Abstract
The CD20 cell surface antigen is expressed at high levels by over 90% of B cell non-Hodgkin lymphomas (NHL), and is the target of the anti-CD20 monoclonal antibody rituximab. To provide more sensitive, tumor-specific positron emission tomography (PET) imaging of NHL, we sought to develop PET imaging agents targeting CD20.
Methods
Two recombinant anti-CD20 rituximab fragments, a minibody (scFv-CH3 dimer, 80 kDa) and a modified scFv-Fc fragment (105 kDa), designed to clear rapidly, were generated. Both fragments were radiolabeled with 124I, and the minibody was additionally radiometal labeled with 64Cu following conjugation to 1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid (DOTA). The radioiodinated fragments and the radiometal labeled minibody were evaluated in mice as microPET imaging agents for in vivo imaging of human CD20-expressing lymphomas.
Results
Rapid and specific localization to CD20-positive tumors was observed with the radioiodinated fragments. However, their tumor uptakes and blood activities differed, resulting in different levels of contrast in the images. The best candidate was the minibody, with superior uptake (2-fold higher than the scFv-Fc) in CD20-positive tumor and low uptake in CD20-negative tumor. Positive tumor to negative tumor ratios were 7.0(±3.1) and 3.9(±0.7) for the minibody and scFv-Fc, respectively at 21 hours. About a 5-fold lower ratio was achieved with the 64Cu-DOTA-minibody at 19 hours due to higher residual background activity in CD20 negative tumor.
Conclusion
Radioiodinated minibody and scFv-Fc fragment produced excellent, high-contrast images in vivo. These new immunoPET agents may prove useful for the imaging CD20 positive lymphomas in preclinical models and in humans with NHL.
Keywords: lymphoma, CD20, antibody fragments, immunoPET, rituximab
INTRODUCTION
Non-Hodgkin lymphoma (NHL) is the fifth most common malignancy in the U.S., accounting for approximately 4% of all cancers. Over 90% of NHL are derived from B cells, and express the B cell differentiation antigen CD20. CD20 is an integral transmembrane protein expressed exclusively by cells in the B-lineage, from normal pre-B-cell precursors through mature B-cells, but not by terminally differentiated plasma cells (1). While the precise functions of CD20 remain unknown, it is thought that CD20 plays a role in B-cell activation events, the regulation of cell growth, and transmembrane calcium flux (2). This 33–37 kDa non-glycosylated, hydrophobic, phosphorylated protein is not shed and undergoes very slow modulation or internalization in response to antibody binding, making it an ideal target for exogenous antibody therapies (2).
The chimeric (mouse/human) anti-CD20 antibody rituximab C2B8 (Rituxan®, Genentech/Biogen-IDEC, San Francisco, CA) has become a mainstay in the treatment of B cell NHL, achieving high response rates in low-grade B cell lymphomas (3), and improvements in survival in both low-grade and aggressive lymphomas when combined with chemotherapy (4, 5). Rituximab’s mechanism of action in vivo appears to be mediated by antibody dependent cell-mediated cytotoxicity (ADCC), complement mediated cell-lysis (CDC), and the induction of apoptosis in tumor cells (6). The efficacy of anti-CD20 antibodies against lymphoma has been further enhanced through combination with therapeutic radionuclides such as 131I (tositumomab, Bexxar®) and 90Y (ibritumomab, Zevalin®) (7, 8).
The fluorine-18 fluorodeoxyglucose ([F-18]-FDG) tracer is currently standard for clinical PET imaging of many malignancies, but its utility in lymphomas can be limited in cases of indolent disease with low metabolic activity. The tumor detection rate for FDG-PET in low-grade small lymphocytic and marginal zone lymphomas can be as low as 50% (9, 10). An imaging agent directed against a cell-surface target could provide more sensitive, tumor-specific imaging. Recently there has been a renewed interest in using antibodies for imaging malignancies (immunoPET). Antibodies radiolabeled with 124I, 64Cu and 89Zr have been evaluated in patients with tumors (11). However, despite promising results, these PET tracers were all based on intact antibodies, and as a result, days were required for the activity levels to drop sufficiently to allow acceptable target-to-background ratios.
Redesigning antibodies, without compromising their specificity by reducing their size, results in rapid clearance from the blood, a desirable property for an imaging agent. We have previously generated engineered antibody fragments including diabodies (dimers of single-chain Fv; scFv; 55 kDa) (12), minibodies (dimers of scFv-CH3; 80 kDa) (13) and scFv-Fc (dimer of single-chain Fv-Fc, 105 kDa) with pharmacokinetics optimized for imaging in vivo (14). MicroPET imaging using 124I- and/or 64Cu-labeled fragments have demonstrated rapid, high level tumor targeting to tumor specific surface molecules such as carcinoembryonic antigen (CEA, colon carcinoma), HER2 (breast cancer) and prostate specific cell antigen (PSCA, prostate cancer) in mice carrying human colon, breast or prostate cancer xenografts (14–18). The major advantage of using non-residualizing labels (i.e.124I) over residualizing labels (i.e. 64Cu) is the low background activity in normal organs (liver, kidneys) obtained with radioiodinated proteins. This difference is due to the different metabolism and clearance of activity after administration: metabolites (e.g. iodide and/or iodotyrosines) of radioiodinated proteins are quickly released from the cells and excreted via the kidneys (19, 20), whereas metabolites of radiometal-chelated proteins are trapped in the cell leading to increased retention of activity over time (21–23). When the anti-CEA T84.66 minibody was labeled with both labels and evaluated by microPET imaging in tumor bearing mice, the tumor to background ratios were 11 with 124I-labeled minibody at 18 hours and almost 2-fold less (6.1) with 64Cu-labeled minibody at 24 hours (16, 17). Here, we describe the generation of two anti-CD20 rituximab fragments, a minibody and a scFv-Fc fragment with mutations of two residues (H310A and H435Q) in the Fc region that have been shown to interfere with the binding to the rodent neonatal Fc recycling receptor (FcRn) (24). The fragments were radiolabeled with 124I and evaluated as microPET imaging agents for the in vivo imaging of human CD20-expressing lymphomas. Rapid and specific localization to CD20-positive tumors was observed. The tumor uptake and blood activities differed between the two fragments, resulting in different levels of contrast in the images. The best candidate was the minibody, owing to its superior uptake in the CD20-positive tumor and rapid blood clearance, producing excellent, high contrast images. The minibody was also radiolabeled with 64Cu and evaluated in the same tumor model as the radioiodinated fragments. However, the stable/residualizing radiometal labeled minibody produced high background signal in the negative tumor and organs, resulting in relatively poor image contrast. These immunoPET agents may prove useful for imaging CD20-positive lymphomas in preclinical models and in humans with NHL.
