IR cell density had markedly decreased (data not shown). Most CB1R-IR cells were nonpyramidal, putative interneurons, based on their small size and round shape (Fig. 2B).
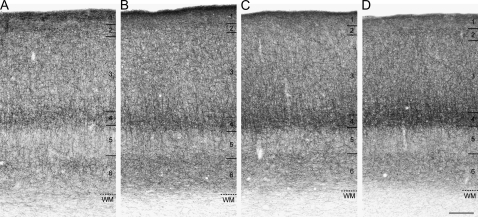
From E132 (Fig. 1B) to E157 (Figs. 1C and 2C), CB1R-IR fibers were present in high density in the white matter and then gradually decreased through the peri- and postnatal periods (Fig. 1D–F). By PN9 months of age (Figs. 1F and 2D), the density of CB1R-IR fibers in the white matter had markedly decreased, making the border between layer 6 and white matter very distinct. Similarly, a high density of CB1R-IR fibers in the white matter was observed in rhesus monkeys at PN7 days (Fig. 3A), and by 4.8 months of age, the density of CB1R-IR fibers in the white matter was markedly decreased (Fig. 3B).
Figure 3.
Brightfield photomicrographs of CB1R immunoreactivity in DLPFC area 46 of rhesus monkeys at PN7 days (A), 4.8 months (B), 3.3 years (C), and 15 years (D) of age. In each panel, numbers and hash marks to the right indicate the boundaries of the cortical layers and the layer 6-white matter (WM) border. Scale bar = 200 μm in D (applies to A–D).
Development of CB1R-IR Structures in Rhesus Monkeys
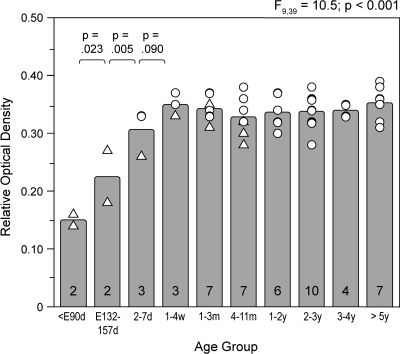
To confirm the findings from the pigtail macaque monkey series, to evaluate changes at later stages of development, and to assess age-related shifts in the laminar distribution of CB1R-IR axons, we also examined CB1R immunoreactivity levels in a cohort of rhesus monkeys (Supplementary Table S2). Like the pigtail monkeys of comparable postnatal ages, the overall level of CB1R immunoreactivity in area 46 of rhesus monkeys did not appear to change with age during postnatal development (Figs. 1D–F and 3). Quantitative assessments of the entire cortical gray matter demonstrated similar age-related changes in mean ROD levels of CB1R immunoreactivity for animals of comparable postnatal ages in both species (Fig. 4). In the combined cohorts, overall levels of CB1R immunoreactivity in area 46 robustly increased during the embryonic and perinatal periods and then did not change from 1 month of age through adulthood (Fig. 4). The effect of age group on CB1R immunoreactivity was highly significant (F9,39 = 10.5; P < 0.001), and post hoc analysis revealed significant increases in CB1R immunoreactivity across the 3 youngest age groups followed by a plateau during the remainder of postnatal development (Fig. 4). Similarly, in the rhesus monkeys alone, mean ROD levels of CB1R immunoreactivity did not differ as a function of postnatal age group (F7,32 = 0.52; P = 0.810).
Figure 4.
Mean (bar) and individual (open triangles, pigtail monkeys; open circles, rhesus monkeys) ROD levels of CB1R immunoreactivity across all cortical layers in DLPFC area 46 are shown for each age group. ANCOVA demonstrated a significant effect of age group on mean ROD levels and post hoc tests revealed significant increases in mean ROD levels between the embryonic and 2–7 day age groups but no change across age groups older than 1–4 weeks of age. Numbers in bars indicate the number of monkeys in each age group. Abbreviations: d, days; E, embryonic; m, months; w, weeks; and y, years.
