Abstract
Frequency facilitation (FF), comprising a rapid and multiple-fold increase in the magnitude of evoked field potentials, is elicited by low-frequency stimulation (LFS) at mossy fiber–CA3 synapses. Here, we show that in freely behaving rats, FF reliably occurs in response to 1 and 2Hz but not in response to 0.25-, 0.3-, or 0.5-Hz LFS. Strikingly, prolonged (∼600 s) FF was tightly correlated to the induction of long-term depression (LTD) in freely moving animals. Although LFS at 2 Hz elicited unstable FF and unstable LTD, application of LFS at 1 Hz elicited pronounced FF, as well as robust LTD that persisted for over 24 h. This correlation of prolonged FF with LTD was absent at stimulation frequencies that did not induce FF. The adenosine-A1 receptor appears to participate in these effects: Application of adenosine-A1, but not adenosine-A3, receptor antagonists enhanced mossy fiber synaptic transmission and occluded FF. Furthermore, adenosine-A1 receptor antagonism resulted in more stable FF at 1 or 2 Hz and elicited more potent LTD. These data support the fact that FF contributes to the enablement of long-term information storage at mossy fiber–CA3 synapses and that the adenosine-A1 receptor may regulate the thresholds for this process.
Keywords: CA3, frequency facilitation, hipocampus, in vivo, long-term depression, mossy fiber, synaptic plasticity
Introduction
Frequency facilitation (FF) has been widely described at mossy fiber–CA3 synapses in vitro (Salin et al. 1996; Toth et al. 2000; Moore et al. 2003; Nicoll and Schmitz 2005) and more recently has been reported in freely behaving rats (Klausnitzer and Manahan-Vaughan 2008). It occurs when the stimulation frequency is altered from a very low rate (e.g., 0.025 Hz) to a low rate (1 Hz) and typically is only sustained for as long as the stimulation is given. It comprises a unique property of the mossy fiber synapse and is not seen at any other hippocampal synapse (Salin et al. 1996; Dobrunz and Stevens 1999; Toth et al. 2000; Klausnitzer and Manahan-Vaughan 2008). FF may fulfill the role of holding synaptic information “online,” in keeping with the contribution of the CA3 region to the processing of working memory (Kesner 2007).
Hippocampal plasticity exists in a multitude of forms, ranging from very short-lasting forms such as FF, paired-pulse depression, or posttetanic potentiation (Lambert and Wilson 1994; Dobrunz et al. 1997; Schmitz et al. 2001; Zucker and Regehr 2002) but can also persist for very long periods of time (long-term potentiation, LTP; long-term depression, LTD) (Bear 1996; Kemp and Manahan-Vaughan 2007). Synaptic plasticity is highly frequency dependent (Malenka and Bear 2004; Huang and Kandel 2005, 2007) and has different properties dependent on the hippocampal subregion in which it is induced (Harris and Cotman 1986; Johnston et al. 1992; Malenka and Nicoll 1993; Martin et al. 2000; Vianna et al. 2000; Straube et al. 2003; Lee et al. 2004). Furthermore, different hippocampal subregions respond with distinct types of synaptic plasticity to novel spatial experience (Kemp and Manahan-Vaughan 2008a). LTD in the dentate gyrus and CA1 regions of freely behaving rats is typically induced by 1 Hz low-frequency stimulation (LFS) although these forms of synaptic plasticity do not share the same induction mechanisms (Dudek and Bear 1992; Mulkey et al. 1993, 1994; O'Mara et al. 1995; Manahan-Vaughan 1997; Wang et al. 1998; Pöschel and Manahan-Vaughan 2005, 2007). Typical studies of FF involve applying stimuli for 30–150 s (Salin et al. 1996; Dobrunz and Stevens 1999; Toth et al. 2000; Klausnitzer and Manahan-Vaughan 2008). We investigated whether prolonged (∼600 s) FF, induced by LFS at mossy fiber–CA3 synapses can also lead to persistent LTD, consistent with a role of FF in the mechanisms underlying persistent synaptic plasticity.
Materials and Methods
Electrophysiology
Male Wistar rats (7–8 weeks, Charles River, Germany) were anaesthetized (Pentobarbital 52 mg/kg) and underwent chronic implantation of a bipolar stimulation electrode and a monopolar recording electrode to enable monitoring of evoked potentials at mossy fiber-CA3 synapses.
The recording electrode was placed above the CA3 pyramidal cell layer of the dorsal hippocampus (anterioposterior (AP): −3.2; mediolateral (ML): 2.2) and the stimulation electrode was implanted at coordinates corresponding to the mossy fiber projections (AP: −3.5; ML: 2.0) (based on Derrick and Martinez 1994) (Fig. 2F). Mossy fiber responses were identified based on their appearance, characterized by negative response with an onset of 3–4 ms and a peak latency of 8–10 ms (Derrick et al. 1991).
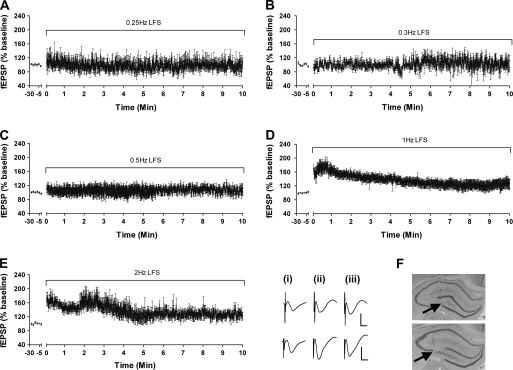
Figure 2.