MATERIAL AND METHODS
Design and Gene Assembly of Anti-CD20 Antibody Fragments
Splice overlap extension PCR (SOE-PCR) was used to create fully synthetic anti-CD20 variable (V) genes based on the V gene sequences of the murine 2B8 (US Patent No. 5,736,137) (25). Full-length 2B8 VL and VH genes were then assembled by SOE-PCR to produce a single chain Fv (scFv) with 18-residue long linker (Whitlow 218 linker; GSTSGSGKPGSGEGSTKG) (26) in VL-VH orientation. Following SOE-PCR which also included a signal peptide to the 5’-end (upstream) to enable secretion, the construct was cloned into pCR®-2.1-TOPO vector (Invitrogen Corp., Carlsbad, CA) and confirmed by sequencing.
The human IgG4 Fc including the hinge (h-CH2-CH3) was amplified by PCR and fused to the 2B8 scFv by another SOE-PCR to make a chimeric scFv-Fc. Using a Quick-Change Site Directed Mutagenesis kit (Stratagene, La Jolla, CA), specific mutations in the CH2 and CH3 domains (H310A and H435Q; EU-index numbering) were introduced to eliminate the binding to the recycling receptor (FcRn), creating the so-called Double Mutant construct (scFv-Fc DM) as described (27). The minibody was assembled by initial amplification of the human IgG4 hinge and CH3 domain that were joined by SOE-PCR and fused to the 2B8 scFv by another SOE-PCR to produce a full length minibody construct. In order to correct for the deficiency of IgG4 in forming inter-heavy-chain bonds (28), a single mutation (S228P) was introduced in the core hinge region (CPSC; residues 226–229) of the scFv-Fc DM and minibody.
Finally, the minibody and scFv-Fc DM fragments were both inserted into the mammalian expression vector pEE12 on XbaI and EcoRI sites as described (14). This vector contains the hCMV promoter and the glutamine synthetase gene for selection.
Expression, Selection and Purification
A total of 2 × 106 NS0 mouse myeloma cells were transfected with 10 µg of linearized (cut with SalI) vector DNA by electroporation and selected in glutamine deficient media (JHR Biosciences, Lenexa, KS) as described (27). After 2–3 weeks, supernatants were screened for expression by ELISA and analyzed by Western blot for size as described (27). The minibody and scFv-Fc DM were captured by goat anti-human Fc specific antibodies and detected by alkaline phosphatase-conjugated goat anti-human Fc specific antibodies (both from Jackson ImmunoResearch Laboratories, West Grove, PA). The highest producing clones were expanded and brought to terminal culture. Supernatants were passed over a protein L agarose column (Thermo Fisher Scientific Inc., Waltham, MA), and bound proteins were eluted using 5, 10 and 5 column volumes of 30%, 50% and 70% 0.2 M Citrate buffer (pH 2.1) in PBS, respectively into 80% v/v 1 M Tris base, pH 8.2. The eluted fractions containing the desired protein were dialysed against PBS using Slide-A-Lyzer Dialysis Cassettes (Thermo Fisher Scientific Inc.) and then concentrated down to 0.5–1.0 mL by Vivaspin 20 (Vivascience AG, Hannover, Germany). The final concentration of purified protein was determined by A280 nm using the extinction coefficient ε 1.4 mg/mL.
Biochemical Characterization of Purified Anti-CD20 Antibody Fragments
Aliquots of purified proteins were analyzed by SDS-PAGE under non-reducing or reducing (1 mM DTT) conditions. Samples were also subjected to size-exclusion high-pressure liquid chromatography (HPLC) on a Superdex-200 HR 10/30 column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) using a 0.5 mL/minute flow rate and 50 mM Na3PO4/0.15 M NaCl (pH 7.0) buffer. Retention time was compared to standards of intact anti-CEA cT84.66 antibody, minibody and diabody as described (14).
Binding of fragments to human CD20 was assessed by indirect immunofluorescence using the murine B-cell lymphoma 38C13-huCD20 line (described below). Cells (5 × 105) were incubated for 1 hour on ice with 500 µl minibody or scFv-Fc at 5µg/mL in PBS plus 1% fetal bovine serum (FBS). Cells were washed and stained with phycoerythrin (PE)-conjugated goat anti-human (Fc specific) antibodies (Jackson ImmunoResearch Laboratories) at 1:100 dilution for detection. Rituximab was used as positive control.
Radioidination with 124I
Purified rituximab minibody and scFv-Fc DM (0.2 mg each) were radioiodinated with 17.1 (0.461 mCi) and 15.6 MBq (0.422 mCi), respectively, of the positron emitting isotope 124I (sodium iodide in 0.02 M NaOH; radionuclide purity >99%) (IBA Molecular, Sterling, VA) using the Iodogen method as previously described (14, 27). Instant thin-layer chromatography using the Monoclonal Antibody ITLC Strips Kit (Biodex Medical Systems, Shirley, NY) was used to determine the labeling efficiency as described (27). Immunoreactivity was assayed by incubating radioiodinated protein with an excess amount of 38C13-huCD20 cells for one hour, centrifuging the cells, and counting activity remaining in the supernatant. Labeling efficiencies were 84.9 and 80.9% and immunoreactivities were 32.8 and 37.7% for scFv-Fc DM and minibody, respectively.
Minibody Conjugation and Radiolabeling with 64Cu
Purified minibody was conjugated to 1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid (DOTA; Macrocyclics, Dallas, TX) by using the water-soluble N–hydroxysuccinimide method as described (29). The extent of modification was evaluated by isoelectric focusing (30).
The positron emitting isotope, 64Cu (copper chloride in 0.1 M HCl; radionuclide purity >99%), was provided by Mallinckrodt Institute of Radiology (Washington University School of Medicine, St. Louis, MO). The anti-CD20 DOTA-conjugated minibody (200 µg) was incubated with 13.0 MBq (0.35 mCi) of 64Cu in 0.1 M NH4 citrate (pH 5.5) for 50 minutes at 43° C. The labeling efficiency was 65.4% as determined by the Monoclonal Antibody ITLC Strips Kit.
