Abstract
1-deoxynojirimycin (1-DNJ) contents in the silkworm, Bombyx mori, at different developmental stages and tissues were investigated by using reverse-phase high-performance liquid chromatography. The 1-DNJ contents of silkworm larvae change significantly with their developmental stages. The male larvae showed higher accumulation efficiency of 1-DNJ than the females and also a significant variation was observed among the silkworm strains. The present results show that tissue distribution of 1-DNJ was significantly higher in blood, digestive juice, and alimentary canal, but no 1-DNJ was observed in the silkgland. Moreover, 1-DNJ was not found in silkworms fed with artificial diet that does not contain mulberry leaf powder. This proves that silkworms obtain 1-DNJ from mulberry leaves; they could not synthesize 1-DNJ by themselves. The accumulation and excretion of 1-DNJ change periodically during the larval stage. There was no 1-DNJ in the newly-hatched larvae and 1-DNJ was mainly accumulated during the early and middle stages of every instar, while excreted at later stages of larval development. Further, it is possible to extract 1-DNJ from the larval feces and it is optimal to develop the 1-DNJ related products for diabetic auxiliary therapy.
Keywords: Silkworm, 1-deoxynojirimycin (1-DNJ), Accumulation, Bombyx mori
1. Introduction
Nojirimycin was firstly discovered from streptomycete. 1-deoxynojirimycin (1-DNJ) is the product of nojirimycin through hydrogenation. The natural DNJ was firstly isolated from mulberry tree (Yoshikaki and Hivonu, 1976), and up to now more than 20 of polyhydroxy alkaloids were identified from mulberry and silkworm (Asano et al., 1994a; 1994b; 2001). As a piperidine alkaloid, DNJ is known as a highly effective α-glycosidase inhibitor (Yoshikuni, 1988; Yoshikuni et al., 1988; Hughes and Rudge, 1994), and is one of the effective chemicals for anti-hyperglycemia. Presently DNJ and its analogs have been abstracted from a wide range of plants and microbes (Asano et al., 1998; 2000; Kim et al., 1999), but the DNJ contents in mulberry tree are the highest compared with other plants (Kimura et al., 2007; Yatsunami et al., 2008).
Mulberry has been utilized as Chinese medicine against diabetes mellitus for long time. Ryu et al. (1997) firstly reported that the silkworm larval powder of the 5th instar (prepared by lyophilization) had a good effect on diabetic patients (Ryu et al., 1997; 1999), and the effect for lowering the blood sugar level was further proved by subsequent research (Han et al., 2007). Recently in Korea, Japan, and China these related products made of mulberry and silkworm larvae are popular for auxiliary therapy on diabetes mellitus.
The silkworm powder has higher DNJ content than mulberry, indicating that the larvae can accumulate and enrich DNJ from mulberry leaves (Kim et al., 2003), and thereby the larval powder displays more effective results on diabetic. However, it is unknown whether the silkworm itself synthesizes DNJ, and little is known about assimilation, enrichment, and excretion of DNJ in silkworm, as well as the factors affecting the DNJ accumulation. Our study provides a basis for the DNJ enrichment and excretion in the silkworm, which may play an active role for preparing DNJ-related products.
2. Materials and methods
2.1. Materials
Silkworm larvae were reared by feeding fresh mulberry leaves or artificial diet at 25 °C. Artificial diets were prepared (Table 1), and six types of diets containing various percentages of mulberry leaf powder were used. The dry diets were mixed with two-fold water and autoclaved.
Table 1.
Ingredients of artificial diets with different contents of mulberry leaf powder
| Group | Mulberry leaf powder (%) | Bean powder (%) | Maize powder (%) | Citric acid (%) | Gallic acid (%) | Sorbic acid (%) | Vitamin C (%) | VB mixture (%) a | Inorganic salt mixture (%) b |
| 1 | 0 | 20.0 | 74.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
| 2 | 10.0 | 20.0 | 64.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
| 3 | 20.0 | 20.0 | 54.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
| 4 | 30.0 | 20.0 | 44.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
| 5 | 40.0 | 20.0 | 34.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
| 6 | 50.0 | 20.0 | 24.4 | 2.5 | 0.5 | 0.3 | 1.0 | 0.3 | 1.0 |
VB mixture consists of cyclohexanhexol, becholine, calcium pantothenate, niacin, benadon, riboflavin, aneurin hydrochloride, bioepiderm, and acidum folicum
Inorganic salt mixture contains K2HPO4·3H2O, CaCO3, KCl, MgSO4·7H2O, and FeSO4·7H2O
2.2. DNJ measurement
For DNJ analysis, the silkworm larvae were collected after starvation for 24 h, and the samples were lyophilized, smashed, and passed through a 100-mesh screen. The artificial diets and mulberry leaves were dried under 60 °C, grounded, and passed through a 100-mesh screen.