FF is highly frequency dependent. LFS at 0.25 (A, n = 6), 0.3 (B, n = 7), or 0.5 (C, n = 6) has no significant effect on the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat. LFS at 1 Hz (D) or 2 Hz (E, n = 9) significantly increases the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat (n = 16). LFS at 2 Hz elicits an FF that is more variable than that seen with 1-Hz LFS. Analogs represent responses evoked with 0.5 Hz (upper traces) or 1 Hz (lower traces) pre-LFS (i), at the 30th (ii), and 300th (iii) pulse. Vertical scale bar: 4 mV; horizontal scale bar: 8 ms. Hippocampal slices showing the position of the stimulating (upper picture) and recording (lower picture) electrodes in the CA3 region and the mossy fiber pathway, respectively (F, black arrows).
To enable drug injections, a guide cannula was placed in the ipsilateral cerebral ventricle, as described previously (Manahan-Vaughan 1997). Experiments were commenced 7–10 days after surgery. During all experiments, the animals could move freely in the recording chamber (40 × 40 × 50 cm) and had free access to food and water. To allow the animals to acclimatize they were transferred to the experiment room the day before the experiment took place.
The head stage was connected to an amplifier and stimulator via a flexible cable with a swivel connector. Recordings were analyzed and stored on a computer and the electroencephalography was monitored throughout experiments.
Measurement of Evoked Potentials and Data Analysis
To evoke field excitatory postsynaptic potentials (fEPSPs), a biphasic pulse was given with a half-wave duration of 0.2 ms. For recordings, the stimulation intensity was set to produce an fEPSP, which was 40% of the maximal obtainable. The intensity was found on the basis of an input–output curve (maximal stimulation 900 μA). Each recording consisted of an average of 5 consecutive pulses at 0.025 Hz (test-pulse stimulation). To ensure stability of recordings, all animals were first tested in a baseline experiment over the same time period as subsequent experiments.
For each time point, 5 consecutively evoked responses at 40-s intervals were averaged. These first 30 min of recording (6 time points) served as baseline, and the data subsequently obtained were expressed as the mean percentage ± the standard error of the mean (SEM) of this average baseline value. Following monitoring of basal synaptic transmission for 30–45 min, drug or vehicle injection was applied. The stability of basal synaptic transmission was then followed for 2.5 h, with further recordings conducted 24 h after injection.
For analysis of differences between groups, a 2-way analysis of variance (ANOVA) was applied. For differences of fEPSPs during FF, the entire stimulus train was compared with test-pulse values. To assess statistical differences in the subsequent synaptic depression, the fEPSPs from the period after stimulation until the end of the experiments were compared. Data correlation was assessed using Spearman's Rho. The level of significance was set at P < 0.05.
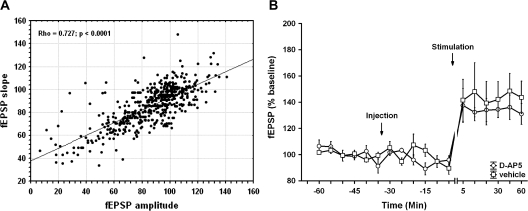
Although we describe relative changes in fEPSP in terms of slope, no differences in the degree or direction of change was seen compared with analysis of changes in fEPSP amplitude (n = 11, Fig. 1A).
Figure 1.
Changes in fEPSP slope and amplitude are tightly correlated at mossy fiber synapses in vivo. LTP is insensitive to NMDA receptor blockade. (A) An analysis of the relative changes in fEPSP slope and fEPSP amplitude revealed that responses are very tightly correlated at mossy fiber synapses of freely moving adult rats (n = 11). (B) Injection of the NMDA receptor antagonist, D-AP5 (3.9 μg) does not alter synaptic responses of LTP at mossy fiber–CA3 synapses after application of HFS in the intact animal. LTP was evoked by tetanic stimulation (100 Hz) of the mossy fiber pathway to CA3. No difference in the profile of LTP was observed when control animals (n = 7) were compared with animals treated with the NMDA receptor antagonist, AP5 (n = 7). Changes in timescale are indicated by line breaks.
Investigations of FF
We attempted to elicit FF using LFS consisting of 600 pulses at frequencies of 0.25, 0.3, 0.5, 1, and of 1200 pulses at 2 Hz. The effects of FF were evaluated during the course of the baseline experiments described above. The change in fEPSP magnitude was calculated relative to the mean of the 6 data points obtained immediately prior to application of LFS. The data obtained during the 600 LFS pulses were thus expressed as the mean percentage ± the SEM of these 6 averaged time points. In the case where high-frequency stimulation (HFS) was given to verify reversibility of LTD, tetanic stimulation at 100 Hz (4 bursts of 100 pulses, with a 5-min interburst interval) was applied.
Paired Pulse Stimulation
To examine the effects of paired-pulse stimulation on fEPSP evoked from mossy fiber synapses, interstimulus intervals (ISIs) of 20, 40, 50, 100, 300, 500, or 1000 ms were applied.
Induction of LTP
LTP was induced by application of HFS at 100 Hz (4 bursts of 100 stimuli, 0.1-ms stimulus duration, and 10-s interburst interval) to mossy fiber synapses.
Drugs
The adenosine-A1 receptor antagonist, 1,3-dipropyl-8-(4-acrylate)phenylxanthine (phenylxanthine), was dissolved in a solution containing 5% 1 N sodium hydroxide (NaOH) (with HCl added to correct for pH) and 95% distilled water. The N-methyl-D-aspartate (NMDA) receptor antagonist D-(−)-2-amino-5-phosphopentanoic acid (D-AP5; Tocris–Cookson Ltd., Bristol, United Kingdom) was first dissolved in 5 μL of 1 N NaOH, then 0.9% sodium chloride (NaCl) was added to make up a solution of 100 μL volume. The selective adenosine-A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, Sigma–Aldrich) was dissolved in 10 mM dimethylsulfoxide (DMSO). The adenosine-A3 receptor antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS 1191, Sigma–Aldrich) was initially dissolved in 100% DMSO and diluted with 0.9% NaCl to obtain a final concentration of DMSO of 0.1%. The group II metabotropic glutamate receptors (mGluR) agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV, Tocris Cookson, Bristol, United Kingdom) was dissolved in isotonic saline (0.9% NaCl) solution. The amount of DCG-IV that was used (20 ng) was chosen because it has no effect on evoked responses in the dentate gyrus (Klausnitzer and Manahan-Vaughan 2008). Phenylxanthine was applied in a 12.5 μg dose, which had been previously shown to elicit significant increases in mossy fiber transmission in vivo (Klausnitzer and Manahan-Vaughan 2008). Drugs were injected in a 5-μL volume, as an acute, single injection, into the ipsilateral lateral ventricle via the implanted cannula.
