Abstract
Aging affects all levels of neural processing including changes of intracortical inhibition and cortical excitability. The paired-pulse stimulation protocol, the application of 2 stimuli in close succession, is used to investigate cortical excitability. The paired-pulse behavior is characterized by the fact that the second response is significantly suppressed at short interstimulus intervals (ISIs) but approaches the first response with increasing ISIs. However, there are controversial reports about the influence of age on paired-pulse behavior. We therefore used pairs of tactile stimuli (ISIs from tens to hundreds of milliseconds) to record extracellular responses of somatosensory cortical neurons of young and aged rats. Paired-pulse behavior was quantified as the ratio of the amplitude of the second response divided by the first. For all ISIs, we found significantly higher ratios in the old animals indicating reduced paired-pulse suppression (PPS). Evaluation of the single response components revealed a significant reduction of the response to the first stimulus for old animals but no age-dependent decrement to the second. Changes in PPS are usually mediated by modulating the second response characteristics. Thus, our data demonstrate reduced PPS due to an overall reduction of the first response as a form of modified PPS developing at old age.
Keywords: aging, cortical excitability, intracortical inhibition, rat, somatosensory cortex
Introduction
Aging exerts major reorganization and remodeling at all levels of brain structure and function. Although there is a growing body of information about age-related changes at the cellular and molecular levels, little is known about how aging affects the way in which neurons process and integrate sensory information.
Numerous lines of evidence converge on the observation that during aging intracortical inhibition is particularly affected. For example, a recent ultrastructural study revealed a significant age-related decline in the numerical density of presumptive inhibitory synapses of sensorimotor cortex (Poe et al. 2001) demonstrating a deficit in the intrinsic inhibitory circuitry of the aging neocortex. This observation is in agreement with recent reports about an improvement of visual cortical function in senescent monkeys after application of γ-aminobutyric acid (GABA) and its agonist muscimol on cells in primary visual cortex (V1) (Leventhal et al. 2003). Comparing stimulus selectivity of cells in V1 in young and old macaque monkeys revealed evidence for a significant degradation of orientation and direction selectivity in old animals (Schmolesky et al. 2000) consistent with an age-related degradation of intracortical inhibition. Reduced inhibition was also reported in the secondary visual cortex of senescent monkeys (Yu et al. 2006).
Studies of primary somatosensory cortex (SI) of aged rats have reported substantial, age-related enlargements of hindpaw receptive fields (RFs) (Spengler et al. 1995; Godde et al. 2002; David-Jurgens et al. 2008). Because γ-aminobutyric acid–mediated (GABAergic) mechanisms are crucially involved in the control of the size and shape of cortical RFs (Hicks and Dykes 1983; Chowdhury and Rasmusson 2002), it is reasonable to conclude that the observed RF enlargement in the old animals is due to alterations in GABAergic transmission. Age-related declines in a subpopulation of GABAergic neurons containing the calcium-binding protein parvalbumin in SI is direct evidence for altered GABAergic transmission (Jürgens and Dinse 1998). Using somatosensory evoked potential mapping in combination with electric source localization, it was shown that the distance between the dipoles of the index and the little fingers increased in elderly subjects, parallel to a decline of tactile acuity (Kalisch et al. 2008). In adults, map expansion is typically associated with a gain in perceptual performance (Elbert et al. 1995; Pleger et al. 2001). To explain this atypical relation between map expansion and perceptual performance, it was suggested that the age-related reduction of intracortical inhibition mechanisms results in the disintegration of cortical maps. Based on computational approaches, it has been demonstrated that less distinctive cortical representations emerge as a typical outcome of age-related alterations, where distinctiveness of cortical maps was assumed to correlate with behavioral measures (Li and Sikstrom 2002).
During the last several years, stimulation with pairs of stimuli in close succession (paired-pulse stimulation) has become a common tool to investigate short-term plasticity. This is a useful technique to investigate changes in, and the balance between, cortical excitation and intracortical inhibition. Paired-pulse suppression (PPS) describes the phenomenon that at short interstimulus intervals (ISIs) neuronal responses to the second stimulus are significantly reduced. PPS is quantified in terms of the ratio of the amplitude of the second response divided by the first. That means that large ratios are associated with reduced PPS, and small amplitude ratios are associated with stronger PPS. However, whether and how aging affects paired-pulse behavior remains largely elusive. The available data from human motor cortex of elderly subjects are controversial. According to one study, the motor-evoked potential (MEP) magnitude of intracortical PPS elicited by transcranial magnetic stimulation (TMS) was significantly smaller in elderly as compared with young adults (Peinemann et al. 2001). In contrast, a study employing a different stimulation device found no evidence for age-related changes of intracortical PPS. The MEP amplitude ratios were similar in young and aged subjects, although the raw amplitudes were significantly smaller in the aged group (Oliviero et al. 2006). Another TMS study based on a large population of young and elderly subjects reached a similar conclusion, reporting a large variability of paired-pulse behavior on the one hand and no influence of age on paired-pulse behavior on the other hand (Wassermann 2002). So far, no data for the somatosensory system have been reported.
We therefore examined paired-pulse behavior at the level of single neurons of somatosensory cortex by studying cortical responsiveness to a second cutaneous stimulus applied at different ISIs in young and aged rats. These experiments revealed substantial similarities in the overall ISI dependence across the age range studied. In contrast to previous reports (Geissler et al. 2007; Hoffken et al. 2007), the reduction in PPS observed at all ISIs tested was due to changes in response magnitude of the first response. Although in aged animals RFs were significantly enlarged, we found no correlation between RF size and PPS, indicating that spatial and temporal aspects of tactile processing are differentially affected by age.
