Abstract
Ischemic preconditioning and postconditioning distinctly attenuate ventricular arrhythmia after ischemia without affecting the severity of myocardial stunning. Therefore, we report the effects of sevoflurane preconditioning and postconditioning on stunned myocardium in isolated rat hearts. Isolated rat hearts were underwent 20 min of global ischemia and 40 min of reperfusion. After an equilibration period (20 min), the hearts in the preconditioning group were exposed to sevoflurane for 5 min and next washout for 5 min before ischemia. Hearts in the sevoflurane postconditioning group underwent equilibration and ischemia, followed immediately by sevoflurane exposure for the first 5 min of reperfusion. The control group received no treatment before and after ischemia. Left ventricular pressure, heart rate, coronary flow, electrocardiogram, and tissue histology were measured as variables of ventricular function and cellular injury, respectively. There was no significant difference in the duration of reperfusion ventricular arrhythmias between control and sevoflurane preconditioning group (P=0.195). The duration of reperfusion ventricular arrhythmias in the sevoflurane postconditioning group was significantly shorter than that in the other two groups (P<0.05). ±(dP/dt)max in the sevoflurane preconditioning group at 5, 10, 15, 20, and 30 min after reperfusion was significantly higher than that in the control group (P<0.05), and there were no significant differences at 40 min after reperfusion among the three groups (P>0.05). As expected, for a 20-min general ischemia, infarct size in heart slices determined by 2,3,5-triphenyltetrazolium chloride staining among the groups was not obvious. Sevoflurane postconditioning reduces reperfusion arrhythmias without affecting the severity of myocardial stunning. In contrast, sevoflurane preconditioning has no beneficial effects on reperfusion arrhythmias, but it is in favor of improving ventricular function and recovering myocardial stunning. Sevoflurane preconditioning and postconditioning may be useful for correcting the stunned myocardium.
Keywords: Inhalation anesthetics, Sevoflurane, Postconditioning, Preconditioning, Ischemia-reperfusion injury, Myocardial stunning
1. Introduction
Anesthetic preconditioning or postconditioning whereby the heart is exposed to a volatile anesthetic before or after prolonged ischemia, exerts a cardioprotective effect (Obal et al., 2005; Feng et al., 2008; Weber and Schlack, 2008), which has triggered an increasing interest in both basic science and clinical research and may ultimately cause an impact on anesthesia practice in patients of cardiac risk. Up to now, most studies have demonstrated similar efficacies on anesthetic postconditioning and ischemic postconditioning in protecting the myocardium (Wang et al., 2006; Tsutsumi et al., 2007) and on anesthetic preconditioning (Riess et al., 2002; Weber and Schlack, 2008) just against prolonged ischemia containing obviously measured infarct size.
Postischemia, a reversible contractile dysfunction termed as myocardial stunning, is a significant phenomenon after cardiac surgery. Transient myocardial ischemia followed by reperfusion may lead to myocardial stunning. As for the stunned myocardium independent of myocardial infarction, it has been reported that ischemic preconditioning (Hagar et al., 1991) and ischemic postconditioning (Kloner et al., 2006; Sasaki et al., 2007; Dow et al., 2008; 2009) markedly attenuate ventricular arrhythmia after ischemia and prevent the recurrence of arrhythmia, and may be useful for correcting the stunned myocardium. Furthermore, the antiarrhythmic protection conferred by ischemic postconditioning is also present in old rats (Dow et al., 2008). The mechanisms have not been studied extensively. One study showed that this protective effect of ischemic preconditioning was not likely to be related to alterations in high-energy phosphate compounds (Hagar et al., 1991). In addition, the mechanism by which ischemic postconditioning reduced reperfusion-induced ventricular arrhythmias in the stunned myocardium was independent of known pathways including adenosine, mitochondrial KATP channel, mitochondrial permeability transition pore, and PI3K pathways (Dow et al., 2009). However, the effects of anesthetic preconditioning or postconditioning on myocardial stunning remain unclear.
With these issues in mind, we investigated the effects of sevoflurane preconditioning and postconditioning on myocardial stunning in isolated rat hearts independent of myocardial infarction.
2. Materials and methods
Male Sprague-Dawley rats (200–250 g) were obtained from the Animal Center of Zhejiang University, Hangzhou, Zhejiang, China. Animals were handled in accordance with the principles of laboratory animal care and all experimental procedures were approved by the Research Commission for the Care and Use of Laboratory Animals of Zhejiang University.