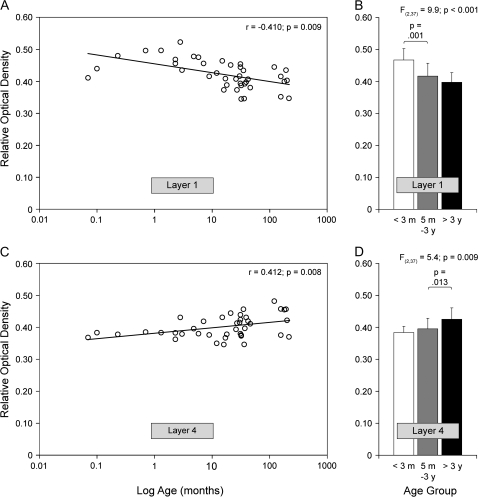
Although the overall level of CB1R immunoreactivity was unchanged during postnatal development, laminar-specific changes in the distribution of CB1R-IR structures occurred with age, with the density of CB1R immunoreactivity decreasing in layers 1–2, increasing in layer 4, and not changing in layers 3, 5, and 6 (Fig. 3; Supplementary Results and Fig. S2). Pearson correlation analyses revealed that ROD levels of CB1R immunoreactivity significantly declined with age in layer 1 (r = −0.410; P = 0.009) but significantly increased with age in layer 4 (r = 0.412; P = 0.008) (Fig. 5A,C). Comparison of neonatal (<3 months; n = 9), juvenile (5 months to 3 years; n = 20), and postpubertal animals (>3 years; n = 11) revealed a significant main effect for age group in layers 1 (F2,37 = 9.9; P < 0.001) and 4 (F2,37 = 5.4; P = 0.009) (Fig. 5B,D). Post hoc tests revealed that mean ROD levels of CB1R immunoreactivity significantly decreased by 14.9% in layer 1 before 5 months of age but significantly increased by 10.8% in layer 4 after 3 years of age (Fig. 5B,D).
Figure 5.
Scatter plots of ROD levels of CB1R immunoreactivity in DLPFC area 46 for individual rhesus monkeys as a function of age in months plotted on a log scale in layers 1 (A) and 4 (C). Pearson correlation analyses revealed a significant decrease and increase in CB1R immunoreactivity with age in layers 1 (A) and 4 (C), respectively. Bar graphs showing the mean (+SD) ROD levels within layer 1 (B) and layer 4 (D) for each of the 3 indicated age groups of rhesus monkeys. The mean ROD levels significantly decreased in layer 1 before 3 months of age but increased in layer 4 after 3 years of age.
Development of CB1R mRNA Expression in Rhesus Monkeys
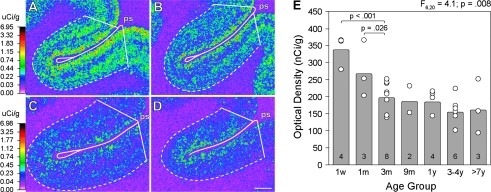
Levels of CB1R mRNA expression in area 46 appeared to be highest qualitatively in animals 1 week of age, lower in animals 1 month of age, and lowest in animals 3 months of age and older (Fig. 6A–D). Quantitative analysis demonstrated a significant (F6,23 = 4.1; P = 0.008) effect of age group on CB1R mRNA OD levels, with post hoc comparisons revealing a significant 41% reduction in CB1R mRNA expression across the 1-week to 3-month age groups followed by no significant change in expression levels across the older age groups (Fig. 7E).
Figure 6.
Representative film autoradiograms illustrating CB1R mRNA expression in DLPFC area 46 of rhesus monkeys at postnatal 1 week (A), 1 month (B), 3 months (C), and 8.7 years (D) of age. The density of hybridization signal is presented in pseudocolor according to the calibration bars to the left of A (applies to A and B) and C (applies to C and D). E illustrates mean (bar) and individual (open circles) OD levels of CB1R mRNA expression across all cortical layers in DLPFC area 46 for each age group of rhesus monkeys. ANCOVA demonstrated a significant effect of age group on mean OD levels and post hoc tests revealed significant decreases in mean OD levels between the 1-week and 3-month age groups but no change across age groups older than 3 months of age; days (d), months (m), nanoCuries per gram (nCi/g), microCuries per gram (μCi/g), years (y).