Verification of Mossy Fiber-CA3 Recordings
The mossy fiber synapse is very sensitive to agonism of group II mGluRs by DCG-IV that selectively inhibits mossy fiber but not associational–commissural EPSPs (Kamiya et al. 1993; Yeckel et al. 1999; Goussakov et al. 2000). Animals were excluded from the mossy fiber-CA3 study when the fEPSP responses evoked in the stratum lucidum failed to show strong sensitivity (i.e., a reduction of test pulse–evoked responses by 60% or greater) to DCG-IV, 1 h after DCG-IV application. Postmortem histological analysis of the electrode localizations was also conducted (Bock 1989; Manahan-Vaughan et al. 1998).
Results
LTP Elicited at Mossy Fiber Synapses Is Not Sensitive to NMDA Receptor Blockade
An important issue to address in conducting experiments in the freely behaving animal is the verification that evoked potentials derive predominantly from the activation of mossy fiber synapses. One strategy that we employed was to assess the sensitivity of the synapses to DCG-IV (see Materials and Methods). A second strategy was to assess if LTP is sensitive to treatment with NMDA receptor antagonists. Previous reports indicate that LTP at commissural associational, but not mossy fiber, synapses can be prevented by application of an NMDA receptor antagonist prior to or during the tetanus (Harris and Cotman 1986; Zalutsky and Nicoll 1990). In control animals, we induced reliable LTP by applying high-frequency tetanization (100 Hz). We then treated animals with a concentration of AP5 that we had previously shown to be effective in blocking LTP at CA1 (Manahan-Vaughan 1997) and dentate gyrus (Manahan-Vaughan et al. 1998) synapses in freely behaving rats. No significant effect on LTP was observed (ANOVA: F(1,47) = 0.40; P = 0.53, n = 7) (Fig. 1B). This supports the likelihood that we recorded predominantly from mossy fiber synapses in our study.
Application of LFS Reveals That FF Is Highly Frequency Dependent
Application of LFS (600 pulses) at a range of frequencies revealed that the successful induction of FF in vivo is tightly dependent on the LFS frequency implemented (Fig. 2). No significant differences were observed between fEPSPs elicited with test-pulse stimulation and fEPSPs elicited with LFS at 0.25 Hz (ANOVA, F(1,35) = 6.13; P = 0.42, n = 6), at 0.3 Hz (ANOVA, F(1,31) = 12.59; P = 0.08, n = 7), and 0.5 Hz (ANOVA, F(1,36) = 24.47; P = 0.16, n = 6, Fig. 2). LFS at 1 Hz elicited robust FF that attained a maximal level of 190 ± 18.2%, declined slightly during LFS, but remained significant from pre-LFS values throughout the 600-s stimulation period (ANOVA, F(1,11) = 395,67; P < 0.0001, n = 16, Fig. 2D). LFS at 2 Hz (1200 pulses) elicited facilitation that was highly variable but still differed significantly from fEPSP responses elicited by test-pulse stimulation (0.025 Hz; ANOVA, F(1,49) = 448,74; P < 0.0001, n = 9) (Fig. 2E). The synaptic depression elicited by 2-Hz LFS was significantly less and more unstable than that elicited by 1-Hz LFS (Fig. 3D,E) (ANOVA, F(1,41) = 14,73; P < 0.001). However, in both cases (600 pulses compared with 1200 pulses), persistent LTD occurred.
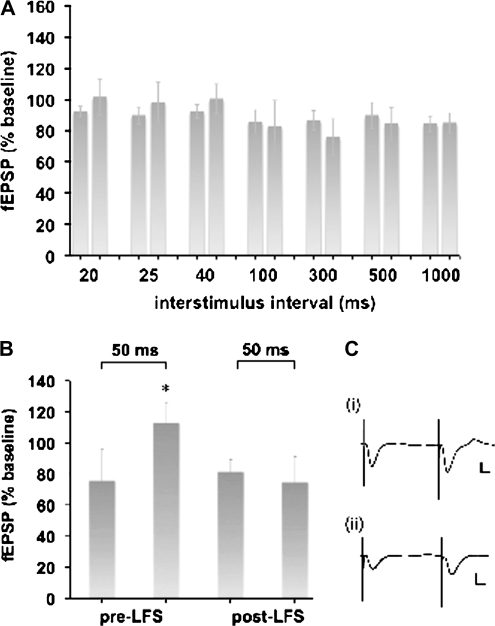
Figure 3.
PPF of mossy fiber responses occurs before but not after induction of LTD. (A) Paired-pulse stimulation at ISIs of either 20, 40, 100, 300, 500, or 1000 ms elicited no significant effects on evoked responses (all n = 3). (B) An ISI of 50 ms, elicited a significant enhancement of the second compared with the first evoked fEPSP (t-test: P = 0.048, n = 3). After application of LFS (1 Hz, 600 pulses) application of paired-pulse stimulation with a 50-ms ISI had no effect on the second fEPSP (t-test: P = 0.65). (C) Analogs represent 1st and 2nd fEPSPs evoked at an ISI of 50 ms (i) before LFS and (ii) after LFS. Vertical scale bar: 2 mV, horizontal scale bar 2 ms.