2.1. Perfusion of isolated rat hearts
Rats were anaesthetized (pentobarbital sodium, 60 mg/kg, i.p.) and the hearts were excised rapidly, placed in ice-cold Krebs-Henseleit (K-H) buffer, mounted on a Langendorff apparatus, and perfused at 37 °C with K-H buffer (Stehr et al., 2007). Perfusion pressure was constant at 75 mmHg. The buffer containing (mmol/L) NaCl 118.0, KCl 4.7, CaCl2 1.25, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0, and glucose 11.0 was equilibrated with 95% O2/5% CO2 (pH 7.39±0.1). Hearts were subjected to global ischemia by stopping the K-H buffer perfusion, while reperfusion was achieved by restarting the perfusion.
Sevoflurane (Abbott Laboratories, Chuoku, Osaka, Japan) was bubbled into the perfusate using an agent specific vaporizer (Vapor 2000; Dräger Medizintechnik GmbH, Lübeck, Germany) placed in the O2-CO2 gas mixture line at a concentration of (1.2±0.02) mmol/L (8%, v/v) measured in the liquid phase by gas chromatography (Agilent Laboratories, Santa Clara, CA, USA). These concentrations, which are too high to maintain general anesthesia but may be used temporarily during mask induction (Epstein et al., 1998; Walpole and Logan, 1999), were chosen to induce sevoflurane preconditioning and postconditioning, as previously described (He et al., 2008; Yan et al., 2008; Zhang et al., 2009).
2.2. Experimental protocol
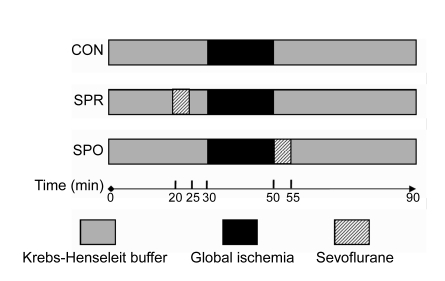
The rats were randomly divided into 3 groups (8 hearts each), 2 for treatment and 1 as control (Fig. 1). The timing of the preconditioning or postconditioning therapy was based on previous reports in necrosis models. Rat hearts in the treatment groups were exposed to sevoflurane. After a 20-min equilibration period, hearts in the sevoflurane preconditioning group (SPR) were exposed to sevoflurane for 5 min, followed by a 5-min washout period before a 20-min global ischemia and a 40-min reperfusion. Hearts in the sevoflurane postconditioning group (SPO) underwent equilibration, then 20 min of global ischemia, followed immediately by sevoflurane exposure in the first 5 min of the 40-min reperfusion period. The control group (CON) received no treatment before the 20-min global ischemia and during the 40 min-reperfusion period.
Fig. 1.
Experimental protocols used in this study
CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
2.3. Assessment of cardiac function
A fluid-filled latex balloon was introduced into the left ventricle through the left atrial appendage and the balloon catheter was linked to a pressure transducer connected to a data-acquisition system (RM6240, Chengdu, China) to measure contractile function. The left ventricular end diastolic pressure (LVEDP) was adjusted to between 4 and 8 mmHg. The cardiac parameters of heart rate (HR), left ventricular-developed pressure (LVDP) (difference between left ventricular end systolic pressure and end diastolic pressure), and maximal rise/fall rate of left ventricular pressure [±(dP/dt)max] were monitored continuously. Electrocardiograms were recorded with carbon leads directly attached to the perfused heart surface. Analysis of arrhythmia incidence was carried out in accordance with the Lambeth Conventions (Walker et al., 1988). Total coronary flow (CF) was measured by timed collection of perfusate dripping from the right heart into a graduated cylinder.
2.4. Tissue histology
At the end of the 40-min reperfusion, the left ventricle was sliced transversely from apex to base into 7 slices. Four hearts in each group were used to determine infarct size by 1% (w/v) 2,3,5-triphenyltetrazolium chloride (TTC) staining, as previously described (Riess et al., 2004; Deyhimy et al., 2007). Slices of the remaining hearts in each group were fixed with 10% (v/v) formalin, embedded in paraffin, and processed for histologic analysis. The slices for histologic analysis were stained with hematoxylin and eosin.