Materials and Methods
Subjects
This study presents the data from 9 young (3–9 months, 200–500 g) and 10 old (20–33 months, 300–500 g) rats (Wistar and Long-Evans). Prior experiments revealed no differences in RF properties and neuronal response characteristics after tactile stimulation between both strains. The 50% probability of survival in an aging colony is about 31 months for Wistar rats (Snyder et al. 1990). The animals were kept under standard housing conditions. Treatment of all animals was within the regulations of the European Communities Council Directive of November 24, 1986 (86/609/EEC), and all experiments were approved by the German Animal Care and Use Committee.
Anesthesia and Animal Preparation
Animals were initially anesthetized with urethane (Sigma, St Louis, MO; 20% in ringer solution, 1.5 g/kg intraperitoneally) together with Ketamine (ketamine-hydrochloride 10%, Pfizer [Karlsruhe, Germany], 0.1 g/kg intramuscularly), and supplements were provided as needed based on eyelid and limb reflexes. Although the use of urethane is experimentally useful (Maggi and Meli 1986), there is still some controversy about its influence on the GABAergic system. For example, modest changes in both the inhibitory and the excitatory system were reported (Hara and Harris 2002), whereas other studies found no alterations of GABAA- or GABAB-mediated inhibitory or glutamate-mediated excitatory synaptic transmission by urethane in cortical neurons of the rat (Sceniak and Maciver 2006). On the other hand, the use of ketamine will introduce interferences with N-methyl-D-aspartic acid receptors (Yamakura et al. 2000). Pentobarbital substantially prolongs evoked inhibitory conductances (Wehr and Zador 2005). Preliminary research in our laboratory suggests that the effects of anesthesia on PPS are small. Somatosensory evoked potentials recorded in elderly human subjects after paired-pulse median nerve stimulation reflect changes in paired-pulse ratios comparable to aged rats (Lenz et al. 2009).
Body temperature was maintained at 37.5 °C by a thermostatically controlled heating pad. The cranial bone was dissected, and the cisterna magna was punctuated, thereby lowering intracranial pressure, minimizing pulsations, and avoiding edema. A craniotomy was performed over the right somatosensory cortex (about 0.5 mm rostral to 1 mm caudal from bregma), and the dura mater was removed. To prevent the cortical surface from drying, the area of the craniotomy was filled with warm silicone oil (Silicone DC 200 fluid 50 cst, Serva, Heidelberg, Germany). The left hindlimb was fixated on the hairy skin to ensure a stable position for tactile stimulation of the glaborous side of the paw.
Stimulation and Recording
Single cells or multiunit activity consisting of a small number of action potentials clearly above background were recorded extracellularly at depths of about 700 μm (layer IV) of the hindpaw representation of primary somatosensory cortex. Recordings were made using glass microelectrodes filled with 3 M NaCl. Electrodes had a resistance of 1–1.5 MΩ. Signals were amplified, high pass filtered, and monitored on an oscilloscope and audio monitor. Microelectrodes were positioned with a DC motor–driven x-, y-micromanipulator (Märzhäuser, Wetzlar, Germany) and run vertically through the cortex using a microstepper with a minimal step length of 1 μ (Physiological Institute, University of Mainz). Data presented in this study are based on a total of 179 recording sites (75 from young, 104 from old animals).
After discriminating action potentials according to amplitude, they were digitalized as transistor–transistor logic pulses and stored in a laboratory PC with a temporal resolution of 1 ms (data acquisition software “korr,” Happy Mutants Bio Corporation, Ltd, Mainz, Germany). Poststimulus time histograms (PSTHs) could be displayed online. Spike acquisition and tactile stimulation were controlled by a Master 8 (A.M.P.I., Jerusalem, Israel) stimulus generator. Tactile stimuli could be applied by means of an electromagnetic stimulator. The diameter of the probe was 2 mm. Probe indentation was approximately 500 μm.
Paired-pulse stimuli were applied to RF centers located on a digit of the hindpaw. Eight-microsecond stimuli with ISIs of 35, 50, 70, 100, 150, and 200 ms were repeated 32 times for each recording. For analysis, we calculated the maximal response strength (amplitude) in terms of spikes per bin to each of both stimuli in the PSTHs using a bin width of 1 ms. The ratio (A2/A1) between the amplitude of the second (A2) and first (A1) response was taken as measure of the PPS of SI neurons. Accordingly, A1 and A2 as well as paired-pulse ratios reflect averages following accumulation of responses from 32 trials.
Measurement of RF Sizes
To define the size of cutaneous RFs on the glabrous skin of the hindpaw of the same neurons that were tested for paired-pulse behavior, we used the handplotting technique (Merzenich et al. 1984). In this technique, RFs are defined as those areas on the skin at which just visible skin indentation using a small probe with a nodular tip of 1-mm diameter evoked a reliable neuronal discharge. Other studies have shown that just visible indentation is in the range of 250–500 μm, which is in the middle of the dynamic range of cutaneous mechanoreceptors (Gardner and Palmer 1989). The location and the extent of RFs were then transferred in a schematic drawing of the paw. RF size was analyzed by calculating the skin area in square millimeter by planimetry.