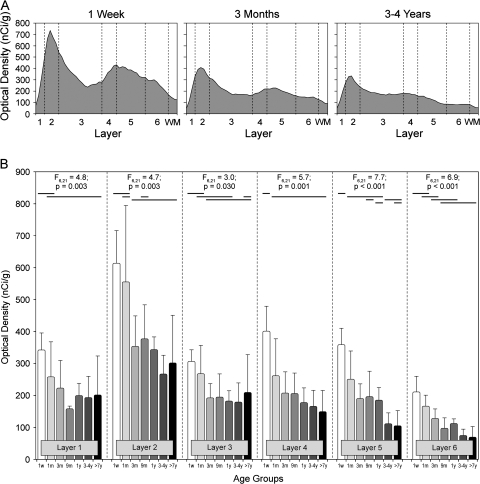
Figure 7.
(A) Plots of mean CB1R mRNA OD as a function of cortical layer in DLPFC area 46 of rhesus monkeys in the postnatal 1 week, 3 month, and 3 to 4 year age groups. CB1R mRNA expression prominently decreased across layers 2–6 with age. (B) Bar graphs of mean (+SD) OD levels of CB1R mRNA expression within each cortical layer for each age group of rhesus monkeys. Multivariate analysis of variance demonstrated a significant effect of age group on mean OD levels of CB1R mRNA in each cortical layer. Within each panel, age groups not sharing horizontal lines on the same level are significantly different at P < 0.05; months (m), nanoCuries per gram (nCi/g), week (w), white matter (WM), years (y).
The expression of CB1R mRNA also changed within cortical layers during postnatal development. In animals 1 week of age, the density of CB1R mRNA-positive neurons was highest in layers 2-superficial 3, intermediate in layers 4 and 5, and lowest in deep layer 3 and layer 6 (Fig. 7A). The subsequent decline in CB1R mRNA levels was associated with a shift in the relative laminar distribution of the transcript such that in older animals only layer 2 contained a prominent peak of CB1R mRNA expression. Quantitative assessments revealed a significant effect for age group in all cortical layers (all F‘s > 3.0; all P < 0.030) (Fig. 7B). Post hoc comparisons in each layer revealed significant decreases in CB1R mRNA expression between each of the age groups from 1 week to 3 months of age in all layers. No significant difference in CB1R mRNA expression levels across the older age groups occurred in layers 1–4, but significant decreases did occur in layers 5 and 6 in the older age groups (Fig. 7B).
Discussion
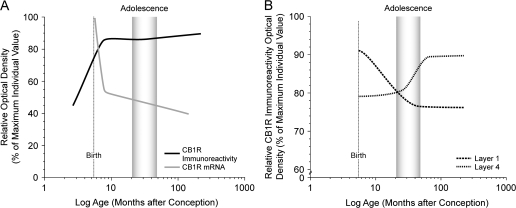
In this study, we sought to understand the involvement of CB1R-mediated signaling in the maturation of primate DLPFC circuitry by examining changes in the levels of CB1R immunoreactivity and mRNA during pre- and postnatal development. The overall level of CB1R immunoreactivity robustly increased during the pre- and perinatal periods and then remained stable (Fig. 8A). However, embedded within these findings were laminar-specific developmental changes in innervation density, with a decrease in layers 1–2 that was most marked during the first postnatal year and an increase in layer 4 that was prominent during adolescence (Fig. 8B). In contrast, CB1R mRNA expression was highest at birth, markedly decreased during the first 3 postnatal months, and then did not change through development into adulthood (Fig. 8A); in association with these changes, a distinct peak in CB1R mRNA expression emerged in layer 2. Thus, the relative levels and laminar distribution of both CB1R immunoreactivity and mRNA exhibited distinctive patterns and different rates of change, eventually achieving peaks of mRNA expression in layer 2 and of CB1R-IR axons in layer 4. These findings, which do not appear to be influenced by potential confounding factors such as sex, levels of sex steroids, or changes in cortical volume (see Supplementary Results), suggest a shifting role of CB1Rs in cortical circuitry that may contribute to the functional maturation of the DLPFC and to age-specific vulnerabilities to cannabis exposure during both the perinatal and adolescent periods of development.