2.5. Statistical analysis
SPSS 10.0 was used for statistical analysis. Data were presented as mean±standard deviation (SD). Differences in functional data at each time point were evaluated using one-way analysis of variance (ANOVA) followed by post hoc analysis with Student-Newman-Keuls for multiple comparisons. The same analysis was used for detection of differences in duration of reperfusion arrhythmias. If variances are not homogeneous, nonparametric tests (Kruskal-Wallis H test) were used to compare the three groups by post hoc analysis with Mann-Whitney U test for multiple comparisons. A value of P<0.05 was considered significant.
3. Results
Twenty-eight rats were instrumented. Three rats were excluded because of severe hypotension during the baseline time (1 CON, 1 SPR, and 1 SPO) and one rat was excluded because of persistent ventricular fibrillation during reperfusion. There were eight successful rats in each group.
3.1. Duration of reperfusion arrhythmias
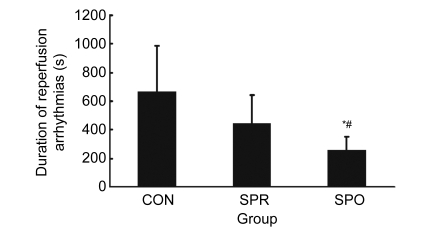
The incidence of reperfusion ventricular arrhythmia was 100% in all three groups. Results of duration of reperfusion arrhythmias are shown in Fig. 2. The variances of duration of reperfusion arrhythmias were not homogeneous, so nonparametric tests were used to compare two or three groups. There was no significant difference in the duration of reperfusion ventricular arrhythmias between the CON group and SPR group (P=0.195). The duration of reperfusion ventricular arrhythmias in the SPO group was markedly less than that in other two groups (SPO vs. CON, P=0.010; SPO vs. SPR, P=0.028). In addition, no ventricular arrhythmia was observed after administration of sevoflurane in the SPR group.
Fig. 2.
Duration of reperfusion arrhythmias
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
3.2. Hemodynamic function
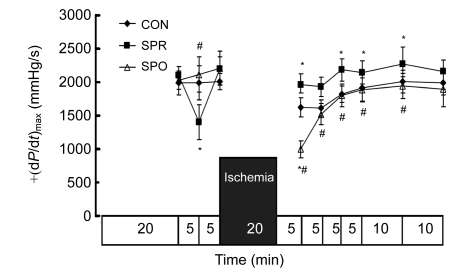
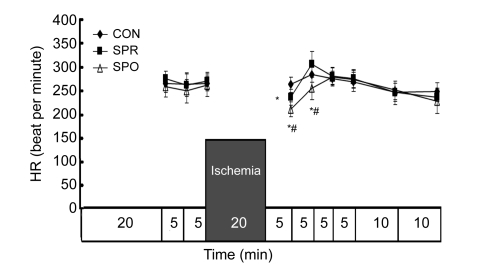
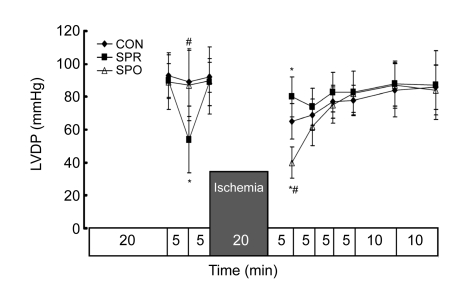
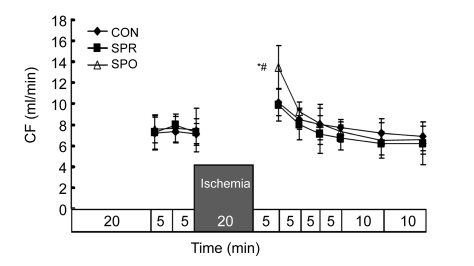
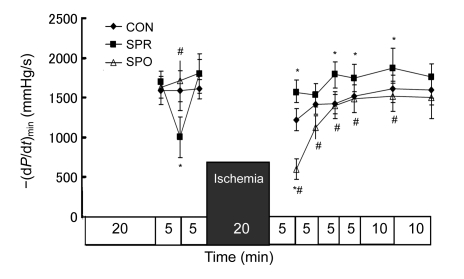
Systemic hemodynamics during baseline was not significantly different among groups (Figs. 3–7). ±dP/dt and LVDP after a 5-min administration of sevoflurane in the SPR were significantly lower than those in the CON and SPO groups, which were recovered 5 min later, i.e., at the moment before the global ischemia (Figs. 3–5). After 20 min of global ischemia, sevoflurane postconditioning, in which hearts were exposed to sevoflurane for 5 min at the onset of reperfusion, significantly inhibited functional recovery when compared with the CON and SPR groups (P<0.05, Figs. 3–5 and 7), and increased the CF at 5 min after reperfusion (Fig. 6). As shown in Figs. 3 and 4, ±dP/dt in the SPR group at 5, 10, 15, 20, and 30 min after reperfusion was significantly higher than that in the CON group (P<0.05). There were no significant differences in ±dP/dt at 40 min after reperfusion between SPR and CON groups [(2164±165) mmHg/s vs. (1993±183) mmHg/s, P>0.05]. ±dP/dt, LVDP, CF, and HR were similar among the three groups at the end of the experiment (P>0.05, Figs. 3–7).