Statistical Analyses
In the present study, values are given as mean ± standard error of the mean, unless otherwise noted. For statistical analyses, values of young and old animals were compared with unpaired, 2-tailed student t-test unless otherwise mentioned. To test the influence of the factor age on the maximal neuronal response strengths of both response components (A1 and A2), we performed a 2-way repeated-measure analysis of variance (ANOVA) with age as between-subject factor and A1 and A2 as within-subject factors. To test for differences in variability between age groups, we used the F-test. For correlation analyses, we calculated Pearson's linear correlation coefficients.
Results
Comparison of PPS in Young and Old Animals
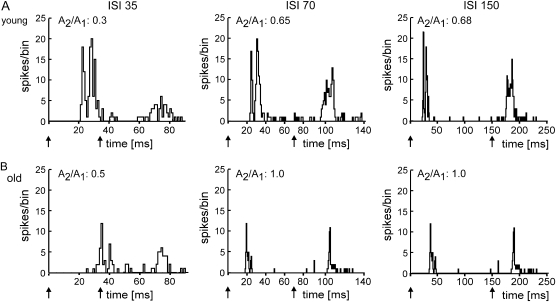
Typical examples of PSTHs from a young and an old animal are shown in Figure 1 for various ISIs. For illustration of the ISI dependence of the PPS, we calculated “paired-pulse curves” (Fig. 2) by plotting the mean amplitude ratios (second response amplitude divided by first response amplitude) obtained from the PSTHs as a function of different ISIs. In general, the PPS of neurons recorded in the hindpaw representation of somatosensory cortex of the old animals resembled that found for young animals: As a rule, the degree of PPS was dependent on the ISI. Substantial PPS was usually present at short ISIs between 35 and 70 ms. At ISIs between 70 and 100 ms, on the other hand, the responses to the paired stimuli became approximately equal in size. On average, we observed very little evidence for significant paired-pulse facilitation, where the second response was larger than the first one (amplitude ratio > 1).
Figure 1.
Examples of PSTHs (A and B). PSTHs of the neuronal responses after tactile paired-pulse stimulation with different ISIs. Stimulus duration was 8 ms. The number of spikes/bin (bin width = 1 ms) is plotted against time. Arrows indicate onset of tactile stimulation. Additionally, the ratio calculated for the amplitudes of the second and first response peaks is shown for each single PSTH. (A) PSTHs of neuron responses recorded in a young animal. (B) PSTHs of neuron responses recorded in an old animal.
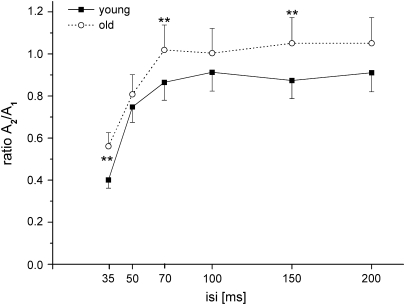
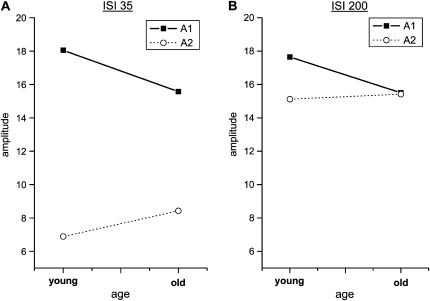
Figure 2.
Mean paired-pulse curves of young and aged rats. Mean amplitude ratios of young (solid line, black squares) and old (dotted line, open circles) animals are plotted against the ISI. Vertical bars show standard error of the mean. Asterisks mark significantly different ratios resulting from comparison of age groups at individual ISIs (*P < 0.05, **P < 0.001).
At all ISIs tested, the mean amplitude ratios of the neurons recorded in old animals were higher than those of the young animals, indicating less PPS in old age (Fig. 2). Repeated-measures ANOVA on ratio values with ISIs as the repeated factor and age group as the between-subject factor revealed significant main effects for the factors age (F13,982, P < 0.0001) and ISI (F53,738, P < 0.0001) with no significant age × ISI interaction (F0,979, P = 0.435). Post hoc comparisons between age groups at each of the individual ISIs revealed significant differences at ISIs of 35, 70, and 150 ms (Bonferroni-adjusted P = 0.0083). Typically, PPS in both young and old animals showed a substantial intra- and interindividual variability. However, data from the aged individuals displayed a larger interindividual variability than young individuals. In fact, high interindividual variability is regarded as a characteristic property of elderly populations (Rapp and Amaral 1992; Gallagher and Rapp 1997). An increase in variability occurred most notably at short ISIs of 35 and 50 ms. Group differences in variability were significant for the ISIs of 35 and 50 ms (F-test; 35 ms: P < 0.001; 50 ms: P < 0.05).