Syngeneic Human CD20-expressing Murine Lymphoma Model
The 38C13 murine B cell lymphoma line (31) was stably transduced with the human CD20 gene using the pRRLsin.hEF1α.CD20.Wpre lentiviral vector provided by Josie Golay (32). Human CD20-positive cells were selected using anti-CD20 MACS beads (Miltenyi Biotec, Auburn, CA) followed by flow cytometric sorting to obtain a population of uniformly high CD20-expressing cells (38C13-huCD20). These cells maintained high-level human CD20 expression during in vitro culture and passage in immunocompetent C3H mice. 38C13 and 38C13-huCD20 tumor cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 50 µM 2-mercaptoethanol (cRPMI). All media and supplements were obtained from Invitrogen. Six to eight week old female C3Hf/Sed/Kam mice were bred and housed at the UCLA Defined Pathogen Colony according to an approved UCLA’s Chancellor’s Animal Research Committee protocol. Tumors were established by s.c. injection of 5 × 103 cells above each shoulder as described (14).
Human CD20 Surface Expression by 38C13-huCD20 Cells
CD20 levels were quantitated on 38C13-huCD20 and three human B cell lymphoma cell lines were used extensively for CD20 xenograft studies (Daudi, Raji, and Ramos) using flow cytometry. Cell lines were stained with anti-human CD20 PE-labeled antibody or a control mouse IgG1, κ-PE labeled antibody (BD Biosciences, San Jose, CA). Samples were run on a FACSCalibur flow cytometer (BD Bioscience) and data was analyzed by FCS Express V3 software (De Novo Software, Los Angeles, CA). To confirm human CD20 expression in vivo, mice were inoculated subcutaneously (s.c.) with 5 × 103 38C13-huCD20 tumor on day 0. Some mice were injected intravenously (i.v.) with 1 mg of rituximab 4 h prior to tumor collection. On day 14 tumors were removed and placed into formalin for 8 h before being transferred to 70% ethanol, and embedded in paraffin. The sections were deparaffinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval (HIER) was carried out for all sections in 0.01 M sodium citrate buffer (pH = 6.0) using a vegetable steamer at 95°C for 25 min. Mouse monoclonal antibody to human CD20 (Dako Corporation, Carpinteria, CA) and rabbit polyclonal antibody to human IgG1 for detection of rituximab were applied at a dilution of 1:1000 and 1:300, respectively, for 45 min at room temperature. The signal was detected using the DAKO horseradish peroxidase EnVision kits (Dako Corporation), and visualized with the diaminobenzidine reaction. The sections were counterstained with hematoxylin.
MicroPET Imaging
Before conducting imaging studies, thyroid uptake was blocked with Lugol’s solution (Sigma-Aldrich, St. Louis, MO) and stomach uptake was blocked by gastric lavage using potassium perchlorate as previously described (16). Mice were serially imaged under anesthesia at around 4 and 20 hours following i.v. injection of radioactivity, using a Focus 220 microPET scanner (Siemens Preclinical Solutions, Knoxville, TN). Acquisition time was 10 minutes (1 bed position), and images were reconstructed using a filtered backprojection (FBP) algorithm (33, 34). After the final scan, mice were euthanized, and tumors and organs were excised, weighed and counted in a Wallac WIZARD Automatic Gamma Counter (PerkinElmer Life and Analytical Sciences Inc., Wellesley, MA). The injected dose was corrected for labeling efficiency and immunoreactivity and from this, the percent injected dose per gram (ID/g) tissue with standard deviation was calculated and decay corrected. Images were displayed using AMIDE (35) and regions of interest (ROIs) were drawn as described (16) and quantitated using a cylinder with known weight and radioactivity to determine a calibration factor (µCi/voxel). ROIs were also drawn to calculate positive tumor and negative tumor and soft tissue ratios. All significance testing was done at the 0.05 level using 1-tail Student’s t-test.
RESULTS
In Vitro Characterization of Engineered Anti-CD20 Antibody Fragments
a) Expression and SDS-PAGE
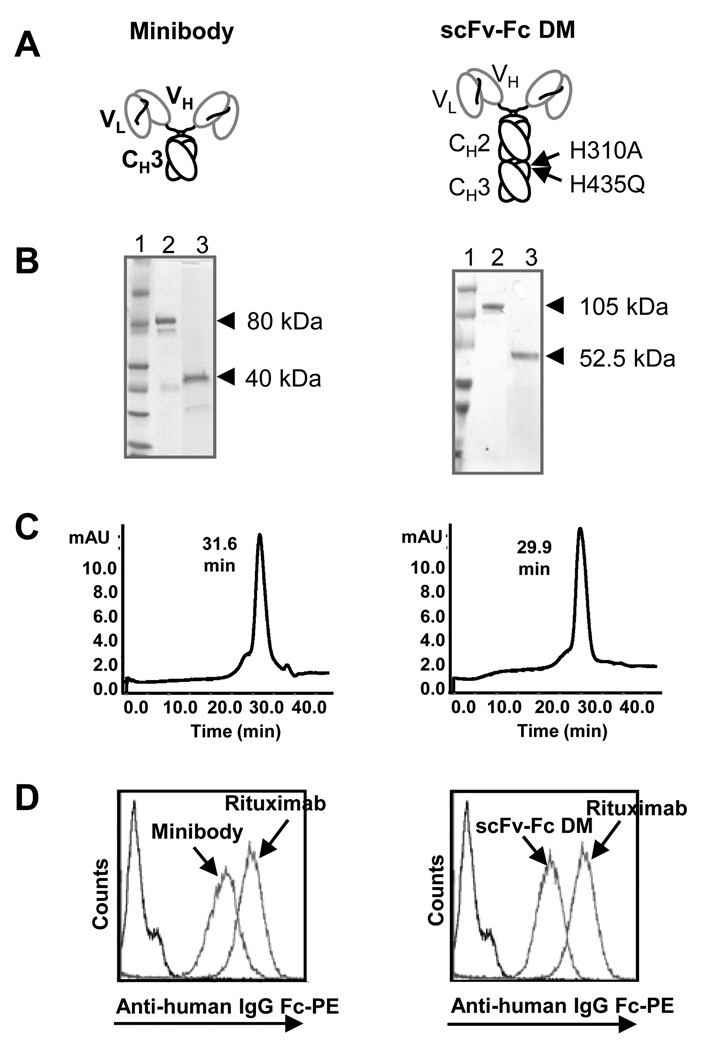
Engineered anti-CD20 antibody fragments (minibody and scFv-Fc DM; Fig. 1A) were expressed at 4–10 µg/mL in terminal cultures of the mouse myeloma cell line NS0 as determined by ELISA. Following affinity chromatography, purified proteins were analyzed by SDS-PAGE (Fig. 1B). Both minibody and scFv-Fc DM migrated as covalent dimers consistent with their predicted molecular mass of approximately 80 kDa and 103 kDa under non-reducing conditions, and as monomers of approximately 40 kDa and 51.5 kDa under reducing conditions.