Figure 8.
Schematic summary Figures illustrating trajectories of overall CB1R immunoreactivity and mRNA levels (A) and density of CB1R-IR axons in layers 1 and 4 (B) across development. Lines were generated by plotting the density as a percent of the maximum density value for individual animals for each marker as a function of age in months after conception on a log scale, fitted by Loess regression analysis, and smoothed by hand. The shaded area indicates the approximate age range corresponding to adolescence (15–42 months; Plant 1988) in macaque monkey. Note the different developmental time courses in overall CB1R immunoreactivity and mRNA levels (A) and in the laminar distribution of CB1R immunoreactivity (B).
Perinatal Development of CB1Rs in Monkey DLPFC
In monkey area 46, the overall levels of CB1R immunoreactivity and mRNA exhibited opposite developmental trajectories during perinatal development (Fig. 8A). Consistent with these findings, other studies using a more limited number of developmental time points reported that CB1R radioligand binding is higher in adult compared with embryonic rats, whereas CB1R mRNA expression is highest at embryonic ages and lower in the adult (Berrendero et al. 1999). In addition, different developmental trajectories for receptor proteins and their cognate mRNAs have been reported for other neurotransmitter systems (Lidow 1995; Jung and Bennett 1996; Weickert et al. 2007; Hashimoto et al. 2009). The dissociation between the developmental trajectories for CB1R mRNA and immunoreactivity may reflect age-related modifications in CB1R protein translation and/or turnover that require high levels of CB1R mRNA expression in order to increase and maintain appropriate levels of CB1R protein early in development but require less mRNA expression later in development.
During perinatal development, marked changes occurred in the types and distribution patterns of structures containing CB1R immunoreactivity or mRNA. In particular, CB1R immunoreactivity and mRNA were transiently present in certain structures that were not labeled later in development. For instance, CB1R mRNA was expressed heterogeneously across all cortical layers in the perinatal period but was restricted to specific layers later in development. In the white matter adjacent to the DLPFC, a transient, high density of CB1R-IR axons was observed at E132 that gradually decreased through the peri- and postnatal periods until 9 months postnatal when CB1R-IR axons were no longer detectable in the white matter. Consistent with these findings, transient localization of CB1R-IR axons and CB1R radioligand binding in cortical white matter have been reported during early development in the rat (Romero et al. 1997; Deshmukh et al. 2007) and human (Mato et al. 2003) brain. The fleeting presence of CB1R-IR axons in the white matter and CB1R mRNA expression in the gray matter could arise from migrating neurons and/or elongating neuronal axons projecting from cell bodies in the DLPFC that transiently express CB1R mRNA. Consistent with this idea, a recent study in the mouse demonstrated that CB1R-IR neurons born in the caudal ganglionic eminence and destined for the hippocampus migrate through the cortical plate (Morozov et al. 2009). Furthermore, CB1Rs regulate cortical neuron axonal pathfinding and guidance early in development (Berghuis et al. 2007), and we found a substantial increase in the branches of CB1R-IR axons and varicosities in the superficial cortical layers during the perinatal period that parallels the marked increase in density of synaptic contacts in monkey DLPFC during this period of development (Bourgeois et al. 1994).
The presence of CB1R mRNA and protein in the monkey DLPFC during the perinatal period suggests that endocannabinoid signaling plays a functional role in early developmental processes, which regulate the formation of cortical circuits, such as neuronal migration, axon growth and pathfinding, and synaptogenesis. Indeed, CB1Rs are localized on neuronal growth cones and activation of CB1Rs in immature CB1R/CCK-IR neurons from the fetal rat cortex regulates the morphological development, migration, and neurite growth of these neurons (Berghuis et al. 2005, 2007). CB1Rs are also located on the membranes of CCK-IR axon terminals where they can suppress GABA release (Morozov and Freund 2003a), and in cell bodies and proximal dendrites where they mediate a form of slow self-inhibition that is generated by Ca2+ dependent autocrine release of endocannabinoids in CCK-IR neurons (Bacci et al. 2004). Thus, CB1Rs may also play an early role in the activity-dependent formation of excitatory synapses by controlling the release of GABA from, and the excitability of, CCK-IR neurons during a period of development when GABA generates depolarizing events that cooperate with glutamate to activate N-methyl-D-aspartate receptors and the subsequent formation of glutamate synapses (Ben-Ari 2002).