DNJ content was measured according to the method reported by Kim et al. (2003). DNJ in the silkworm larva or mulberry leaf powder was extracted with 0.05 mol/L HCl, treated with 9-fluorenylmethyl (FMOC) in pH 8.5 borate buffer solution to produce DNJ-FMOC complex compound, and finally detected by ultraviolet-reverse-phase high-performance liquid chromatography (UV-RP-HPLC).
Preparation of DNJ crude extract: About 30 mg of larva or mulberry leaf powder was mixed with 1 ml of 0.05 mol/L HCl, followed by vortexing for 1 min, and then treated with ultrasonic for 40 min. After centrifugation at 12 000 r/min for 30 min, the supernatant was collected. The pellet was treated again by repeating the above steps, and then supernatants were combined and diluted to 2 ml by adding distilled water. Thus obtained samples were used as crude extract.
DNJ derivation: The crude extract (35 μl of larval powder crude extract and 140 μl of mulberry leaf powder crude extract) was mixed with 169 μl of 0.4 mol/L potassium borate buffer (pH 8.5) in a 1.5-ml microtube. 20 μl of 5 mmol/L 9-fluorenylmethyl chloroformate (FMOC-Cl) dissolved in 50% (v/v) CH3CN was added, and mixed thoroughly. The mixture was placed in 20 °C water bath for 20 min, and subsequently 25 μl of 1 mol/L glycine was added to quench the remaining FMOC-Cl. Finally, 66 μl of 0.1% (v/v) acetic acid was added to stabilize the DNJ-FMOC formed in the reaction, and distilled water was added to final volume 707 μl. The sample was ready for DNJ measurement by filtering through a 0.2-μm nylon syringe filter.
DNJ determination: RT-HPLC was applied for DNJ content determination. Dionex 500E HPLC was procured from Dionex Co. (USA). DNJ-HCl was obtained from Sigma Chemicals (USA), and FMOC-Cl was from Fluca. HPLC system consisted of a VENUS-C18-5u column (250 mm×4.60 mm i.d.), a UVD170U detector (excitation 254 nm), and a Dionex Chromeleon working station as data processing software. The column was eluted with a mobile phase of acetonitrile: 0.1% (v/v) aqueous acetic acid (11:16, v/v) at 1.0 ml/min. 20 μl of sample was added to the column and the concentration was calculated by using external standards.
2.3. Data analysis
All the data were treated by statistical analysis applying to SAS 9.0 DA software. The absorption and enrichment of DNJ in silkworm larvae were calculated by the following formulas: R a (%)=M 1/M 2×100, where R a is the absorption ratio of DNJ during 1st to 4th instar or every day of the 5th instar, M 1 is the increased DNJ amount in body at every instar or every day of the 5th instar, and M 2 is the DNJ amount ingested at that instar or that day of the 5th instar; R e (%)=M 3/M 4×100, where R e is the accumulated enrichment ratio of DNJ by larvae, M 3 is the DNJ amount in larvae, and M 4 is the accumulated DNJ amount ingested.
3. Results
3.1. Relationship between DNJ contents in diets and in larvae
The silkworm larvae were fed with artificial diets varying with different contents of mulberry leaf powder or fresh mulberry leaves. The DNJ contents of artificial diets and the larvae of the 3rd day in the 5th instar were measured, and are presented in Table 2. The chromatograms of silkworm DNJ are shown in Fig. 1. The results show that the DNJ content in the artificial diets depended on the content of mulberry leaf powder. No DNJ could be detected in the diet without mulberry powder, implying that mulberry powder was the sole source for DNJ. In the larvae, the DNJ content increased with the amount of DNJ in the diets. Statistical analysis demonstrated that there was a closely positive and linear relationship between the DNJ content in the diets (X) and the one in the larval body (Y) when euryphagous or general silkworm race was tested: Y=7.2111X+0.0232, R 2=0.9154 (Group 1, a euryphagous silkworm Guangshi); Y=5.2633X+0.0129, R 2=0.9526 (Group 2, general hybrid silkworm Guangshi×Fengyi). No DNJ was detected in the larvae raised with artificial diets without mulberry leaf powder, indicating that the larva could not synthesize DNJ by itself.