Paired Pulse Facilitation (PPF) of Mossy Fiber Responses Occurs Before but not After Induction of LTD
PPF has been described at mossy fiber synapses in the hippocampal slice preparation (Salin et al. 1996; Henze et al. 2000). To assess whether PPF is present at mossy fibers of freely moving rats, we tested the effects of a range of ISIs. We found that ISIs of either 20,40, 100, 300, 500, or 1000 ms elicited no significant effects on evoked responses (Fig. 3A, all n = 3).
An ISI of 50 ms, elicited a significant enhancement of the second compared with the first evoked fEPSP, however (t-test: P = 0.048, n = 3, Fig. 3B,C) in line with previous in vitro reports (Kamiya and Ozawa 1998). We then applied paired-pulse stimulation at a 50-ms ISI after LFS (1 Hz, 600 pulses) to induce LTD, to evaluate the presynaptic involvement in synaptic plasticity at mossy fiber-CA3 synapses. After LFS, application of paired-pulse stimulation with a 50-ms ISI had no effect on the second fEPSP (t-test: P = 0.65, Fig. 3B,C).
Application of DCG-IV Suppresses Evoked Responses at Mossy Fiber–CA3 Synapses In Vivo
Application of DCG-IV (20 ng) significantly suppressed field responses evoked by test-pulse stimulation evoked at mossy fiber-CA3 synapses, compared with basal (preinjection) levels (n = 21; P < 0.001) (Fig. 4A).
Figure 4.
FF in freely moving adult rats is tightly associated with the successful induction of LTD. Evoked responses elicited by test-pulse stimulation (0.025 Hz) are significantly inhibited by application of the group II mGluR agonist DCG-IV (A, 20 ng, n = 21). Analogs show responses taken 30 min after treatment with vehicle (solid line) or DCG-IV (dashed line). Vertical scale bar: 2 mV; horizontal scale bar: 8 ms. (B). The maximal level of FF correlated significantly to the subsequent degree of synaptic depression. (C). LFS at 0.25 (n = 9), 0.3 (n = 7), or 0.5 Hz (n = 6) does not result in lasting synaptic depression at mossy fiber–CA3 synapses in the intact freely behaving rat. LFS at 1 Hz (D, n = 10) or 2 Hz (E, n = 9) significantly induces lasting depression (>24 h) of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat (n = 10). LTD elicited by 2-Hz LFS is weaker and less stable than that elicited by 1-Hz LFS (P < 0.01). (F) Analogs represent responses obtained pre-LFS (i) and 5-min post-LFS (ii) using stimulation at 0.25, 0.3, and 0.5 Hz or (G) pre-LFS (i), 5 -in post-LFS (ii), and 24h post-LFS (iii) evoked using stimulation at 2 Hz. Vertical scale bar: 4 mV; horizontal scale bar: 8 ms.
FF in Freely Moving Adult Rats Is Associated with the Successful Induction of LTD
Application of LFS (600 pulses) did not induce LTD, when frequencies of 0.25, 0.3, or 0.5 Hz were applied. Application of 1-Hz LFS induced persistent LTD, whereas 2-Hz induced LTD that was less pronounced and more unstable than that seen with 1-Hz LFS (P < 0.01). A distinct correlation between the maximal level of FF by LFS (600 pulses) and the subsequent synaptic depression was observed (Rho: 0.824, P = 0.0005) (Fig. 4B).
LFS at 0.25 Hz had no lasting effects on the fEPSP (ANOVA, F(1,94) = 0.18; P = 0.67, n = 9, Fig. 4C). Five minutes after termination of LFS at 0.3 Hz, no synaptic depression was observed (ANOVA, F(1,70) = 3.40; P = 0.70, n = 7, Fig. 3C). LFS at 0.5 Hz also had no significant effect on the fEPSP (ANOVA, F(1,54) = 8.14; P = 0.91, n = 7, Fig. 4C).
LFS at 1 Hz (600 pulses) was characterized by an initial FF of maximally 190 ± 18.2% that endured for approximately 300 s. After the initial 5 min, the evoked potentials declined to an average of 133 ± 7%. Five minutes after the termination of LFS, a significant synaptic depression was observed with a maximal depression of the fEPSP of 64 ± 4.4% (compared with baseline values) that lasted for over 24 h (Fig. 4D). Reducing the number of pulses to 30 results in FF that has no significant effects on basal synaptic transmission (Klausnitzer and Manahan-Vaughan 2008), suggesting that the duration of FF is a decisive factor in determining whether LTD occurs.
A 2-Hz LFS induced a significant but unstable FF compared with that seen with 1-Hz LFS. Five minutes after termination of 2-Hz LFS, an fEPSP depression to 80 ± 10% (n = 9) compared with baseline values was noted that lasted for over 24 h (Fig. 4E). This response was significantly different compared with values obtained during application of test pulses (ANOVA, F(1,64) = 48.34; P < 0.0001) but was also significantly less than LTD elicited with 1-Hz LFS (P < 0.01).
LTD Induced by LFS of Mossy Fiber Synapses In Vivo Is Reversed by Tetanic Stimulation
To exclude that LTD induced as a consequence of LFS of mossy fibers is not a product of synaptic run-down, or other factors unrelated to plasticity, we assessed whether responses could be reversed by HFS at 100 Hz (n = 6). HFS was given 2 h after induction of LTD when responses had reached a steady plateau. A full reversal of LTD with a tendency toward synaptic potentiation became evident (Fig. 5). The potentiation effects endure for as long as recordings were conducted (>25 h after application of LFS. Effects were highly significant (ANOVA, F(1.78) = 70.81; P < 0.0001).
Figure 5.