FIGURE 1.
Characterization of purified rituximab minibody and scFv-Fc DM. A) Schematic presentations of the minibody and scFv-Fc DM. Both proteins assemble into covalent bound homodimers through cysteines in the hinge region. The two mutations (H310A and H435Q) present in the Fc region are indicated. VL = variable light. VH = variable heavy. CH = constant heavy. B) Coomassie stained SDS-PAGE of purified proteins under non-reducing (lanes 2) and reducing (lanes 3) conditions. Lane 1 = molecular marker. C) Size-exclusion chromatography analysis of purified minibody and scFv-Fc DM. D) Flow cytometric analysis of CD20 binding by rituximab antibody fragments. Purified proteins were assayed for binding to 38C13-huCD20 cells. Bound protein was detected with phycoerythrin (PE) conjugated goat anti-human (Fc specific) antibodies. Rituximab was used as positive control and secondary antibody alone as negative control.
b) Size-Exclusion Chromatography
Size exclusion chromatography verified that the anti-CD20 minibody and scFv-Fc DM eluted at times corresponding to correctly folded dimers of expected molecular weights (Fig. 1C). As expected, the minibody eluted slightly later than the scFv-Fc DM at 31.6 versus 29.9 minutes which is consistent with their size difference. The purity of the proteins was determined from the size-exclusion chromatography to be above 90%.
c) CD20 Binding Studies
Binding to target antigen was demonstrated by indirect immunofluorescence cell surface staining of 38C13-huCD20 cells incubated with purified protein using flow cytometry (Fig. 1D). CD20 binding by the minibody and scFv-Fc DM shifted the cells to the right, indicating specific binding. The binding of rituximab to the cells is also shown.
Expression of target CD20 antigen by 38C13-huCD20 lymphoma cells In Vivo and In Vitro
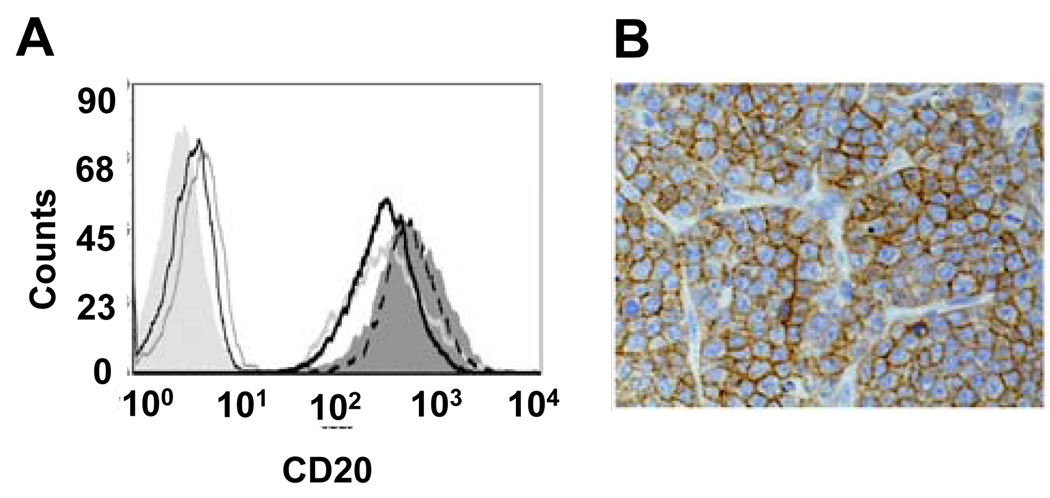
To evaluate our anti-CD20 immunoPET imaging agents in vivo, we chose to use the 38C13-huCD20 B cell lymphoma model. This fully syngeneic model allows for physiologic tumor growth in a natural immunocompetent host, where tumor cell adhesion, tumor metastasis, and adaptive immune effector mechanisms are fully functional. As shown in Fig 2A, cultured 38C13-huCD20 cells expressed high levels of human CD20, comparable to those found on human B cell lymphoma lines commonly used as lymphoma xenografts (36). Upon s.c. injection of 38C13-huCD20 cells into C3H mice, tumors grew rapidly, and immunohistochemical staining of excised tumor demonstrated high-level human CD20 expression (Fig. 2B). Penetration of intact rituximab into growing tumors was demonstrated by i.v. injection, followed by tumor excision 4 hours later and staining with anti-human IgG1 antibody, after which prominent staining was observed (data not shown). Together these results show that human CD20 is expressed at levels similar to those seen with typical human B cell lymphoma lines and that in vivo rituximab can recognize the human CD20 expressed on this murine B cell lymphoma.
FIGURE 2.
38C13-huCD20 cells express surface human CD20 at levels comparable to human B cell lymphoma xenograft cell lines. A) Flow cytometric analysis of cells stained with anti-human CD20-PE antibody, showing surface staining of 38C13-huCD20 (dark grey histogram), Daudi (dotted black line), Raji (thick light grey line), Ramos (thick black line), and wild-type, non-CD20 expressing 38C13 (thin black line) cell lines. Controls include unstained 38C13-huCD20 cells (light gray shaded histogram), and isotype control PE-labeled antibody (thin light grey line). B) Immunohistochemistry for human CD20 expression on 38C13-huCD20 in vivo. Day 14-established 38C13-huCD20 tumors grown in wild-type C3H mice were excised and stained with anti-human CD20 antibody, followed by peroxidase detection.