Table 2.
Effects of mulberry leaf powder on DNJ contents in artificial diets and silkworm
| Mulberry leaf powder content in artificial diets (%) | DNJ content in artificial diets (%) | DNJ content in euryphagous silkworm (%) a | DNJ content in general hybrid silkworm (%) b |
| 0 | 0 | 0 | 0 |
| 10 | 0.0148±0.0005 | 0.1862±0.0022 | 0.1244±0.0060 |
| 20 | 0.0333±0.0004 | 0.2062±0.0128 | 0.1670±0.0049 |
| 30 | 0.0363±0.0004 | 0.2984±0.0203 | 0.1906±0.0019 |
| 40 | 0.0439±0.0006 | 0.3228±0.0006 | 0.2380±0.0075 |
| 50 | 0.0482±0.0010 | 0.3982±0.0257 | 0.2863±0.0033 |
| Fresh leaves | 0.0918±0.0028 | 0.5706±0.0142 | 0.4608±0.0001 |
A euryphagous silkworm race, Guangshi, was used
General hybrid silkworm race was used, commercial name: Guangshi×Fengyi
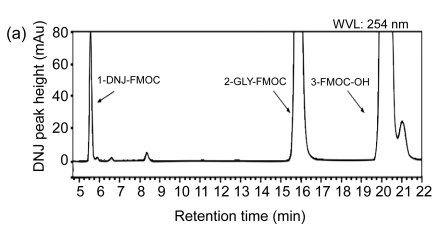
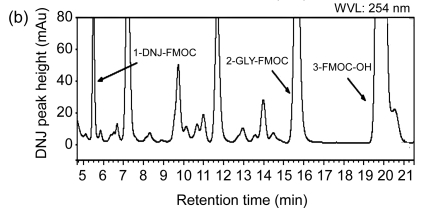
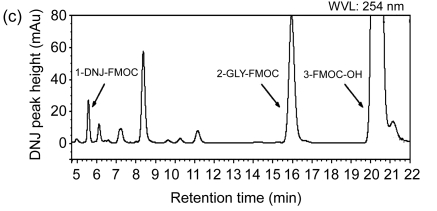
Fig. 1.
High-performance liquid chromatograms of DNJ analysis
(a) Chromatogram of separation of DNJ standard solution; (b) Chromatogram of separation of mulberry powder solution; (c) Chromatogram of separation of silkworm powder solution. 1-GLY-FMOC: 1-glycine-fluorenyl-methyl chl-oroformate
3.2. Variation of DNJ accumulation between male and female silkworm larvae
The results show that the DNJ content in male larvae was relatively higher than that in the females. There was a significant increase in DNJ content in both sexes when the larvae were fed with Nongsang 14 over Dajiguan and Husang 32 mulberry varieties (Table 3). The present results show that the male larvae could absorb and accumulate DNJ from diets more efficiently than females.
Table 3.
Variation of DNJ contents between the female and male larvae *
| Morus species | DNJ content in mulberry leaf powder (%) | DNJ content in larval powder (%) |
|
| Female | Male | ||
| Nongsang 14 | 0.121±0.025 | 0.371±0.007 | 0.465±0.018 |
| Dajiguan | 0.116±0.010 | 0.286±0.032 | 0.341±0.006 |
| Husang 32 | 0.107±0.060 | 0.257±0.010 | 0.316±0.036 |
Silkworm variety: Chunlei×Zhenzhu
The larvae of 3rd day in 5th instar were tested
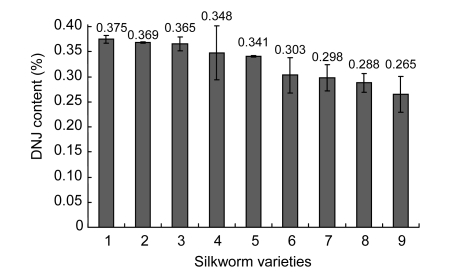
3.3. DNJ accumulation among silkworm varieties
The DNJ contents among various silkworm varieties were investigated (Fig. 2). The results show that there was a significant difference in DNJ content among various silkworm varieties. Among the races, the larvae of Jingsong showed a relatively high content of DNJ (0.375%), and a lower one was observed in hybrid silkworm 973×974.