Application of HFS reverses LTD at mossy fiber synapses in vivo. LFS at 1 Hz (n = 6) significantly induces lasting depression (>24 h) of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat (n = 6). Application of HFS at 10 Hz 120 min after induction of LTD, successfully and significantly reversed the synaptic depression.
Antagonism of Adenosine-A1 Receptors Enhances Mossy Fiber Transmission and Prevents FF Induced by Low-Frequency Stimulation
Application of the adenosine-A1 receptor antagonist phenylxanthine (12.5 μg) significantly increased synaptic responses at mossy fiber–CA3 synapses compared with vehicle-injected controls (ANOVA, F(1,84) = 29.86; P < 0.0001, n = 7 and ANOVA, F(1,95) = 24.24; P < 0.0001, n = 8. Fig. 6A,B, respectively). This is consistent with previous reports using the same concentration in vivo (Klausnitzer and Manahan-Vaughan 2008). FF during stimulation at 1 Hz at mossy fiber–CA3 synapses was significantly occluded (ANOVA, F(1,16) = 130.17; P < 0.0001, n = 7) (Fig. 6C). Maximal FF reached similar levels to that seen under control conditions, but in contrast to controls, FF did not decline and was sustained at these high levels for the duration of LFS. LTD elicited by 1-Hz LFS in the presence of phenylxanthine was significantly enhanced (ANOVA, F(1,36) = 31.70; P < 0.0001). Furthermore, phenylxanthine significantly stabilized FF at 2 Hz (Fig. 6D) and enabled an LTD that was significantly larger and more stable (ANOVA, F(1,83) = 44.35; P < 0.0001, n = 5) (Fig. 6B).
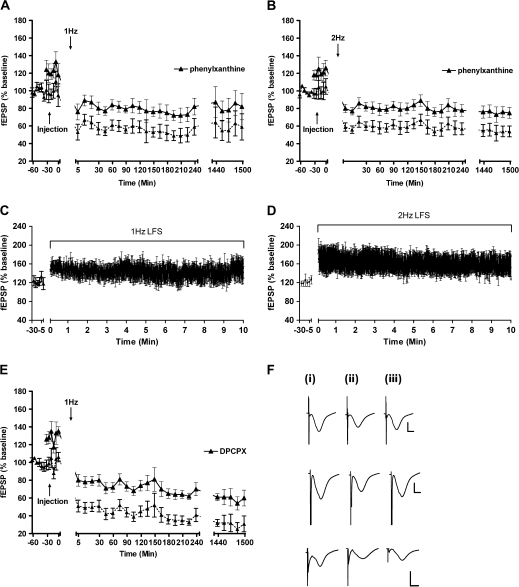
Figure 6.
Adenosine-A1 receptor blockade increases evoked fEPSPs and enhances LTD. Solid lines show the original potentials after application of phenylxanthine in 1 Hz (A) and 2 Hz (B) experiments. Dashed lines show the potentials normalized to levels corresponding to field potentials prior to application of phenylxanthine (12.5 μg). Evoked responses obtained during 1 Hz (C) and 2 Hz (D) stimulation (600 pulses) after application of phenylxanthine. Phenylxanthine elevated evoked responses to approximately 120% of basal levels. FF elicited by LFS in the presence of phenylxanthine was no larger than that seen in controls, providing evidence of occlusion. FF became nondecremental and more stable however in the presence of the adenosine-A receptor antagonist. (E) In the presence of the adenosine-A1 receptor antagonist DPCPX (76 μg), evoked synaptic responses are significantly increased. Normalization of the responses to values obtained before application of DPCPX indicate a significant LTD that lasts over 24 h (n = 6). (F) Analogs represent responses obtained in the presence of phenylxanthine during application of 1 Hz (top row), 2 Hz (middle row), and during the DPCPX experiment (bottom row) (i) 5-min pre-sLFS, (ii) 5-min post-sLFS, and (iii) 24-h post-sLFS. Vertical scale bar: 4 mV, horizontal scale bar 2 ms.
We examined whether we could replicate these findings using another adenosine-A1 receptor antagonist. We found a concentration of 76 μg DPCPX to be effective in the occlusion of FF at mossy fibers in vivo (Klausnitzer and Manahan-Vaughan 2008). Application of this compound at the same concentration also effectively occluded FF in the present study. Similar to the application of phenylxanthine, DPCPX (76 μg, n = 6) showed a significant increase of responses, evoked with test-pulse stimulation, immediately after injection compared with controls (ANOVA: F(1,56) = 75.76; P < 0.0001) (Fig. 6E). The evoked potentials elicited by 1-Hz LFS did not differ compared with responses evoked in the presence of phenylxanthine (ANOVA: F(1,62) = 1.21; P = 0.28).
The A3 adenosine receptor antagonist MRS 1911 was used to address the questions as to whether an adenosine receptor other than the A1 receptor is involved in regulating synaptic plasticity at the mossy fiber–CA3 synapse. Application of 10-μM MRS 1191 significantly decreased test pulse–evoked responses compared with vehicle-injected controls (ANOVA, F(1,36) = 11.90; P < 0.01, n = 4) (Fig. 7A). However, no effect on LTD was obtained: LTD induced in the presence of MRS 1191 did not differ significantly from LTD responses evoked in the presence of vehicle (ANOVA, F(1,20) = 4.63; P = 0.03, n = 4).
Figure 7.
Adenosine-A3 receptor blockade depresses evoked fEPSPs and elicits no net effect on LTD. (A) Injection of the adenosine-A3 receptor antagonist, MRS 1191 (10 μM), significantly reduces fEPSPs after intracerebral injection. After normalization of the evoked responses to baseline (pretreatment) levels, no significant effect was observable (n = 4). Changes in timescale are indicated by line breaks. Analogs represent responses obtained during the MRS 1191 experiment (i) 5-min pre-sLFS, (ii) 5-min post-sLFS, and (iii) 24-h post-sLFS. Vertical scale bar: 4 mV, horizontal scale bar 2 ms.