These marked changes in expression and distribution of CB1R mRNA and protein suggest that perinatal development is a sensitive period for exposure to exogenous cannabinoids, and consequently that cannabis use by pregnant or nursing mothers could disrupt the normal development of DLPFC circuits. Consistent with this idea, prenatal exposure to cannabinoids produces alterations in the development of a number of neurotransmitter systems, including dopamine and serotonin (Fernandez-Ruiz et al. 2000). Furthermore, in the rat hippocampus, prenatal Δ9-THC exposure increases the density of CCK-IR neurons (Berghuis et al. 2005), whereas activation of CB1Rs during the perinatal period inhibits GABA release and disrupts neuronal network activity (Bernard et al. 2005), which could alter the activity-dependent formation of inhibitory and excitatory synapses. Similar types of effects in the DLPFC could, in part, contribute to the impaired cognitive functions that develop later in life in the offspring of cannabis-using mothers (Fride 2004).
Development of CB1Rs during Adolescence in Monkey DLPFC
The differences in amount and distribution of CB1R mRNA and immunoreactivity in the monkey DLPFC with development suggest a shift in the functional role of CB1Rs from the early regulation of cortical circuit formation to a later, more restricted role in modulating neurotransmission from specific neuron types. In particular, although a small percentage of CB1R-IR axons may arise from neurons containing the calcium-binding protein calbindin (Bodor et al. 2005), the majority of axons containing CB1R immunoreactivity in the monkey DLPFC most likely arise from inhibitory interneurons that contain the neuropeptide CCK, as indicated by studies in the rodent hippocampus (Morozov and Freund 2003a). For example, in the cerebral cortex of both rodents and primates, 1) CB1R-IR cells have morphologic features characteristic of GABA neurons, such as round cell bodies and axon terminals that form classic symmetric synapses (Bodor et al. 2005; Eggan and Lewis 2007); 2) CB1Rs are highly expressed by, and contained in the terminals of, CCK-IR basket neurons (Marsicano and Lutz 1999; Katona et al. 2000; Bodor et al. 2005); and 3) activation of CB1Rs suppresses both the release of GABA from CCK-IR neuron axon terminals and inhibitory postsynaptic currents in pyramidal neurons (Galarreta et al. 2004; Trettel et al. 2004; Bodor et al. 2005). In addition, comparison of the current findings with previous studies indicate that CB1R- and CCK-IR structures in macaque monkey DLPFC exhibit similar overall distribution patterns in the adult and undergo nearly identical changes in laminar distribution during postnatal development. In particular, both CB1R- (Fig. 8B) and CCK-IR (Oeth and Lewis 1993) axons gradually decrease in superficial layers and increase in layer 4 during postnatal development. In contrast, this pattern of developmental refinements of CB1R- and CCK-IR axons in the monkey DLPFC is distinctly different from those exhibited by other neuronal subpopulations, such as chandelier neurons (Cruz et al. 2003, 2009), PV-IR basket cells (Erickson and Lewis 2002; Cruz et al. 2003), dopamine axons (Rosenberg and Lewis 1995), or excitatory inputs to layer 3 pyramidal neurons (Anderson et al. 1995; Gonzalez-Burgos et al. 2008).