Fig. 3.
Maximal rise/fall rate of left ventricular pressure [+(dP/dt)max]
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
Fig. 7.
Heart rate (HR)
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
Fig. 5.
Left ventricular developed pressure (LVDP)
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
Fig. 6.
Coronary flow (CF)
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
Fig. 4.
Minimal rise/fall rate of left ventricular pressure [−(dP/dt)min]
Data are mean±SD, n=8 in each groups; * P<0.05 vs. CON; # P<0.05 vs. SPR. CON: control group; SPR: sevoflurane preconditioning group; SPO: sevoflurane postconditioning group
3.3. Infarct size and histologic analysis
As expected, for a 20-min general ischemia, infarct size in heart slices by TTC staining among the groups was not obvious. Histologic analysis revealed intact architecture of the cardiomyocytes. Specifically, contraction band necrosis or sarcolemmal blebs were not observed. Occasionally, cells showed mild edema and waviness.
4. Discussion
In this study, we investigated the effects of sevoflurane preconditioning and postconditioning on myocardial stunning using isolated rat hearts as the ischemia reperfusion model. We found that sevoflurane postconditioning reduces reperfusion arrhythmias without affecting the severity of myocardial stunning. In contrast, sevoflurane preconditioning has no beneficial effects on reperfusion arrhythmias, but it is in favor of improving ventricular function and recovering myocardial stunning.
Myocardial stunning, whose diagnosis is based on experimental findings, is defined as a reversible contractile dysfunction during reperfusion after brief ischemia, and it happens in absence of necrosis (Braunwald and Kloner, 1982). In isolated perfused heart preparations, myocardial stunning can be generated after 20–25 min of global no-flow ischemia followed by reperfusion (Palmer et al., 2004; Chow et al., 2007; Tiwari et al., 2008). If the period of ischemia extends to 30 min, irreversible functional impairment occurs as cells undergo necrosis, i.e., infarction (Hansen, 1995; Kloner et al., 1998; Bolli and Marban, 1999; Park and Lucchesi, 1999; Palmer et al., 2004; Grund et al., 2006; Chow et al., 2007). All hearts in the experiments underwent 20 min of ischemia and 40 min of reperfusion, and absence of infarction was confirmed by TTC staining. Therefore, the experimental model used can also be considered as an experimental model of the stunned myocardium. There are, to our knowledge, no published data examining the effects of sevoflurane preconditioning and postconditioning on the stunned myocardium.
Arrhythmias developed by reperfusion after ischemia are also a type of ischemia reperfusion injury. The mechanisms of reperfusion arrhythmias are complex and are incompletely understood. Reperfusion arrhythmias occur due to heterogeneous recovery of conduction and the refractory period caused by incomplete reperfusion, reentry, abnormal automaticities, and activities triggered by Ca2+ overload (Dennis et al., 1990). Another common reperfusion arrhythmia mechanism is formation of free radicals and accentuation of preexisting heterogeneity of refractoriness occurring during reperfusion (Wit and Janse, 2001).