In Vivo Characterization of 124I-labeled Engineered Anti-CD20 Antibody Fragments
a) MicroPET Imaging using 124I-labeled Anti-CD20 ScFv-Fc DM
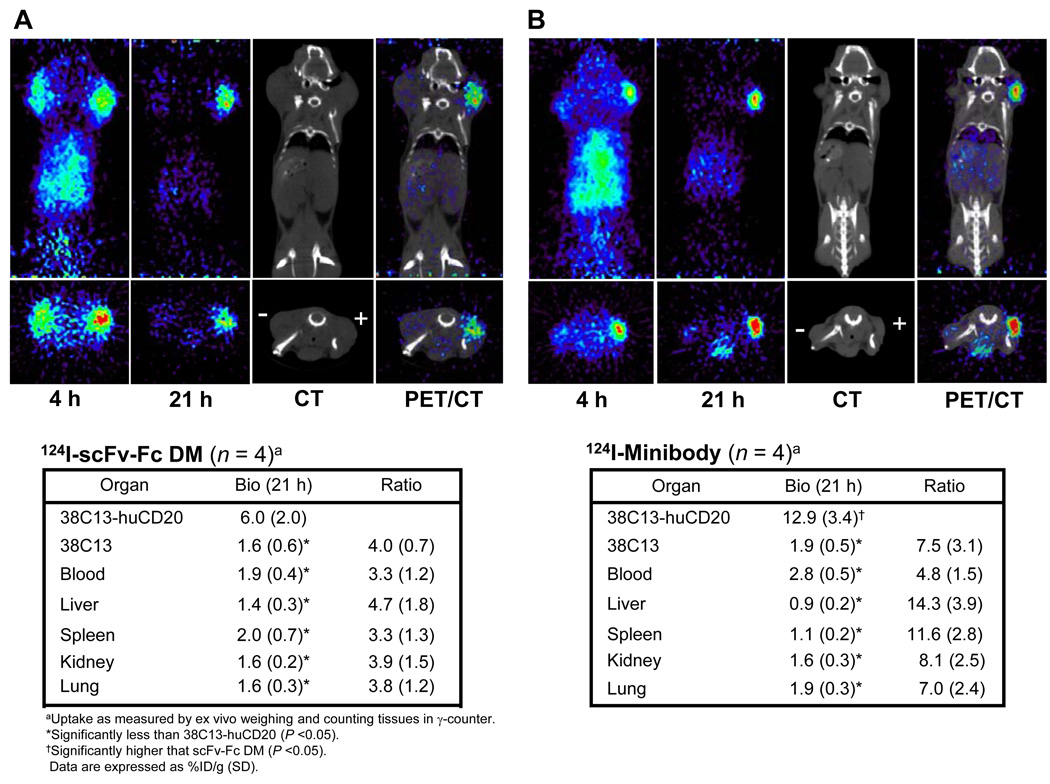
The scFv-Fc DM was evaluated in C3H mice bearing 38C13-huCD20 and 38C13 tumors averaging 91±92 mg (range: 28–225 mg) and 321±199 (range: 93–565 mg) in weight, respectively. These mice were each injected with approximately 3.5 MBq of 124I- scFv-Fc DM (specific activity = 0.066 MBq/µg), and at 4 and 21 hours after administration whole body scans were obtained. At 4 hours radioactivity in both tumors as well as in normal organs were seen (Fig. 3A). By 21 hours the non-specific activity in tumor and organs had cleared and only the positive tumor showed a strong signal. Following the last scan, tumors and organs were harvested and the %ID/g was calculated and shown in Fig. 3A. The average uptake in the CD20-positive tumors was 6.0(±2.0)%ID/g, and significantly higher than the uptake by control 38C13 (CD20-negative) tumors, 1.6(±0.6)%ID/g (P = 0.007) and blood 1.9(±0.4)%ID/g (P = 0.011). Liver, kidney, spleen and kidney were all below 2%ID/g and also significantly lower (P < 0.05). In these mice, the positive tumor to negative tumor ratio was 3.9(±0.7) and the positive tumor to blood ratio was 3.1(±1.2). The positive tumor to negative tumor ratios determined by ROIs were 1.2(±0.2) and 2.4(±0.8), whereas the positive tumor to soft tissue ratios were 4.3(±1.4) and 9.9(±4.6) at 4 and 21 hours, respectively (Table 1).
FIGURE 3.
Serial microPET images of mice bearing 38C13-huCD20 (+) and wild-type 38C13 (−) tumors at 4 and 21 h after administration of radioiodinated anti-CD20 scFv-Fc DM (A) and minibody (B). Both coronal (upper panels) and transverse (lower panels) slices are shown. PET/CT scan overlay images are shown for 21 h. The biodistribution of the radioiodinated proteins at the time of sacrifice (21 h) and the tumor to organ ratios are shown below. Abbreviations: ID = injected dose; SD = standard deviation.
Table 1.
Ratios derived from PET images
| 38C13-huCD20 to 38C13 |
38C13-huCD20 to soft tissue |
|||
|---|---|---|---|---|
| 4 h | 21 h | 4 h | 21 h | |
| scFv-Fc DM (n = 4) | 1.2 ± 0.2 | 2.4 ± 0.8* | 4.3 ± 1.4 | 9.9 ± 4.6* |
| Minibody (n =4) | 2.2 ± 0.8 | 4.0 ± 0.7* | 4.6 ± 0.4 | 17.0 ± 6.7* |
Significantly higher from 4 h (P< 0.05)
Data are mean ± S.D.
b) MicroPET Imaging using 124I-labeled Anti-CD20 Minibody
Tumor targeting of the anti-CD20 minibody was evaluated in mice bearing 38C13-huCD20 and 38C13 tumors averaging 59±32 mg (range: 34–106 mg) and 71±54 mg (range 10–134 mg), respectively. The mice were each injected with approximately 3.9 MBq of 124I-minibody (specific activity = 0.069 MBq/µg), and whole body scan were obtained at 4 and 21 hours after administration. Similar to the scFv-Fc DM, activity in both tumors and normal organs was seen at 4 hours, but at 21 hours persisted only in the CD20-positive tumor (Fig. 3B). At 21 hours, mice were sacrificed, tumors were excised, and the %ID/g was calculated (Fig. 2B). The average uptake in the CD20-positive tumors was 12.9(±3.4)%ID/g and the uptake by control 38C13 xenografts was significantly lower at 1.9(±0.5)%ID/g (P = 0.003). Also liver, kidney spleen and lung had activities (all were below 2%ID/g) that were significantly lower than that in the positive tumor (P < 0.05). The activity in the blood was 2.8(±0.5)%ID/g which was also significantly less than the activity in the positive tumor (P = 0.004). Here, the positive tumor to negative tumor ratio was 7.0(±3.1) and positive tumor to blood ratio was 4.7(±1.4). ROIs resulted in positive tumor to negative tumor ratios of 2.2(±0.8) at 4 hours and 4.0(±0.7) at 21 hours. The positive tumor to soft tissue ratios were 4.6(±0.4) at 4 hours, which increased more than 4-fold at 21 hours to 17.0(±6.7) (Table 1). Thus, both anti-CD20 antibody fragments offered cell surface target-specific imaging in vivo, with the minibody achieving higher contrast images.