CB1R immunoreactivity has also been observed in asymmetric, excitatory synapses in the neocortex (Katona et al. 2006; Kawamura et al. 2006), and CB1R agonists modulate glutamate release (Chevaleyre et al. 2006). However, the antibody utilized in the present study exclusively labels symmetric, inhibitory synapses by electron microscopy in both the monkey DLPFC (Eggan and Lewis 2007) and rodent hippocampus (Katona et al. 1999; Hajos et al. 2000), probably because the level of CB1Rs in excitatory terminals is below the threshold of detectability (Katona et al. 2006; Eggan and Lewis 2007). This interpretation is supported by findings that 1) CB1R mRNA levels are much higher in GABA neurons than in pyramidal cells (Marsicano and Lutz 1999), 2) the density of CB1Rs is 20- to 30-fold higher in inhibitory terminals than in excitatory terminals (Kawamura et al. 2006), and 3) a ∼30 times higher concentration of CB1R agonist is necessary for 50% suppression of neurotransmitter release from excitatory terminals than from GABA terminals (Ohno-Shosaku et al. 2002). Furthermore, the CB1R antibody used in Katona et al. (2006) revealed dense axon labeling in the strata radiatum and oriens of the rodent hippocampus, which contain the axon arbors of excitatory CA3 pyramidal neurons, whereas these hippocampal layers contained the lowest density of axons labeled by the antibody used in the present study in both the rodent (Katona et al. 1999; Hajos et al. 2000) and monkey (Eggan and Lewis 2007). Consistent with a selective labeling of GABAergic axon terminals in the present study, the observed developmental pattern of CB1R immunoreactivity does not match that of excitatory synapse density in monkey DLPFC (Bourgeois et al. 1994; Anderson et al. 1995). Thus, the observed changes in CB1R immunoreactivity across postnatal development in the present study likely reflect changes in the expression of CB1R protein specifically in inhibitory neurons and axon terminals.
The postnatal refinements in the laminar distribution of CB1R-IR axons suggest that CB1Rs may play a role in the development of cognitive functions reliant on the DLPFC. For example, the age-related improvements in performance on working memory tasks (Goldman 1971; Alexander and Goldman 1978; Diamond 2002) parallels the increase of CB1R-IR axons in layer 4. Working memory is thought to be mediated by recurrent excitatory connections between discrete populations of DLPFC pyramidal cells in nonadjacent cortical columns (Goldman-Rakic 1995). Thus, the developmental increase of CB1R-IR terminals in layer 4, and the associated greater capacity for depolarized induced suppression of inhibition (Pitler and Alger 1992; Wilson and Nicoll 2002), could allow for an activity-dependent disinhibition of pyramidal neurons that share the same segregated excitatory inputs (Carter and Wang 2007), contributing to the neural substrate for improved working memory function.
The age-related improvement in working memory performance is also associated with the maturation of other components of GABA circuitry in the DLPFC (Lewis et al. 2004). In particular, pre- and postsynaptic markers of perisomatic GABAergic inputs to DLPFC pyramidal cells from PV-IR neurons, which do not express CB1Rs, undergo considerable changes during postnatal development, with these changes particularly marked during adolescence (Erickson and Lewis 2002; Cruz et al. 2003, 2009). In the monkey DLPFC, pyramidal neurons receive convergent perisomatic input from PV-IR basket and chandelier neurons and CB1R/CCK-IR basket neurons (Melchitzky et al. 1999; Eggan and Lewis 2007). These convergent sources of perisomatic inhibition in the rodent play specific roles in shaping network activity (Freund 2003). For example, CB1R/CCK-IR and PV-IR neurons fire at different phases of network oscillations (Klausberger et al. 2005), generate temporally distinct epochs of somatic inhibition (Glickfeld and Scanziani 2006), and play complementary roles in regulating gamma band oscillations (Hajos et al. 2000). Furthermore, recent evidence suggests that CB1R/CCK-IR neurons regulate the activity of PV-IR neurons (Foldy et al. 2007; Karson et al. 2008, 2009). Thus, the developmental refinements in both CB1R/CCK- and PV-IR neurons during adolescence may be involved in the maturation of prefrontal gamma band synchrony (Uhlhaas et al. 2009), which in humans, increases with, and in proportion to, increasing working memory load (Howard et al. 2003).