Fig. 2.
DNJ contents among silkworm varieties
Silkworm varieties: 1, Jingsong; 2, Chunlei×Zhenzhu; 3, Luqi; 4, Haoyue; 5, 9202×Luqi; 6, 9202; 7, Luhuang; 8, Jingsong×Haoyue; 9, 973×974. DNJ was measured using the larvae of the 3rd day in the 5th instar. Each value is the mean±SE of replications
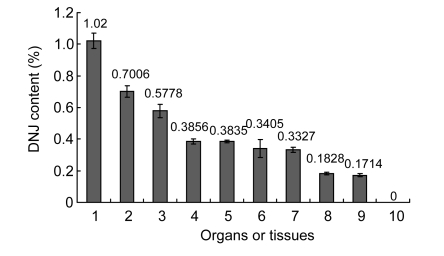
3.4. Distribution of DNJ in larval organs or tissues
As silkworm variety Zhg×Chun 54 is the most popular and a commercial variety in production, this variety has better characteristics, such as a big body and being easy to raise for a large scale. Considering a medical use in the future, we chose this variety for the current study, except specially indicated. The distribution of DNJ in the larval body was investigated, and the results (Fig. 3) show that, except the silk gland, all organs and tissues contained DNJ. Among the tissues, there was a significant increase in DNJ content in blood, followed by intestinal juice and alimentary canal.
Fig. 3.
DNJ distribution in larval organs and tissues
DNJ was measured using the larvae of the 3rd day in the 5th instar. Larval organs or tissues: 1, blood; 2, intestinal juice; 3, alimentary canal; 4, Malpighian tubule; 5, midgut peritrophic membrane; 6, cuticles; 7, body wall; 8, fat body; 9, trachea; 10, silk gland
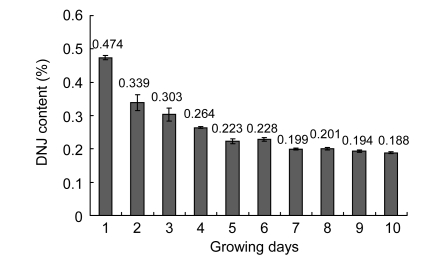
3.5. DNJ content in mulberry leaf during growing stage
The DNJ contents in mulberry leaves of various growing stages were analyzed and are present in Fig. 4. The DNJ content was relatively high in tender leaves and decreased as the leaves matured into the medium-sized and coarse ones.
Fig. 4.
DNJ contents in mulberry leaves during growing stages
Mulberry variety: Nongsang 14. Growing days refer the day order since the sprouting
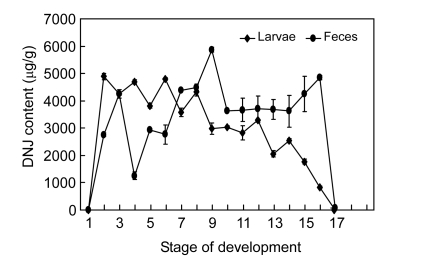
3.6. Accumulation and excretion of DNJ during larval stages
The results showed that the newly-hatched silkworm contained no DNJ, and then the DNJ content gradually increased until the middle of every instar, and then gradually decreased. The DNJ contents in the molting larvae were relatively low than that in the feeding larvae. The DNJ contents were sharply decreased from the 3rd or 4th day of the 5th instar, and no DNJ could be detected in the mature larvae.
DNJ content in the larval feces changed significantly during the growth period (Fig. 5). A significant increase in the DNJ content was observed during the 4th instar in feces over the other instars. In the 5th instar, the DNJ contents were stable for the first three days, and increased in the following two days and reached the peak one day before maturing larvae.
Fig. 5.
DNJ change in the larval body and feces during the growing stages
Stage of development: 1, newly-hatched larva; 2, 3rd day of 1st instar; 3, molting stage of 1st instar; 4, larva in the middle of 2nd instar; 5, molting larva of 2nd instar; 6, larva in the middle of 3rd instar; 7, molting larva of 3rd instar; 8, larva in the middle of 4th instar; 9, molting larva of 4th instar; 10, 1st day of 5th instar; 11, 2nd day of 5th instar; 12, 3rd day of 5th instar; 13, 4th day of 5th instar; 14, 5th day of 5th instar; 15, 6th day of 5th instar; 16, 7th day of 5th instar; 17, mature larvae
Based on the above results, it was noticed that DNJ contents in the larvae and the feces changed periodically and there was a close relationship of DNJ contents between the larvae and feces. DNJ content increased in larvae during the early and middle stages of every instar, while decreased during later stages, i.e., molting and mature larval stages. The present results indicate that early and middle instars were the main stages for DNJ accumulation in larvae, while at later stages DNJ content was mostly excreted and increased in the feces.