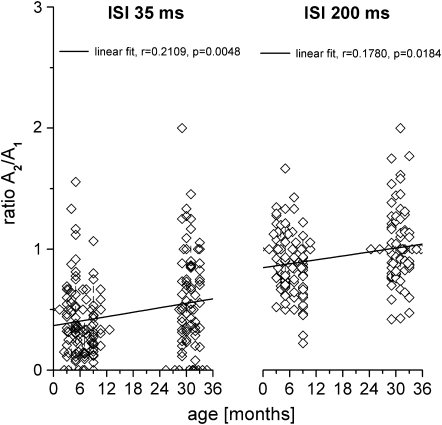
To test the influence of age on PPS, we calculated Pearson's linear correlation coefficients between the amplitude ratio (A2/A1) and age parameters. Scatterplots depicting the relation between age and amplitude ratio are shown for the ISIs of 35 and 200 ms in Figure 3. For both ISIs, we found a significant positive correlation with age (A2/A1 35 ms: r = 0.210, P < 0.001; A2/A1 200 ms: r = 0.178, P < 0.05). One-way ANOVA of the factors age (months) and paired-pulse ratios revealed significant age effects for both ISIs (ISI 35 ms: F = 3,392; P < 0.001; ISI 200 ms: F = 2,636; P < 0.05).
Figure 3.
Amplitude ratios as a function of age. Amplitude ratios of all recording sites of young and old animals are plotted against age (overlapping data are separated by a small upright line). The black line represents the best linear fit. Amplitude ratios obtained at ISI 35 ms (left) and at ISI 200 ms (right).
Impact of Age on Stimulus Number
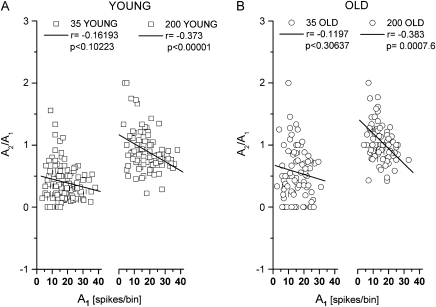
In principle, an increase of the amplitude ratio between the first and second response (i.e., a reduction of PPS) can be achieved by 2 different types of alterations of the response behavior: either by an increase of the second response or by a reduction of the first response. Analyzing our data revealed that the amplitude of the response to the first stimulus plays a crucial role in controlling PPS in general and in mediating changes of PPS during aging in particular. In both young and old animals, small first peak amplitudes were associated with minimal PPS, whereas large first peak amplitudes were linked to stronger PPS (Fig. 4). Perhaps, due to strongly suppressed responses to the second stimulus and/or a large degree of variability, the relation between the magnitude of the first response and the degree of PPS was not significant at an ISI of 35 ms; however, our data are significant at an ISI of 200 ms.
Figure 4.
Amplitude ratios as a function of the response amplitude to the first stimulus (A and B). Amplitude ratios of all recording sites of young (squares) and old (circles) animals are plotted against the size of the first response amplitude (overlapping data are separated by a small upright line). The black lines represent the best linear fit. (A) Data of the young animals. Amplitude ratios obtained at ISI 35 ms (left) and at ISI 200 ms (right). (B) As in (A) for the old animals.
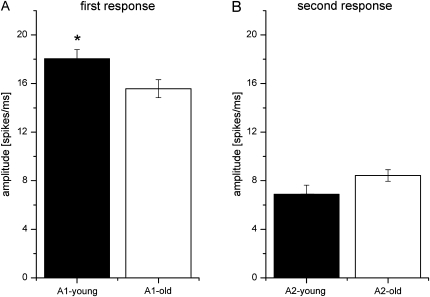
To test the impact of age on the first and second individual response amplitudes, we performed a 2-way repeated-measure ANOVA with age as between-subject factor and A1 and A2 as within-subject factors. There is a significant interaction of the factors age and amplitude for ISIs 35 and 200 ms, indicating that the amplitudes of A1 and A2 diverge as age increases (35 ms: F1,176 = 13.719, P < 0.001; 200 ms: F1,173 = 10.449, P < 0.001). We also compared the mean amplitudes of the first and second response peaks of the young and the old rats (Fig. 5). We found significantly smaller amplitudes of the first response in old animals (t-test, P < 0.05) but no significant age group differences in the size of the amplitude of the second response peak (t-test, P = 0.083). Interaction between age and first and second response amplitude is illustrated in Figure 6.
Figure 5.
Mean response amplitudes in young and old animals (A and B). Mean amplitudes of young (black) and old (white) animals. Error bars show standard error of the mean. (A) Mean values for the first response amplitude and (B) for the second response amplitude. Significant group differences are indicated by asterisks (*P < 0.05).
Figure 6.
Interaction between age and response magnitude (A and B). Interaction of the factors age and amplitude of the first (A1, solid black line) and second (A2, dotted line) response peak. (A) Data refer to ISI 35 ms. (B) Data refer to ISI 200 ms.
Comparison of RF Size in Young and Old Animals
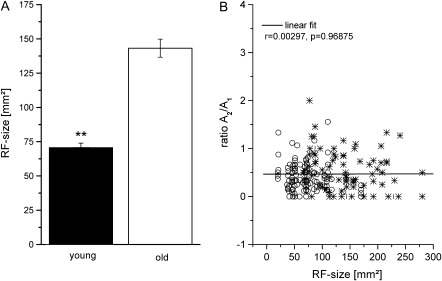
In the present study, in addition to assessing PPS, we measured RF size at each recording site. The averaged RF size recorded in young animals was 70.76 and 143.24 mm2 in the group of aged animals (Fig. 7A, t-test, P < 0.0001). Age and RF size were highly correlated (r = 0.65, P < 0.0001). Similar to the increased interindividual paired-pulse ratio variability of aged individuals, we observed a significant increase in variability for the RF size factor in the aged animal group (F-test: P < 0.0001).
Figure 7.