Taken together, these observations suggest a mechanism by which cannabis exposure during adolescence could serve as a risk factor for the later development of schizophrenia. Specifically, because stimulation of CB1Rs strongly suppresses inhibitory input to pyramidal neurons from CCK-IR basket neurons, the indiscriminate activation of these receptors associated with cannabis use during adolescence may alter the normal balance of CB1R/CCK-IR and PV-IR inhibitory inputs to the perisomatic region of DLPFC pyramidal neurons. This imbalance during a sensitive period may disrupt the developmental trajectories of these GABAergic inputs, producing persistent circuitry alterations that impair the mechanisms of neural synchrony required for the maturation of working memory performance. Consistent with this interpretation, markers of both PV-IR (Pierri et al. 1999; Hashimoto et al. 2003) and CB1R/CCK-IR (Eggan et al. 2008) neurons are altered in the DLPFC of subjects with schizophrenia, including in the middle cortical layers, where the density of CB1R-IR axons increases during adolescence (Fig. 8B). Thus, the increased risk of schizophrenia associated with cannabis exposure during adolescence (Moore et al. 2007; Murray et al. 2007) might reflect the disruption of the normal refinements in CB1R axon innervation patterns, and in PV neurons whose activity is regulated by the output of CB1R-IR axons (Foldy et al. 2007; Karson et al. 2008), during this sensitive period of development.
Supplementary Material
Supplementary Material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (grant numbers DA023109, MH051234 to D.A.L.); National Institutes of Mental Health Undergraduate Fellowship Program in Mental Health Research grant R-25 (grant number MH54318-13 to S.R.S.).
Supplementary Material
Acknowledgments
The authors thank Dr Ken Mackie for kindly donating the CB1 receptor antibody, Mary Brady for assistance with the graphics, and Jim Kosakowski for Matlab program development. Conflict of Interest: David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007–2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, and Neurogen. All other authors have no conflicts of interest to disclose.
References
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci USA. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di MV, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J-P, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Carter E, Wang XJ. Cannabinoid-mediated disinhibition and working memory: dynamical interplay of multiple feedback mechanisms in a continuous attractor model of prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i16–i26. doi: 10.1093/cercor/bhm103. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund JS, Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Dev Brain Res. 1996;96:261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009;514:353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh S, Onozuka K, Bender KJ, Bender VA, Lutz B, Mackie K, Feldman DE. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience. 2007;145:279–287. doi: 10.1016/j.neuroscience.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. London: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Akil M, Levey AI, Lewis DA. Postnatal development of tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in monkey rostral entorhinal cortex. Cereb Cortex. 1998;8:415–427. doi: 10.1093/cercor/8.5.415. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernaandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fride E. The endocannabinoid-CB(1) receptor system in pre- and postnatal life. Eur J Pharmacol. 2004;500:289–297. doi: 10.1016/j.ejphar.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Exp Neurol. 1971;32:366–387. doi: 10.1016/0014-4886(71)90005-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG. Marijuana use among adolescents. Pediatr Clin North Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Jung AB, Bennett JP. Development of striatal dopaminergic function .1. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Brain Res Dev Brain Res. 1996;94:109–120. doi: 10.1016/0165-3806(96)00033-8. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54:117–128. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lidow MS. D1- and D2 dopaminergic receptors in the developing cerebral cortex of macaque monkey: a film autoradiographic study. Neuroscience. 1995;65:439–452. doi: 10.1016/0306-4522(94)00475-k. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: laminar, regional and target specificity of type I and type II synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Postnatal development and migration of cholecystokinin-immunoreactive interneurons in rat hippocampus. Neuroscience. 2003a;120:923–939. doi: 10.1016/s0306-4522(03)00409-3. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci. 2003b;18:1213–1222. doi: 10.1046/j.1460-9568.2003.02852.x. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;1(Suppl 19):i78–i89. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Oeth KM, Lewis DA. Postnatal development of the cholecystokinin innervation of monkey prefrontal cortex. J Comp Neurol. 1993;336:400–418. doi: 10.1002/cne.903360307. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo T-U, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM. Neuroendocrine basis of puberty in the rhesus monkey (Macaca mulatta) In: Martini L, Ganong WF, editors. Frontiers in neuroendocrinology. Vol. 10. New York: Raven Press; 1988. pp. 215–238. [Google Scholar]
- Romero J, GarciaPalomero E, Berrendero F, GarciaGil L, Hernandez ML, Ramos JA, Fernandezruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Sundram S. Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol. 2006;21:245–254. doi: 10.1002/hup.762. [DOI] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, Kleinman JE, Akil M. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.