This study demonstrates that sevoflurane postconditioning markedly reduces duration of reperfusion arrhythmias. The degree of reduction of arrhythmia by sevoflurane postconditioning has been little investigated, but in the present study it was found quite striking. As for ischemic postconditioning, some studies (Kloner et al., 2006; Sasaki et al., 2007; Dow et al., 2008; 2009) reported that ischemia postconditioning markedly attenuates ventricular arrhythmias after ischemia-reperfusion in the necrosis-free model. Ischemic postconditioning’s benefit on reperfusion ventricular arrhythmias was also maintained in the senescent heart (Dow et al., 2008). In addition, the mechanism by which ischemic postconditioning-reduced reperfusion induced ventricular arrhythmias was independent of known pathways including adenosine, mitochondrial KATP channel, mitochondrial permeability transition pore, and PI3K pathways (Dow et al., 2009). The proposed mechanism for anesthetic postconditioning’s cardioprotection includes many of the same pathways proposed for postconditioning’s benefits, including adenosine (Weihrauch et al., 2005), opening of the mitochondrial KATP channel (Obal et al., 2005), nitric oxide (Krolikowski et al., 2006), closing of the mitochondrial permeability transition pore (He et al., 2008), and intracellular survival pathways such as ERK1/2 and PI3K/Akt (Chiari et al., 2005; Feng et al., 2006; Krolikowski et al., 2006). In addition, administration of sevoflurane may make the myocardium more electrically homogenous and, therefore, affect such a mechanism of arrhythmia as reentrant circuits. However, exact mechanisms underlying the described effect remain to be investigated, especially in the stunned myocardium. The mechanism of anesthetic postconditioning’s ability to reduce arrhythmias was not the focus of the present study; further studies are needed in the future.
On the other hand, sevoflurane preconditioning performed until 5 min before the 20-min ischemia did not affect the duration of reperfusion arrhythmias in this study. As for ischemia preconditioning, Hagar et al. (1991) reported that ischemic preconditioning of hearts could reduce the incidence of reperfusion arrhythmias, but another study (Sasaki et al., 2007) suggested that ischemic preconditioning had no effects on reperfusion arrhythmias. The effect of preconditioning on reperfusion arrhythmias has not been studied extensively. No matter what the effect of sevoflurane preconditioning on arrhythmias is like, sevoflurane preconditioning improved ventricular function in the early reperfusion 20 min after ischemia, which may be also useful for correcting the stunned myocardium. Although the pathogenesis of myocardial stunning has not been definitively established, the two major hypotheses can be that it is caused by the generation of oxygen-derived free radicals (oxyradical hypothesis) or that by a transient calcium overload (calcium hypothesis) on reperfusion (Bolli and Marban, 1999). In the necrosis model, sevoflurane preconditioning could reduce formation of oxygen-derived free radicals during reperfusion (Novalija et al., 2002; 2003; Kevin et al., 2003) and enhance Ca2+ handling and mechanical and metabolic functions elicited by Na+-Ca2+ exchange inhibition (An et al., 2006; Bouwman et al., 2006). However, there are no published data examining these events in the stunned myocardium.
Limitation of the present study is that only one dose and single time points of administration of sevoflurane preconditioning or postconditioning were used. In addition, we have not tested the potential mechanisms, which, indeed, could be an important approach to further explore. The implementation of the isolated rat heart system of ischemia-reperfusion injury has been considered necessary for the intrada for future in vivo studies. However, the ex vivo system of ischemia-reperfusion injury has several significant limitations such as its dependence on a physiological (non-blood based) buffer for perfusion purposes.