In Vivo Characterization of 64Cu-DOTA-Minibody by MicroPET Imaging
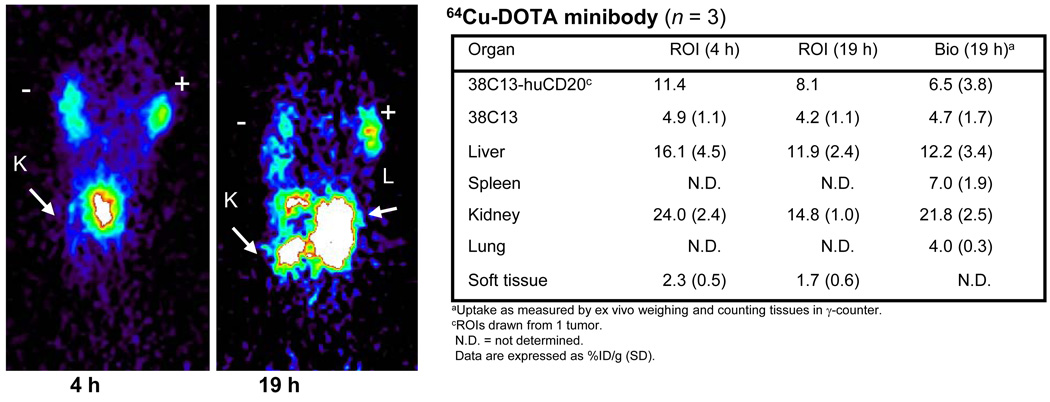
The minibody was also evaluated in the same tumor model following labeling with a residualizing radiometal in order to evaluate CD20-positive tumor targeting and normal organ uptake. The minibody was conjugated to DOTA, radiolabeled with 64Cu and evaluated in mice bearing 38C13-huCD20 (n = 2) and control 38C13 tumors (n = 3) averaging 26(±20) mg and 79(±38) mg in weight, respectively. The mice were each injected with approximately 3.7 MBq of 64Cu-DOTA rituximab minibody (specific activity = 0.042 MBq/µg). Whole body images were obtained at 4 and 19 hours, and after the last scan mice were sacrificed, organs harvested and counted in order to calculate %ID/g. The mean uptake in the 38C13-huCD20 tumor was 6.5(±3.8)%ID/g and the uptake by control 38C13 xenograft was 4.7(±1.7)%ID/g, resulting in a ratio of 1.4, which was 5 times lower than the ratio obtained with 124I-labeled minibody. The images obtained at 4 and 19 hours showed activity in both tumors (Fig. 4). The activities in the tumors, liver, and kidneys calculated from biodistribution and ROIs are shown in the Fig. 4 table. ROIs were drawn only on the largest, most clearly-imaged CD20-positive tumor in the mouse shown in Fig. 4. The uptakes in the positive tumor at 4 and 19 hours, determined by ROI analyses, were 11.4 and 8.1%ID/g, respectively, which corresponded to the activity measured at the time of sacrifice (9.1%ID/g at 19 h). ROIs of the negative tumors (n =3) resulted in 4.9 (±1.1) and 4.2(±1.1)%ID/g at 4 and 19 hours, respectively. This resulted in positive tumor to negative tumor ratios of 2.3 at 4 hours and 1.9 at 19 hours. Thus, compared to the 124I-labeled minibody, CD20-positive tumor-specific imaging was less favorable with the 64Cu-DOTA-minibody, owing to high background signal in the control tumor and normal organs.
FIGURE 4.
Coronal serial microPET images of a mouse bearing 38C13-huCD20 (+) and wild-type 38C13 (−) tumors at 4 and 19 h after administration of 64Cu-labeled anti-CD20 DOTA-minibody. K = kidney; L = Liver. Estimated activities from drawn regions of interest (ROIs) in 38C13, liver, spleen, kidney, lung and soft tissue at 4 and 19 h are shown below as well as activities present in the tissues at the time of sacrifice (19 h).
DISCUSSION
In this work, we sought to develop novel immunoPET imaging agents targeting the CD20 cell surface antigen expressed by most human lymphomas. We describe the generation of two antibody fragments of different sizes and compare their properties as imaging agents for CD20 expressing tumors. Although both fragments had low immunoreactivity (32–38%) following radioiodination, they were both able to target the tumors well in vivo. Since the low immunoreactivity could be attributed to modified tyrosines in the antigen binding region, site-specific radiolabeling may be a better labeling approach for these fragments. Still in this study, the minibody exhibited better (2-fold) tumor targeting than that of the scFv-Fc DM at 21 hours, producing excellent contrast images. This was unexpected as the iodinated anti-CEA scFv-Fc DM has been shown to have a slightly longer serum half-life (Tβ = 8.0) than the iodinated anti-CEA minibody (Tβ = 5.3–7.0 hours) (13, 14, 37). This discrepancy may be due to the Fc region in the fragments of the two systems, namely IgG1 and IgG4 for the anti-CEA and anti-CD20 fragments, respectively. We chose to use IgG4 Fc region in order to minimize interaction with other immune cells as IgG1, as opposed to IgG4, permits complement activation and strong effector mediated cell killing by antibody mediated cytotoxicity (38). In humans the mean serum half-life of IgG1 is 36.3 ± 9.2 days and 15.6 ± 4.5 days for IgG4 (39). This difference in serum half-lives was also observed in mice with a residence time of 199.0 ± 9.8 hours for IgG1 and 77.3 ± 10.1 hours for IgG4 (40). When the IgG4 CH2-CH3 domains were exchanged to IgG1 CH2-CH3 domains, the serum half-life increased to 281.5 ± 10.1 hours which interestingly was higher than either parental IgG. Mutations in the CH2-CH3 domains have identified residues important for high affinity binding to the rodent neonatal Fc receptor (FcRn) (24). The histidine residues at position 435 in CH3 domain was shown to have a dramatic effect on this interaction for human IgG1, whereas the H433 has little effect. However, for human IgG4, H433 has been implicated to be more important than H435 (41). The scFv-Fc DM used in this work contains two mutations in the Fc region, H310A in CH2 and H435A in CH3. The shorter serum half-life and the presence of these mutations in IgG4 Fc region may explain the lower activity observed in the blood at 21 hours relatively to that of the minibody. Since the minibody contains no mutation in the CH3 domain, it may be possible that the minibody has some interaction with the FcRn that enable it to stay in the blood slightly longer than the larger scFv-Fc DM fragment.