Discussion
These data indicate that FF at mossy fiber–CA3 synapses may comprise an intrinsic component of the induction of LTD in the intact freely behaving rat. The occurrence of prolonged FF correlated with the successful induction of LTD, indicating that FF may play a role in both short-term and long-term information storage at these synapses. This LTD was reversed by HFS supporting that synaptic plasticity genuinely occurred. Antagonism of the adenosine-A1 receptor occluded FF, rendered unstable FF more stable, and facilitated LTD suggesting that the adenosine-A1 receptor may play a critical role in regulating the thresholds for FF-dependent information storage.
FF has been discussed as a means for holding synaptic information online (Bischofberger et al. 2006), as in in vitro studies FF endures only for as long as LFS is given (Salin et al. 1996; Chen et al. 2001). Typically, FF is applied for 30–150 pulses, in vitro, and results in an immediate and potent increase in the amplitude of the fEPSP (Salin et al. 1996; Toth et al. 2000; Nicoll and Schmitz 2005). FF has therefore been discussed as a mechanism for the involvement of the CA3 region in the processing of working (i.e., short-term) memory (Kesner 2007). This form of brief FF has no lasting effect on basal synaptic transmission (Klausnitzer and Manahan-Vaughan 2008). To our knowledge, the relationship between prolonged LFS, the appearance of FF, and the induction of LTD has not yet been studied. Our study, reveals that prolonged (600 s) LFS at 1 Hz induces FF that endures for 300 s, is succeeded by a return of evoked potentials to baseline levels and a subsequent induction of LTD that lasts for over 24 h in freely behaving animals. This suggests that the duration of FF determines whether synaptic information is retained for brief or prolonged periods, reflecting the difference between “online” or long-term memory.
LTD comprises an information storage mechanism that may enable the persistent encoding of aspects of spatial context (Kemp and Manahan-Vaughan 2007, 2008a, 2008b). The type of spatial information encoded is dependent on the hippocampal subregion examined. Exploration of small spatial features facilitates LTD in the CA1 region (Manahan-Vaughan and Braunewell 1999; Lemon and Manahan-Vaughan 2006) but not in the dentate gyrus (Kemp and Manahan-Vaughan 2008a), whereas large objects that serve as landmark cues lead to an LTD in the dentate gyrus but not in CA1 (Kemp and Manahan-Vaughan 2008a). This suggests that depending on the hippocampal synapse concerned, very different aspects of spatial information are encoded and that these different synapses contribute jointly, to the creation of a spatial map.
Brief periods (30 pulses) of LFS at perforant path synapses to the dentate gyrus do not induce FF, whereas the same brief LFS of mossy fiber–CA3 synapses via the perforant path elicits an FF of up to 200% (Klausnitzer and Manahan-Vaughan 2008). Commissural-associational CA3 synapses (Klausnitzer and Manahan-Vaughan 2008) and CA1 synapses (Dobrunz and Stevens 1999) are unaffected by this kind of LFS. This suggests that the mossy fiber-CA3 synapse is uniquely sensitive to very brief periods of LFS and that a selective bolstering of information flow from the entorhinal cortex via the mossy fibers takes place, which perhaps corresponds with the postulated role of the CA3 region in pattern separation and/or completion (Guzowski et al. 2004; Gilbert and Kesner 2006). Persistent LFS results in LTD at all hippocampal synapses (Dudek and Bear 1992; Manahan-Vaughan and Braunewell 1999; Chen et al. 2001; Kemp and Manahan-Vaughan 2007), suggesting that this is a fundamental property of hippocampal synaptic information encoding. The precession of mossy fiber LTD by FF suggests that in this context it may subserve an increase in signal-to-noise ratio that supports long-term information storage in the form of LTD. FF may thus support the processing of spatial and temporal information in accordance with the relevant range of granule cell firing patterns (Salin et al. 1996).
It was striking that LFS at frequencies of 0.25, 0.3, or 0.5 Hz neither elicited FF nor enabled the induction of synaptic plasticity. Two Hertz elicited FF that was significantly different from test-pulse stimulation and also induced LTD. In both cases, effects were less stable than that obtained with 1-Hz LFS. The induction of LTD following successful FF was not due to synaptic exhaustion or run-down: Application of HFS successfully potentiated synapses that had previously expressed LTD.
The very narrow frequency range with which LTD was induced is in contrast to other hippocampal synapses, where it has been reported that a variety of LFS frequencies will induce LTD (Bear and Abraham 1996; Manahan-Vaughan 1998; Nakao et al. 2002; Izaki et al. 2004) or even LTP (Staubli and Lynch 1990; Calixto et al. 2003; Lanté et al. 2006) at CA1 and dentate gyrus synapses. On the other hand, FF is unique to mossy fiber synapses and is not seen at any other hippocampal synapses (Salin et al. 1996; Dobrunz and Stevens 1999). The finding of the present study not only supports a close relationship between the appearance of FF and the subsequent induction of LTD, but it also suggests that the mossy fiber-CA3 synapse exhibits a very narrow range of sensitivity to LFS. This, on the one hand, underlines the importance of the 1-Hz frequency for information encoding and on the other hand may confer a very specific information encoding property on the CA3 region. The latter may not only reflect the postulated role of the CA3 region in pattern separation and/or completion (Guzowski et al. 2004; Gilbert and Kesner 2006), but may also reflect the role of the CA3 region as a sensory information filter (Bischofberger et al. 2006; Gundlfinger et al. 2007; Scharfman 2007). Computer models suggest that the mossy fiber circuitry may function as a “high pass” filter (Zalay and Bardakjian 2006). This may have the consequence that LTD is expressed after modest, but not after lower, frequencies of afferent activity, and may explain the very narrow frequency range with which LTD is induced at this synapse.