In summary, we were able to characterize an ischemia reperfusion-induced myocardial stunning in a rat model. Sevoflurane postconditioning reduces reperfusion arrhythmias without affecting the severity of myocardial stunning. In contrast, sevoflurane preconditioning has no beneficial effects on reperfusion arrhythmias, but it is in favor of improving ventricular function and recovering myocardial stunning. Therefore, this study helps broaden the understanding of effects of sevoflurane preconditioning and postconditioning on the stunned myocardium. Sevoflurane preconditioning and postconditioning may be useful for correcting the stunned myocardium.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30772090), the Natural Science Foundation of Zhejiang Province (No. Y204141), the Foundation from Science and Technology Department of Zhejiang Province (No. 2007R10034), and the Foundation from the Health Bureau of Zhejiang Province (No. 2007QN007), China
References
- 1.An J, Rhodes SS, Jiang MT, Bosnjak ZJ, Tian M, Stowe DF. Anesthetic preconditioning enhances Ca2+ handling and mechanical and metabolic function elicited by Na+-Ca2+ exchange inhibition in isolated hearts. Anesthesiology. 2006;105(3):541–549. doi: 10.1097/00000542-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79(2):609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 3.Bouwman RA, Salic K, Padding FG, Eringa EC, van Beek-Harmsen BJ, Matsuda T, Baba A, Musters RJ, Paulus WJ, de Lange JJ, et al. Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation. 2006;114(1Suppl.):I226–I232. doi: 10.1161/CIRCULATIONAHA.105.000570. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 5.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102(1):102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152(2):189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis SC, Coetzee WA, Cragoe EJ, Opie LH. Effects of proton buffering and of amiloride derivatives on reperfusion arrhythmias in isolated rat hearts. Possible evidence for an arrhythmogenic role of Na+-H+ exchange. Circ Res. 1990;66(4):1156–1159. doi: 10.1161/01.res.66.4.1156. [DOI] [PubMed] [Google Scholar]
- 8.Deyhimy DI, Fleming NW, Brodkin IG, Liu H. Anesthetic preconditioning combined with postconditioning offers no additional benefit over preconditioning or postconditioning alone. Anesth Analg. 2007;105(2):316–324. doi: 10.1213/01.ane.0000267524.71445.e7. [DOI] [PubMed] [Google Scholar]
- 9.Dow J, Bhandari A, Kloner RA. Ischemic postconditioning’s benefit on reperfusion ventricular arrhythmias is maintained in the senescent heart. J Cardiovasc Pharmacol Ther. 2008;13(2):141–148. doi: 10.1177/1074248408317705. [DOI] [PubMed] [Google Scholar]
- 10.Dow J, Bhandari A, Kloner RA. The mechanism by which ischemic postconditioning reduces reperfusion arrhythmias in rats remains elusive. J Cardiovasc Pharmacol Ther. 2009;14(2):99–103. doi: 10.1177/1074248408329606. [DOI] [PubMed] [Google Scholar]
- 11.Epstein RH, Stein AL, Marr AT, Lessin JB. High concentration versus incremental induction of anesthesia with sevoflurane in children: a comparison of induction times, vital signs, and complications. J Clin Anesth. 1998;10(1):41–45. doi: 10.1016/S0952-8180(97)00218-3. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Fischer G, Lucchinetti E, Zhu M, Bestmann L, Jegger D, Arras M, Pasch T, Perriard JC, Schaub MC, et al. Infarct-remodeled myocardium is receptive to protection by isoflurane postconditioning: role of protein kinase B/Akt signaling. Anesthesiology. 2006;104(5):1004–1014. doi: 10.1097/00000542-200605000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res. 2008;80(1):20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- 14.Grund F, Treiman C, Ilebekk A. How to discriminate between hibernating and stunned myocardium. J Cardiovasc Surg (Torino) 2006;47(3):323–328. [PubMed] [Google Scholar]
- 15.Hagar JM, Hale SL, Kloner RA. Effect of preconditioning ischemia on reperfusion arrhythmias after coronary artery occlusion and reperfusion in the rat. Circ Res. 1991;68(1):61–68. doi: 10.1161/01.res.68.1.61. [DOI] [PubMed] [Google Scholar]
- 16.Hansen PR. Myocardial reperfusion injury: experimental evidence and clinical relevance. Eur Heart J. 1995;16(6):734–740. doi: 10.1093/oxfordjournals.eurheartj.a060991. [DOI] [PubMed] [Google Scholar]
- 17.He W, Zhang FJ, Wang SP, Chen G, Chen CC, Yan M. Postconditioning of sevoflurane and propofol is associated with mitochondrial permeability transition pore. J Zhejiang Univ-Sci B. 2008;9(2):100–108. doi: 10.1631/jzus.B0710586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96(4):949–955. doi: 10.1213/01.ANE.0000052515.25465.35. [DOI] [PubMed] [Google Scholar]