A) Mean RF size in young (black) and old (white) rats. Error bars show standard error of the mean. Significant group differences are indicated by asterisks (**P < 0.001). (B) Amplitude ratios as a function of RF size. Amplitude ratios obtained at ISI 35 ms of young (open circles) and old (stars) animals are plotted against RF size. The black line represents the best linear fit.
No Correlation between PPS and RF Size in Young and Old Animals
The enlargement of RFs in old animals demonstrated in our present and previous studies might be a consequence of reduced, presumably, GABAergic inhibition. A similar argument can be made for the reduction of PPS found in the old animals. We calculated correlation coefficients to analyze a possible relation between RF size and PPS from both young and old animals (Fig. 7B). There was no correlation between PPS and RF size (r = 0.0029, P = 0.968).
Discussion
We used a paired-pulse protocol consisting of paired tactile stimulation with different ISIs to study age-related alterations in PPS of SI neurons recorded in young and old rats. We analyzed neuronal responses in terms of maximal response strength (amplitude). PPS was quantified as the ratio of the amplitude of the second response divided by the first. For all ISIs, we found significantly higher ratios in the old animals as compared with young rats, indicating reduced PPS. However, the dependence of the neural responsiveness on different ISIs was similar in young and old animals. Our results provide new evidence of an age-specific modulation of PPS: In old animals, the response to the first stimulus is reduced, whereas the response to the second stimulus is not affected by age.
Terminology
Many related terms are used to describe aspects of neural excitability and inhibition. We use the term “paired-pulse behavior” to describe the overall response dependency on different ISIs. We use the term “PPS” to refer to a reduction in the neuronal response to the second of a pair of 2 successive stimuli, a phenomenon often referred to as “forward suppression.” We will use “inhibition” to refer to one possible candidate for this suppression, namely, GABAergic intracortical inhibition arising from postsynaptic activation of GABAA receptor– or GABAB receptor–gated channels. By “synaptic depression,” we refer to a reduction in synaptic drive. Mechanisms mediating synaptic depression include postsynaptic receptor desensitization, presynaptic depletion of releasable vesicles, or other presynaptic mechanisms depressing vesicle release (Bellingham and Walmsley 1999). The fact that the second response of 2 stimuli given in short succession is strongly suppressed has been denoted as “short-term depression,” a special form of short-term plasticity, to describe changes of neural behavior resulting from prior activity (Zucker and Regehr 2002).
Potential Mechanisms Underlying Paired-Pulse Behavior
Our data provide evidence that aging affects PPS. However, the question remains, whether GABAergic or which other mechanisms contribute to the observed age-related alterations. Despite substantial experimental and theoretical work, even in young animals, the mechanisms mediating paired-pulse behavior are not fully understood. There is agreement that presynaptic mechanisms play a crucial role in PPS (Hashimoto and Kano 1998). As to the contribution of GABAergic inhibition, Wehr and Zador (2005) reported that in rat auditory cortex GABA receptor–mediated inhibition does not play a major role in forward suppression for ISIs beyond 100 ms. For longer ISIs, synaptic depression is assumed to be responsible for the observed PPS (Wehr and Zador 2005). In somatosensory cortex, synaptic depression is responsible for a form of rapid sensory adaptation that is similar and perhaps analogous to forward suppression in the auditory system (Chung et al. 2002). In the visual cortex, suppression is also more consistent with thalamocortical synaptic depression than with inhibition (Carandini et al. 2002; Freeman et al. 2002). Because of differences in the time course of recovery between cortical and thalamic cells, however, it seems unlikely that inheritance of thalamic response properties fully accounts for long-lasting forward suppression (Wehr and Zador 2005). Other lines of evidence suggest alterations in the composition of synaptic connections to explain changes in PPS. For example, in auditory cortex slices, low-probability connections were associated with either paired-pulse facilitation or PPS and a nondecremental response to 20-Hz spike trains, whereas high-probability connections were characterized by marked suppression (Atzori et al. 2001). In addition, there is evidence for the involvement of GABAB receptors in regulation of PPS, as presynaptic blockade of GABAB receptors causes a decrease in synaptic release probability consistent with the presynaptic inhibition of glutamate release (Porter and Nieves 2004). Furthermore, it was reported that the amount of paired-pulse facilitation is inversely related to the initial release probability of the synapse (Dobrunz and Stevens 1997). Under the assumption that the response amplitudes used in our study can be equated with synaptic release probability measurements, a decrease in the amplitude of the first response can account for the decrease in PPS.
Besides the contribution of GABAergic mechanisms, there is also evidence for the involvement of glutamatergic transmission in the paired-pulse phenomenon. For example, presynaptic group II and III metabotropic glutamate receptors can have a strong inhibitory effect on transmitter release (Takahashi et al. 1996; von Gersdorff et al. 1997) implying a crucial role in modulating paired-pulse behavior. Beyond direct effects, it is possible that more subtle feed-forward circuits of excitatory and inhibitory interneurons influence paired-pulse behavior. Because our experiments used paired-pulse protocols at a systemic level to investigate aging effects on cortical excitability, our data do not provide information about the possible underlying mechanisms.
Other Conditions Affecting PPS
Besides the influence of age, other conditions such as learning, environmental enrichment, or brain injury affect PPS. Percaccio et al. (2005) studied temporal information processing in the auditory cortex of rats that were housed in an enriched environment. The authors reported that enrichment increased the response to the first tone thereby enhancing the degree of PPS. With respect to our data, this study is important because it shows that PPS can be modulated by altering the response strength of the first stimulus. In a subsequent paper, this group provided evidence that the increase of response strength to the first stimulus as a result of housing animals under enriched conditions is most presumably mediated by changes in glutamatergic but not in GABAergic transmission (Nichols et al. 2007).