Granule cells tend to exhibit low mean firing rates that are as low as 0.01–0.5 Hz in vivo (Jung and McNaughton 1993). Our data suggest that these firing frequencies would not be adequate to elicit lasting changes in synaptic strength at mossy fiber-CA3 synapses. In effect, a firing frequency of 1–2 Hz would reflect an increase above basal activity in the granule cell population. A study that examined place cell activity granule cells, which reflect phases of information encoding, indicates that it occurs in the range of 1.7–26 Hz, whereby the latter is considered a “high” rate of activity (Henze et al. 2002). These observations are quite intriguing as they suggest that phases of information encoding in the dentate gyrus might translate into FF and LTD induction at mossy fiber synapses in the CA3 region, similar to that induced by afferent stimulation in our study.
PPF is a form of short-term synaptic plasticity, which is mediated by an increase in the probability of neurotransmitter release of the presynaptic synapse (Thomson et al. 1993). PPF is considerably enhanced at the mossy fiber-CA3 synapse (Salin et al. 1996; Henze et al. 2000). Paired-pulse stimulation at an interstimulus interval of 50, but not 20, 40, 100, 300, 500, or 1000 ms, elicited facilitation of the second evoked fEPSP compared with the first. This is in line with in vitro findings that reported that PPF was most effective at an ISI of 50 ms (Salin et al. 1996; Kamiya and Ozawa 1998).
It was striking that this effect was evident before application of LFS to induce LTD and not afterward. An ISI of 50 ms enhances action potential–driven Ca2+ influx into the presynaptic terminal, a process mediated by presynaptic kainate receptors (Kamiya et al. 2002), which are abundant at the mossy fiber–CA3 synapse.
PPF seen in this study, before application of LFS, may thus be mediated by the activation of presynaptic kainate receptors and facilitation of Ca2+ influx into the mossy fiber terminal, which results in an increase of probability of neurotransmitter release. After LFS, the ready-releasable pool of transmitters may be depleted such that paired-pulse stimulation may lead to an enhanced Ca2+-influx but not to a higher probability of transmitter release. Subsequent presynaptic plastic changes may then lead to a sustained decrease in the amount of released neurotransmitter, which in turn enables the persistency of LTD, as observed in our study. Our findings with regard to PPF support the involvement of presynaptic mechanisms mediated by kainate receptors in the formation of FF and subsequent LTD at mossy fiber synapses in the freely behaving rat.
The adenosine-A1 receptor plays a critical role in the regulation of mossy fiber FF (Moore et al. 2003; Klausnitzer and Manahan-Vaughan 2008). We investigated to what extent the adenosine-A1 receptor is involved in the induction of LTD by prolonged FF. Consistent with previous reports, antagonism of adenosine-A1 receptors occluded FF (Klausnitzer and Manahan-Vaughan 2008). Interestingly, receptor antagonism was also associated with a stabilization of FF and facilitation of LTD. This suggests that the adenosine-A1 receptor may not only regulate FF but may also set the thresholds for long-term information storage through LTD at the mossy fiber synapse. FF effects were less pronounced than that reported using in vitro preparations (Salin et al. 1996; Dobrunz and Stevens 1999; Toth et al. 2000; Nicoll and Schmitz 2005). This may be due to the fact that the degree of intrinsic excitability and tonic control is less in the intact, awake animal, than in the isolated hippocampal slice. Activation of adenosine-A1 receptors is believed to maintain low transmitter release probability at mossy fiber synapses (Moore et al. 2003). One mechanism contributing to the low release probability and thus to a reduction in synaptic transmission involves the direct inhibition of presynaptic voltage-dependent calcium channels by adenosine (Gundlfinger et al. 2007). Treatment with adenosine-A1 antagonists in the present study resulted in similar increases in fEPSPs as that seen following 1 Hz–mediated FF. Thus this suggests, that in vivo, less tonic control may be mediated by adenosine-A1 receptor than in the slice preparation.
The effects on FF and LTD that were observed following adenosine-A1 receptor antagonism were confirmed with 2 different antagonists (phenylxanthine and DPCPX) but could not be emulated by the application of an adenosine-A3 receptor antagonist. In the latter case, LTD was not significantly affected although a depressant effect on fEPSP was observed.
Research conducted in recent years on adenosine-A3 receptors has shown a widespread involvement of adenosine-A3 receptors in hippocampal functions such as activation of phospholipase C (Abbracchio et al. 1995), modulation of LTP and LTD in CA1 (Costenla et al. 2001), and protective effects on the CA1 region after inhibition of adenosine-A3 receptors (Pugliese et al. 2006). To our knowledge, no study so far has addressed the effects of adenosine-A3 receptor antagonism on mossy fiber plasticity. Recently, it was proposed that adenosine-A3 receptor activation reduced the sensitivity of presynaptic adenosine-A1 receptors in area CA1 in hippocampal slices of rats although no effect on synaptic plasticity was observed (Dunwiddie et al. 1997). In our study, antagonism of adenosine-A3 receptors reduced fEPSPs at mossy fiber–CA3 synapses but had no direct effect on LTD.
Two observations support that recordings derived predominantly from the mossy fiber synapse: Firstly, evoked potentials could be profoundly suppressed by application of the mGlu receptor agonist DCG-IV (Kamiya et al, 1993; Yeckel et al, 1999; Goussakov et al, 2000). Secondly, LTP evoked at these synapses was not sensitive to application of the NMDA receptor antagonist, AP5. The same concentration of AP5 was used that is effective in blocking LTP at medial perforant path-dentate gyrus and Schaffer collateral-CA1 synapses in the freely behaving rat. Finally, postmortem histological analysis was used to conform correct electrode localization. Taken together, these findings suggest that recordings were obtained from the mossy fiber synapse in vivo.