- 19.Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998;97(18):1848–1867. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- 20.Kloner RA, Dow J, Bhandari A. Postconditioning markedly attenuates ventricular arrhythmias after ischemia-reperfusion. J Cardiovasc Pharmacol Ther. 2006;11(1):55–63. doi: 10.1177/107424840601100105. [DOI] [PubMed] [Google Scholar]
- 21.Krolikowski JG, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Role of Erk1/2, p70s6K, and eNOS in isoflurane-induced cardioprotection during early reperfusion in vivo [Le rôle des Erk1/2, p70s6K et eNOS dans la cardioprotection induite par l'isoflurane pendant la reperfusion in vivo précoce] Can J Anesth. 2006;53(2):174–182. doi: 10.1007/BF03021824. [DOI] [PubMed] [Google Scholar]
- 22.Novalija E, Varadarajan SG, Camara AK, An J, Chen Q, Riess ML, Hogg N, Stowe DF. Anesthetic preconditioning: triggering role of reactive oxygen and nitrogen species in isolated hearts. Am J Physiol Heart Circ Physiol. 2002;283(1):H44–H52. doi: 10.1152/ajpheart.01056.2001. [DOI] [PubMed] [Google Scholar]
- 23.Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98(5):1155–1163. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101(5):1252–1260. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- 25.Palmer BS, Hadziahmetovic M, Veci T, Angelos MG. Global ischemic duration and reperfusion function in the isolated perfused rat heart. Resuscitation. 2004;62(1):97–106. doi: 10.1016/j.resuscitation.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68(5):1905–1912. doi: 10.1016/S0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 27.Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283(1):H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 28.Riess ML, Kevin LG, Camara AK, Heisner JS, Stowe DF. Dual exposure to sevoflurane improves anesthetic preconditioning in intact hearts. Anesthesiology. 2004;100(3):569–574. doi: 10.1097/00000542-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki H, Shimizu M, Ogawa K, Okazaki F, Taniguchi M, Taniguchi I, Mochizuki S. Brief ischemia-reperfusion performed after prolonged ischemia (ischemic postconditioning) can terminate reperfusion arrhythmias with no reduction of cardiac function in rats. Int Heart J. 2007;48(2):205–213. doi: 10.1536/ihj.48.205. [DOI] [PubMed] [Google Scholar]
- 30.Stehr SN, Pexa A, Hannack S, Heintz A, Heller AR, Deussen A, Koch T, Hubler M. Insulin effects on myocardial function and bioenergetics in L-bupivacaine toxicity in the isolated rat heart. Eur J Anaesthesiol. 2007;24(4):340–346. doi: 10.1017/S0265021506002109. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari M, Hemalatha T, Ganesan K, Nayeem M, Manohar BM, Balachandran C, Vairamuthu S, Subramaniam S, Puvanakrishnan R. Myocardial ischemia and reperfusion injury in rats: lysosomal hydrolases and matrix metalloproteinases mediated cellular damage. Mol Cell Biochem. 2008;312(1-2):81–91. doi: 10.1007/s11010-008-9723-7. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci. 2007;81(15):1223–1227. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22(7):447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 34.Walpole R, Logan M. Effect of sevoflurane concentration on inhalation induction of anaesthesia in the elderly. Br J Anaesth. 1999;82(1):20–24. doi: 10.1093/bja/82.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Neff DA, Krolikowski JG, Weihrauch D, Bienengraeber M, Warltier DC, Kersten JR, Pagel PS. The influence of B-cell lymphoma 2 protein, an antiapoptotic regulator of mitochondrial permeability transition, on isoflurane-induced and ischemic postconditioning in rabbits. Anesth Analg. 2006;102(5):1355–1360. doi: 10.1213/01.ane.0000202463.28618.64. [DOI] [PubMed] [Google Scholar]
- 36.Weber NC, Schlack W. Handbook of Experimental Pharmacology. Modern Anesthetics. Vol. 182. Springer Berlin Heidelberg; 2008. Inhalational Anaesthetics and Cardioprotection; pp. 187–207. [DOI] [PubMed] [Google Scholar]
- 37.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101(4):942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 38.Wit AL, Janse MJ. Reperfusion arrhythmias and sudden cardiac death: a century of progress toward an understanding of the mechanisms. Circ Res. 2001;89(9):741–743. [PubMed] [Google Scholar]
- 39.Yan M, Chen C, Zhang F, Chen G. Lidocaine abolishes the myocardial protective effect of sevoflurane post-conditioning. Acta Anaesthesiol Scand. 2008;52(1):111–116. doi: 10.1111/j.1399-6576.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang FJ, Chen G, Chen C, Yan M. Sevoflurane postconditioning converts persistent ventricular fibrillation into regular rhythm. Eur J Anaesthesiol. 2009;26(9):766–771. doi: 10.1097/EJA.0b013e32832a58fa. [DOI] [PubMed] [Google Scholar]