In the recent years, PPS has been increasingly studied in humans as well. Application of tactile coactivation, a passive stimulation protocol, has been shown to improve tactile discrimination performance of the fingers in parallel to an expansion of the cortical maps in SI finger representation (Pleger et al. 2001, 2003; Dinse et al. 2003). Hoffken et al. (2007) used electrical median nerve stimulation and tactile coactivation to demonstrate reduced PPS of somatosensory evoked potentials. Specifically, they demonstrated that the individual gain in perceptual performance was positively correlated with the degree of suppression. In contrast to the results shown here, the response magnitude of the first stimulus remained unaffected. Instead, the coactivation-induced suppression was due to an increased response to the second stimulus.
Damage of cortical tissue is known to lead to hyperexcitability that dramatically alters PPS. Autoradiography studies in which cortical lesions were induced by photothrombosis or freezing revealed a downregulation of GABAA receptors and PPS (Qu et al. 1998; Que et al. 1999; Schmidt et al. 2006). In another study, reduced PPS was demonstrated in 7-week-old rats after hypoxic ischemic brain injury indicative of hyperexcitability (Geissler et al. 2007). In contrast to the current results, the magnitude of the first peak remained unaffected, but the response to the second stimulus was substantially enhanced. This implies that the alterations of PPS measured after cortical lesions or tactile coactivation are controlled by other mechanisms than those involved in changes induced by environmental enrichment or aging.
Taken together, PPS can be altered in at least 3 qualitatively different ways: first by changing the response to the first stimulus or, alternatively, by changing the response magnitude of the second stimulus. A third possibility to influence PPS arises from changes in the effectiveness of the inhibitory influence of the first response on the second response.
Reduced First Response Amplitudes—Subcortical or Cortical Effects?
Although postnatal developmental changes of thalamocortical transmission are well investigated, little is known about possible changes developing at old age. Although paired-pulse behavior most presumably reflects intracortical processing, the observation of reduced first amplitudes at old age most likely reflects alterations of thalamocortical transmission and/or changes developing along the entire sensory pathway. According to an unpublished study in our laboratory performed in the ventroposteriolateral (VPL) nucleus of the thalamus of aged rats, response magnitudes of VPL neurons were similarly reduced as reported here for SI. However, due to the substantial corticothalamic projections, it is difficult to conclude whether this is a true subcortical alteration or a reflection of cortical changes. To show that age-related changes of cortical processing are not a simple reflection of changes occurring already at the periphery, the effects of aging on cutaneous mechanoreceptors had been investigated in adult and old rats. Evoked action potentials revealed comparable shapes and amplitudes in all animals of all age groups, and there were no differences in RF size and in threshold between old and adult animals (Reinke and Dinse 1996). Similarly, for the visual system, little age-related changes had been reported for retinal and thalamic levels (Spear et al. 1994; Kim et al. 1997). However, conduction is significantly slowed down in aged animals resulting in a lengthening of response latencies by up to 35% (David-Jurgens et al. 2008). It is conceivable to assume that as a consequence the scatter of the incoming pulses is increased that in turn impairs synaptic integration. As a result, in the old rats, the temporal precision of spiking is reduced that causes more diffuse responses characterized by smaller amplitudes.
Relation between RF Size and PPS
In this study on age-related changes of PPS, we additionally confirmed previous findings about a substantial enlargement of RFs in the hindpaw representation in aged rats (Spengler et al. 1995; Godde et al. 2002; David-Jurgens et al. 2008). In young animals, GABAergic mechanisms are known to control the size and shape of RFs (Hicks and Dykes 1983). Accordingly, as possible alterations of GABAergic processes might play a role in age-related reduction of PPS, we asked whether age-related alterations of PPS and of RFs covary. If this is the case, one would expect to find a strong correlation between age-related enlargement of RFs and a reduction of PPS. Surprisingly, the correlation analysis revealed a complete lack of relationship, indicating that both parameters are regulated independently. One possible explanation for the lack of correlation is that the different GABA receptors involved in mediating spatial and temporal inhibition are differentially affected by aging. Spatial inhibition, which modulates RF size, is mediated to a large extent postsynaptically via GABAA and GABAB receptors (Lee et al. 1994; Schwark et al. 1999; Chowdhury and Rasmusson 2002, 2003). On the other hand, PPS is to a substantial degree presynaptically controlled (Hashimoto and Kano 1998; Bellingham and Walmsley 1999). As a result, no obvious relationship between inhibition acting on RF size and on temporal aspects should result. Alternatively, it is reasonable that during aging particular GABAA receptor subunits change, thereby yielding receptors with different subunit composition (Rissman et al. 2007; Schmidt et al. 2008). In cases where these changes affect surround inhibition and PPS differently, no correlation would be found. Another explanation for the lack of correlation is that the inhibition constraining RF size is likely mediated by superficial intracortical connections, whereas the inhibition contributing to paired-pulse effects is more likely thalamocortical. Furthermore, it is conceivable that GABAergic transmission involved in controlling PPS is not fundamentally altered in the aged animals.