Conclusion
This study provides the first observation that FF at mossy fiber–CA3 synapses of freely behaving adults rats is closely correlated with the appearance of persistent (>24 h) LTD. This suggests that FF may comprise an important functional component of long-term information storage at these synapses. The finding that antagonism of adenosine-A1 receptors occludes FF and facilitates mossy fiber LTD suggests that this receptor is an intrinsic element in the mechanisms underlying these effects.
Funding
Deutsche Forschungsgemeinschaft grant (to D.M.V.).
Acknowledgments
We thank A. Bikbaev for help with statistics, J. Klausnitzer and B. Krenzek for technical assistance, and N. Gomell for help with animal care.
Conflict of Interest: None declared.
References
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflugers Arch. 2006;453:361–372. doi: 10.1007/s00424-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Bock P. Romeis, Mikroskopische Technik. München (Germany): Urban und Schwarzenberg; 1989. pp. 575–576. [Google Scholar]
- Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber–long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Huang CC, Hsu KS. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J Neurosci. 2001;21:3705–3714. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenla AR, Lopes LV, de Mendonça A, Ribeiro JA. A functional role for adenosine A3 receptors: modulation of synaptic plasticity in the rat hippocampus. Neurosci Lett. 2001;302:53–57. doi: 10.1016/s0304-3940(01)01633-0. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Martinez JL. Opioid receptor activation is one factor underlying the frequency dependence of mossy fiber LTP induction. J Neurosci. 1994;14:4359–4367. doi: 10.1523/JNEUROSCI.14-07-04359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick BE, Weinberger SB, Martinez JL. Opioid receptors are involved in an NMDA receptor-independent mechanism of LTP induction at hippcampal mossy fiber-CA3 synapses. Brain Res Bull. 1991;27:219–223. doi: 10.1016/0361-9230(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Huang EP, Stevens CF. Very short-term plasticity in hippocampal synapses. Proc Natl Acad Sci USA. 1997;94:14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron. 1999;22:157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Kim HO, Jiang JL, Jacobson KA. Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Goussakov IV, Fink K, Elger CE, Beck H. Metaplasticity of mossy fiber synaptic transmission involves altered release probability. J Neurosci. 2000;20:3434–3441. doi: 10.1523/JNEUROSCI.20-09-03434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J Physiol. 2007;582:263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neurosci. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–793. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Theta frequency stimulation induces a local form of late phase LTP in the CA1 region of the hippocampus. Learn Mem. 2005;12:587–593. doi: 10.1101/lm.98905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Low-frequency stimulation induces a pathway-specific late phase of LTP in the amygdala that is mediated by PKA and dependent on protein synthesis. Learn Mem. 2007;14:497–503. doi: 10.1101/lm.593407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki Y, Takita M, Nomura M, Akema T. Effects of ventral hippocampal long-term potentiation and depression on the gamma-band local field potential in anesthetized rats. Exp Brain Res. 2004;157:147–151. doi: 10.1007/s00221-004-1828-y. [DOI] [PubMed] [Google Scholar]
- Johnston D, William S, Jaffe D, Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol. 1998;509(3):833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S, Manabe T. Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J Neurosci. 2002;22:9237–9243. doi: 10.1523/JNEUROSCI.22-21-09237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol (Lond) 1993;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008a;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. {beta}-Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008b;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Klausnitzer J, Manahan-Vaughan D. Frequency facilitation at mossy fiber–CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors. J Neurosci. 2008;28:4836–4840. doi: 10.1523/JNEUROSCI.3729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophys. 1994;72:121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- Lanté F, de Jésus Ferreira MC, Guiramand J, Récasens M, Vignes M. Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus. 2006;16:345–360. doi: 10.1002/hipo.20146. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Priming of group 2 metabotropic glutamate receptors facilitates induction of long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 1998;37:1459–1464. doi: 10.1016/s0028-3908(98)00150-6. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Behnisch G, Vieweg S, Reymann KG, Behnisch T. Semi-automated analysis of NMDA-mediated toxcicity in digitised colour images from rat hippocampus. J Neurosci Methods. 1998;82:85–95. doi: 10.1016/s0165-0270(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci. USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Nakao K, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N. Hippocampal long-term depression as an index of spatial working memory. Eur J Neurosci. 2002;16:970–974. doi: 10.1046/j.1460-9568.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Rowan MJ, Anwyl R. Metabotropic glutamate receptor-induced homosynaptic long-term depression and depotentiation in the dentate gyrus of the rat hippocampus in vitro. Neuropharmacology. 1995;34:983–989. doi: 10.1016/0028-3908(95)00062-b. [DOI] [PubMed] [Google Scholar]
- Pöschel B, Manahan-Vaughan D. Group II mGluR-induced long term depression in the dentate gyrus in vivo is NMDA receptor-independent and does not require protein synthesis. Neuropharmacology. 2005;49:1–12. doi: 10.1016/j.neuropharm.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Pöschel B, Manahan-Vaughan D. Persistent (>24 h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology. 2007;52:46–54. doi: 10.1016/j.neuropharm.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol. 2006;147:524–532. doi: 10.1038/sj.bjp.0706646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1-5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Frey JU. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 2003;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem. 2000;7:333–340. doi: 10.1101/lm.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu J, Rowan MJ, Anwyl R. Role of protein kinase C in the induction of homosynaptic long-term depression by brief low frequency stimulation in the dentate gyrus of the rat hippocampus in vitro. J. Physiol. 1998;513:467–475. doi: 10.1111/j.1469-7793.1998.467bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalay OC, Bardakjian BL. Simulated mossy fiber associated feedforward circuit functioning as a highpass filter. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4979–4982. doi: 10.1109/IEMBS.2006.260702. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]