The fact that the minibody has a longer residence time in the blood also explains the much higher tumor uptake observed with this fragment at 21 hours. The radioactivity measured in the tumors with the minibody was about 2-fold higher than that of the scFv-Fc DM. Such difference is not observed with the anti-CEA minibody and scFv-Fc DM, with a tumor uptakes of 12.6, 15.7 and 29.1%ID/g reported for the iodinated minibody (12, 13, 37) and 18.6%ID/g for the iodinated scFv-Fc (42) at 24 hours. The anti-CD20 minibody exhibited other superior properties over the scFv-Fc DM, such as high CD20-positive tumor to negative tumor and soft tissue ratios. The minibody was also labeled with 64Cu and evaluated for imaging in tumor bearing mice. However, even at 19 hours, this agent showed inferior positive tumor to negative tumor uptake ratios, and persistent high-level uptake in normal kidney, liver, and bowel. Since the molecular weight of the minibody is above the threshold clearance of the kidneys, significant retention of activity over the tumor in the liver was not unexpected as this had been observed previously in other tumor models (Wu et al 2000; Olafsen et al 2005). However, this phenomenon could also possibly be due to an alteration of the biodistribution properties of the minibody by the DOTA chelator. Such alteration was observed for an anti-neuroblastoma F(ab’)2 fragment when it was chelated to two analogous copper chelators with different charge (43). Thus, for further development of CD20 immunoPET imaging, we favor the 124I-labeled minibody format.
The human CD20-specific PET imaging agents described here offer new opportunities to image B cell lymphomas in both preclinical animal models and humans. Our specific imaging of human CD20-positive lymphomas in mice demonstrates that 124I-labeled rituximab minibody fragments can serve as a tool for non-invasive in vivo monitoring of lymphoma growth and metastasis. This imaging technique should allow assessment of disease spread in mice without the high background levels of signal within the brain, heart, bowel, kidneys, and bladder seen with FDG-PET imaging of lymphoma in mice (44). In the immunocompetent model we describe here, anti-CD20 immunoPET imaging could be used to study response to therapies depending on an intact adaptive immune system, such as tumor antigen vaccines (45). We have recently transduced another murine B cell lymphoma (A20, BALB/c) with the human CD20 gene, for propagation in BALB/c human CD20 transgeneic mice (unpublished observations). In the clinic, anti-CD20 immunoPET imaging might be used to increase the sensitivity of PET imaging in cases of low tumor burden, or in cases of indolent lymphomas, in which the sensitivity of FDG-PET can be as low as 50% (9, 10).
CD20-specific imaging might also have use in imaging the B cell compartment in other experimental or pathologic states. With further refinements in sensitivity, anti-CD20 immunoPET might be used to image the B cell compartment in human CD20 transgenic mice, either during immune reconstitution after B lymphocyte-depleting therapies (46) or in autoimmune diseases where collections of autoimmune B cells contribute to pathogenesis (47). Such imaging might increase our understanding of B cell-mediated disease states.
In future studies, it will be important to determine if pre-treatment with rituximab interferes with subsequent anti-CD20 immunoPET imaging of lymphomas via blockade of rituximab binding sites on CD20. Studies in human CD20 transgenic mice will help clarify this issue. As rituximab levels fall after cessation of therapy, it is likely that anti-CD20 immunoPET reagents would be able to bind to an increasing number of available sites on the tumor cell surface. Indeed, CD20 immunoPET may be useful in determining if tumor CD20 sites are saturated after a given dose of, or at specific intervals following rituximab administration.
CONCLUSION
This work demonstrates rapid targeting to CD20-positive lymphoma in vivo by two engineered antibody fragments; minibody and scFv-Fc DM. Both fragments produced high-contrast, target-specific PET images in tumor-bearing mice at 21 hours. The best candidate was the minibody, that exhibited superior tumor uptake, and combined with rapid clearance gave higher positive tumor to negative tumor/tissue ratios. When the minibody was radiolabeled with 64Cu, lower ratios were obtained due to residual activity in the negative tumor and liver. Thus, 124I is the preferred radiolabel due to the lower background activity in normal tissues, enhancing the overall image.
ACKNOWLEDGMENTS
Grant support: This work was supported by NIH Grants P50 CA107399, P50 CA086306, CA119367, and the Margaret Early Medical Research Trust. The production of Copper-64 at Washington University School of Medicine was supported by the NCI grant R24 CA 86307.
AAR and AMW are members of the City of Hope Comprehensive Cancer Center (CA 33572). JMT and AMW are members of Jonsson Comprehensive Cancer Center (CA 16042). JMT is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-26-05).
The authors would like to thank Karl B. Bauer for excellent technical support. We are especially grateful to Dr. David Stout, Waldemar Ladno, and Judy Edwards at the Crump Institute for Molecular Imaging at UCLA for their assistance with the microPET/CT scans. Thanks are also extended to the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility at UCLA.