Possible Impact on Sensory Function in Aged Individuals
From a functional perspective, paired-pulse behavior serves as a marker of the overall status of intracortical excitability (Kujirai et al. 1993; Ziemann et al. 1996; Ilic et al. 2002; Fedi et al. 2008; Schmidt et al. 2008). Conceivably, the observed increase of excitability with age might affect multiple levels of processing and has been discussed in the context of a loss of neuron specificity and the resulting deterioration of sensory processing (Leventhal et al. 2003; Yang et al. 2008). Similarly, the expansion of somatosensory maps and associated reduction of tactile performance have been linked to reduced levels of suppression (Kalisch et al. 2008). Using a computational mean field approach, we have recently shown that map expansion can be paralleled by either a perceptual impairment or an improvement dependent on changes of lateral interaction processes based on Mexican hat interaction. The “aging” model reveals broader distributed representations together with reduced tactile acuity due to a broader excitatory and a weaker inhibitory component (Wilimzig et al. 2006). On the other hand, paired-pulse behavior has been linked to forward masking and temporal processing (Atzori et al. 2001; Percaccio et al. 2005; Wehr and Zador 2005). There is compelling evidence that temporal processing in elderly individuals is impaired (Mendelson and Ricketts 2001; Mendelson and Wells 2002; Conlon and Herkes 2008). Single-cell data obtained for tactile train stimulation in old rats revealed a substantial inability to faithfully follow rates of 20–30 Hz. Interestingly, paired-pulse behavior turned out to be a rather poor predictor of train behavior (David-Jurgens and Dinse 2008). Accordingly, more studies are needed to explore the relation between paired-pulse behavior and how rapidly presented sequences of stimuli are perceived.
Conclusion
The paired-pulse ratios from the old animals were higher than those from the young rats at all ISIs, indicating less suppression. The shape of the paired-pulse curve, however, remained unaltered. Accordingly, the ISI dependency characterized by substantial suppression at short ISIs and less suppression at longer ISIs is similar in young and old animals. Thus, our results revealed no evidence for age-related changes of paired-pulse behavior itself. On the other hand, we found an age-related reduction of the first response amplitude. In the case of unchanged PPS, the second response amplitude should be reduced as well as compared with young animals, which was, however, not the case. Instead, the ratio between the first and second response was higher for old animals relative to young animals. This indicates that an age-related reduction of activity as apparent in a decreased first response amplitude most likely causes less suppression of the second response amplitude as evident in an increased paired-pulse ratio in old animals. Changes of PPS might be one substrate responsible for impaired temporal processing in aged individuals.
Funding
Deutsche Forschungsgesellschaft (Da 1116/1-1 DFG, Di DFG 334/19-1).
Acknowledgments
Conflict of Interest: None declared.
References
- Atzori M, Lei S, Evans DIP, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–1237. doi: 10.1038/nn760. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci. 2002;22:10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Rasmusson D. Comparison of receptive fields expansion produced by GABAA and GABAB receptor antagonists in raccoon primary somatosensory cortex. Exp Brain Res. 2002;144:114–121. doi: 10.1007/s00221-002-1035-7. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Corticocortical inhibition of peripheral inputs within primary somatosensory cortex: the role of GABAA and GABAB receptors. J Neurophysiol. 2003;90:851–856. doi: 10.1152/jn.01059.2002. [DOI] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory sesponses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Conlon E, Herkes K. Spatial and temporal processing in healthy aging: implications for perceptions of driving skills. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:446–470. doi: 10.1080/13825580701878008. [DOI] [PubMed] [Google Scholar]
- David-Jurgens M, Churs L, Berkefeld T, Zepka RF, Dinse HR. Differential effects of aging on fore- and hindpaw maps of rat somatosensory cortex. PLoS ONE. 2008;3:e3399. doi: 10.1371/journal.pone.0003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Jurgens M, Dinse HR. Age related alterations of response properties of cortical somatosensory neurons after presentation of train stiumli—influence of age on temporal processing. 2008 In: Society for Neuroscience Abstract pp 177. 120/PP125. Neuroscience Meeting Planner. Society for Neuroscience. Online. [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Fedi M, Berkovic SF, Macdonell RAL, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18:664–669. doi: 10.1093/cercor/bhm100. [DOI] [PubMed] [Google Scholar]
- Freeman TCB, Durand S, Kiper DC, Carandini M. Suppression without inhibition in visual cortex. Neuron. 2002;35:759–771. doi: 10.1016/s0896-6273(02)00819-x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Palmer CI. Simulation of motion on the skin. II. Cutaneous mechanoreceptor coding of the width and texture of bar patterns displaced across the OPTACON. J Neurophysiol. 1989;62:1437–1460. doi: 10.1152/jn.1989.62.6.1437. [DOI] [PubMed] [Google Scholar]
- Geissler M, Neuhoff S, Kreikemeier K, Meier C, Dinse HR. Human umbilical cord blood cells restore cortical maps and cortical excitability after hypoxic ischemia in rats. 2007 In: Society for Neuroscience Abstract pp 899. 814/W897 Neuroscience Meeting Planner. Society for Neuroscience. Online. [Google Scholar]
- Godde B, Berkefeld T, David-Jurgens M, Dinse HR. Age-related changes in primary somatosensory cortex of rats: evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev. 2002;26:743–752. doi: 10.1016/s0149-7634(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J Physiol. 1998;506(Pt 2):391–405. doi: 10.1111/j.1469-7793.1998.391bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks TP, Dykes RW. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983;274:160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Hoffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol. 2007;584:463–471. doi: 10.1113/jphysiol.2007.140079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens M, Dinse HR. Distribution of the calcium-binding protein parvalbumin in the fore- and hindpaw representation of somatosensory cortex of adult and aged rats. Soc Neurosci Abstr. 1998;24:633. [Google Scholar]
- Kalisch T, Ragert P, Schwenkreis P, Dinse HR, Tegenthoff M. Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex. 2008;19:1530–1538. doi: 10.1093/cercor/bhn190. [DOI] [PubMed] [Google Scholar]
- Kim CB, Pier LP, Spear PD. Effects of aging on numbers and sizes of neurons in histochemically defined subregions of monkey striate cortex. Anat Rec. 1997;247:119–128. doi: 10.1002/(SICI)1097-0185(199701)247:1<119::AID-AR14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. J Neurophysiol. 1994;71:1716–1726. doi: 10.1152/jn.1994.71.5.1716. [DOI] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Stude P, Hoffken M, Kalisch T, Dinse HR. Annual meeting of the German Section of the International Federation of Clinical Neurophysiology (DGKN) Abstract. 2009. Median nerve paired-pulse stimulation reveals age-related changes of cortical excitability of human somatosensory cortex; p. 395. DGKN Abstracts Online. [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Li SC, Sikstrom S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: general considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Ricketts C. Age-related temporal processing speed deterioration in auditory cortex. Hear Res. 2001;158:84–94. doi: 10.1016/s0378-5955(01)00294-5. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Wells EF. Age-related changes in the visual cortex. Vision Res. 2002;42:695–703. doi: 10.1016/s0042-6989(01)00307-8. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Nichols JA, Jakkamsetti VP, Salgado H, Dinh L, Kilgard MP, Atzori M. Environmental enrichment selectively increases glutamatergic responses in layer II/III of the auditory cortex of the rat. Neuroscience. 2007;145:832–840. doi: 10.1016/j.neuroscience.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neuroscience Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neuroscience Lett. 2001;313:33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Percaccio CR, Engineer ND, Pruette AL, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment increases paired-pulse depression in rat auditory cortex. J Neurophysiol. 2005;94:3590–3600. doi: 10.1152/jn.00433.2005. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci USA. 2001;98:12255–12260. doi: 10.1073/pnas.191176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster A-F, Ragert P, Dinse HR, Schwenkreis P, Malin J-P, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40:643–653. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- Poe BH, Linville C, Brunso-Bechtold J. Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical disector. J Comp Neurol. 2001;439:65–72. doi: 10.1002/cne.1335. [DOI] [PubMed] [Google Scholar]
- Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813:374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- Que M, Witte OW, Neumann-Haefelin T, Schiene K, Schroeter M, Zilles K. Changes in GABAA and GABAB receptor binding following cortical photothrombosis: a quantitative receptor autoradiographic study. Neuroscience. 1999;93:1233–1240. doi: 10.1016/s0306-4522(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Reinke H, Dinse HR. Functional characterization of cutaneous mechanoreceptor properties in aged rats. Neuroscience Lett. 1996;216:171–174. doi: 10.1016/0304-3940(96)13039-1. [DOI] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM. GABAA receptors in aging and Alzheimers disease. J Neurochem. 2007;103:1285–1292. doi: 10.1111/j.1471-4159.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95:3865–3874. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Bruehl C, Hagemann G, Witte OW, Redecker C. Impairment of functional inhibition in the contralateral cortex following perinatally acquired malformations in rats. Exp Neurol. 2006;201:270–274. doi: 10.1016/j.expneurol.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW. Age-related decline of functional inhibition in rat cortex. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.006. doi:10.1016/j.neurobiolaging.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schwark HD, Tennison CF, Ilyinsky OB, Fuchs JL. Inhibitory influence on receptive field size in the dorsal column nuclei. Exp Brain Res. 1999;126:439–442. doi: 10.1007/s002210050750. [DOI] [PubMed] [Google Scholar]
- Snyder DL, Pollard M, Wostmann BS, Luckert P. Life span, morphology, and pathology of diet-restricted germ-free and conventional Lobund-Wistar rats. J Gerontol. 1990;45:B52–B58. doi: 10.1093/geronj/45.2.b52. [DOI] [PubMed] [Google Scholar]
- Spear PD, Moore RJ, Kim CB, Xue JT, Tumosa N. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys. J Neurophysiol. 1994;72:402–420. doi: 10.1152/jn.1994.72.1.402. [DOI] [PubMed] [Google Scholar]
- Spengler F, Godde B, Dinse HR. Effect of ageing on topographic organization of somatosensory cortex. NeuroReport. 1995;6:469–473. doi: 10.1097/00001756-199502000-00016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wilimzig C, Pleger B, Ragert P, Kalisch T, Tegenthoff M, Dinse HR. Differential effects of aging and leraning processes on cortical interaction. 2006 In: Society for Neuroscience Abstract pp 53. 56/N14 Neuroscience Meeting Planner. Society for Neuroscience. Online. [Google Scholar]
- Yamakura T, Chavez-Noriega LE, Harris RA. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology. 2000;92:1144–1153. doi: 10.1097/00000542-200004000-00033. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Li G, Wang Y, Zhou Y, Leventhal AG. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience. 2008;156:748–757. doi: 10.1016/j.neuroscience.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]