REFERENCES
- 1.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15(9):450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 2.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Current directions in autoimmunity. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 6.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104(9):2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 7.Witzig TE, White CA, Wiseman GA, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin's lymphoma. J Clin Oncol. 1999;17(12):3793–3803. doi: 10.1200/JCO.1999.17.12.3793. [DOI] [PubMed] [Google Scholar]
- 8.Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2000;18(6):1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 9.Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer. 2006;107(1):175–183. doi: 10.1002/cncr.21967. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto N, Kojima M, Hasegawa M, et al. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer. 2007;110(3):652–659. doi: 10.1002/cncr.22807. [DOI] [PubMed] [Google Scholar]
- 11.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer journal (Sudbury, Mass. 2008;14(3):191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 12.Wu AM, Williams LE, Zieran L, et al. Anti-carcinoembryonic antigen (CEA) diabody for rapid tumor targeting and imaging. Tumor Targeting. 1999;4:47–58. [Google Scholar]
- 13.Hu S, Shively L, Raubitschek A, et al. Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 1996;56(13):3055–3061. [PubMed] [Google Scholar]
- 14.Kenanova V, Olafsen T, Crow DM, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res. 2005;65(2):622–631. [PMC free article] [PubMed] [Google Scholar]
- 15.Olafsen T, Kenanova VE, Sundaresan G, et al. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005;65(13):5907–5916. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundaresan G, Yazaki PJ, Shively JE, et al. 124I-Labeled Engineered Anti-CEA Minibodies and Diabodies Allow High-Contrast, Antigen-Specific Small-Animal PET Imaging of Xenografts in Athymic Mice. J Nucl Med. 2003;44(12):1962–1969. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu AM, Yazaki PJ, Tsai S, et al. High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc Natl Acad Sci U S A. 2000;97(15):8495–8500. doi: 10.1073/pnas.150228297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyton JV, Olafsen T, Lepin EJ, et al. Humanized Radioiodinated Minibody for Imaging of Prostate Stem Cell Antigen-Expressing Tumors. Clinical Cancer Res. 2008;14(22):7488–7496. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissler F, Anderson SK, Venkatesan P, Press O. Intracellular catabolism of radiolabeled anti-mu antibodies by malignant B-cells. Cancer Res. 1992;52(10):2907–2915. [PubMed] [Google Scholar]
- 20.Xu FJ, Yu YH, Bae DS, et al. Radioiodinated antibody targeting of the HER-2/neu oncoprotein. Nucl Med Biol. 1997;24(5):451–459. doi: 10.1016/s0969-8051(97)00065-6. [DOI] [PubMed] [Google Scholar]
- 21.Buchsbaum D, Randall B, Hanna D, Chandler R, Loken M, Johnson E. Comparison of the distribution and binding of monoclonal antibodies labeled with 131-iodine or 111-indium. European journal of nuclear medicine. 1985;10(9–10):398–402. doi: 10.1007/BF00256578. [DOI] [PubMed] [Google Scholar]
- 22.Schott ME, Milenic DE, Yokota T, et al. Differential metabolic patterns of iodinated versus radiometal chelated anticarcinoma single-chain Fv molecules. Cancer Res. 1992;52(22):6413–6417. [PubMed] [Google Scholar]
- 23.Sharkey RM, Gold DV, Aninipot R, et al. Comparison of tumor targeting in nude mice by murine monoclonal antibodies directed against different human colorectal cancer antigens. Cancer Res. 1990;50(3 Suppl):828s–834s. [PubMed] [Google Scholar]
- 24.Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158(5):2211–2217. [PubMed] [Google Scholar]
- 25.Yazaki PJ, Sherman MA, Shively JE, et al. Humanization of the anti-CEA T84.66 antibody based on crystal structure data. Protein Eng Des Sel. 2004;17(5):481–489. doi: 10.1093/protein/gzh056. [DOI] [PubMed] [Google Scholar]
- 26.Whitlow M, Bell BA, Feng SL, et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993;6(8):989–995. doi: 10.1093/protein/6.8.989. [DOI] [PubMed] [Google Scholar]
- 27.Olafsen T, Kenanova VE, Wu AM. Tunable pharmacokinetics: modifying the in vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nature Protocols. 2006;1(4):2049–2060. doi: 10.1038/nprot.2006.322. [DOI] [PubMed] [Google Scholar]
- 28.Angal S, King DJ, Bodmer MW, et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30(1):105–108. doi: 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MR, Kao JY, Anderson AL, Shively JE, Raubitschek A. An improved method for conjugating monoclonal antibodies with N-hydroxysulfosuccinimidyl DOTA. Bioconjug Chem. 2001;12(2):320–324. doi: 10.1021/bc0000886. [DOI] [PubMed] [Google Scholar]
- 30.Robertson EF, Dannelly HK, Malloy PJ, Reeves HC. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- 31.Bergman Y, Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977;7(7):413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- 32.Golay J, Cittera E, Di Gaetano N, et al. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;91(2):176–183. [PubMed] [Google Scholar]
- 33.Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. IEEE Trans NS. 1989;36(1):964–968. [Google Scholar]
- 34.Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16(2):145–158. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- 35.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2(3):131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 36.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 37.Yazaki PJ, Wu AM, Tsai SW, et al. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12(2):220–228. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]
- 38.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert opinion on biological therapy. 2007;7(9):1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 39.Alyanakian MA, Bernatowska E, Scherrmann JM, Aucouturier P, Poplavsky JL. Pharmacokinetics of total immunoglobulin G and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency syndromes. Vox sanguinis. 2003;84(3):188–192. doi: 10.1046/j.1423-0410.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 40.Zuckier LS, Chang CJ, Scharff MD, Morrison SL. Chimeric human-mouse IgG antibodies with shuffled constant region exons demonstrate that multiple domains contribute to in vivo half-life. Cancer Res. 1998;58(17):3905–3908. [PubMed] [Google Scholar]
- 41.Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34(45):14649–14657. doi: 10.1021/bi00045a005. [DOI] [PubMed] [Google Scholar]
- 42.Kenanova V, Olafsen T, Williams LE, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res. 2007;67(2):718–726. doi: 10.1158/0008-5472.CAN-06-0454. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann K, Gianollini S, Schubiger PA, Novak-Hofer I. A triglycine linker improves tumor uptake and biodistributions of 67-Cu-labeled anti-neuroblastoma MAb chCE7 F(ab')2 fragments. Nucl Med Biol. 1999;26(8):943–950. doi: 10.1016/s0969-8051(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 44.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 45.Betting DJ, Kafi K, Abdollahi-Fard A, Hurvitz SA, Timmerman JM. Sulfhydryl-based tumor antigen-carrier protein conjugates stimulate superior antitumor immunity against B cell lymphomas. J Immunol. 2008;181(6):4131–4140. doi: 10.4049/jimmunol.181.6.4131. [DOI] [PubMed] [Google Scholar]
- 46.Gong Q, Ou Q, Ye S, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174(2):817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 47.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179(5):3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]