The data of DNJ ingestion and accumulation in the larvae are presented in Table 4. The results show that the total DNJ content ingested during the whole larval stage was 9625 μg. DNJ accumulation in larval body reached the maximum on the 5th day of the 5th instar, and every larva contained 1825 μg of DNJ. From the 6th day of the 5th instar, no DNJ was observed in silkworm larvae; i.e., it was excreted. The absorption ratio on DNJ was higher on the 1st to 3rd days of the 5th instar, and the highest (44.41%) on the 2nd day. The accumulation ratio reached the peak on the 3rd day of the 5th instar by 33.80%.
Table 4.
Enrichment of DNJ in larvae during different stages
| Every instar and every day of the 5th instar | Ingested DNJ (μg/larva) | Cumulative ingested DNJ (μg/larva) | DNJ content in larva (μg/larva) | Increased DNJ in larva (μg/larva) | Absorption ratio (%) | Cumulative enrichment ratio (%) |
| Newly hatched | 0.00 | 0.00 | ||||
| 1st instar | 17.19 | 17.19 | 3.11 | 3.11 | 18.09 | 18.09 |
| 2nd instar | 66.48 | 83.67 | 17.49 | 14.38 | 21.63 | 20.90 |
| 3rd instar | 272.21 | 355.88 | 96.54 | 79.05 | 29.04 | 27.13 |
| 4th instar | 1416.80 | 1772.68 | 379.14 | 282.60 | 19.95 | 21.39 |
| Day 1, 5th instar | 367.12 | 2139.80 | 537.54 | 158.40 | 43.15 | 25.12 |
| Day 2, 5th instar | 717.85 | 2857.65 | 856.37 | 318.83 | 44.41 | 29.97 |
| Day 3, 5th instar | 1279.99 | 4137.64 | 1398.65 | 542.28 | 42.36 | 33.80 |
| Day 4, 5th instar | 1211.22 | 5348.86 | 1643.24 | 244.59 | 20.19 | 30.72 |
| Day 5, 5th instar | 1365.08 | 6713.94 | 1825.46 | 182.22 | 13.35 | 27.19 |
| Day 6, 5th instar | 1392.66 | 8106.60 | 1815.85 | −9.61 | −0.69 | 22.40 |
| Day 7, 5th instar | 1060.13 | 9166.73 | 1037.01 | −778.84 | −73.47 | 11.31 |
| Day 8, 5th instar | 457.84 | 9624.57 | 631.74 | −405.27 | −88.52 | 6.56 |
| Mature larva | 0.00 | 9624.57 | 0.00 | −631.74 | 0.00 |
4. Discussion and conclusion
There are many reports on the DNJ in Morus, and it was found that DNJ contents in mulberry leaves were affected by many factors, such as Morus species and seasons (Kimura et al., 2007; Yatsunami et al., 2008). However, almost no studies were done in the silkworm, and DNJ accumulation and excretion in the larva are not clear. This investigation showed that DNJ in the silkworm was ingested from diets, and its contents depended on the DNJ content in the diets, indicating that a silkworm larva could not synthesize DNJ by itself.
Our results indicate that the accumulation of DNJ in the larvae was affected by the sex, varieties, and developing stages of silkworms. The male larvae can accumulate DNJ more efficiently than the females. DNJ accumulation and excretion in the larvae were periodically changed. We conclude that it is the most effective and economic to develop DNJ-related products by using the male larvae of the 3rd day of the 5th instar, fed with leaves containing higher DNJ. In addition, the feces of silkworms before ecdysis and mature stage contain higher DNJ content and it can also be a resource for DNJ extraction.
References
- 1.Asano N, Tomioka E, Kizu H, Oseki K, Matsui K. Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis . Carbohydr Res. 1994;253(3):235–245. doi: 10.1016/0008-6215(94)80068-5. [DOI] [PubMed] [Google Scholar]
- 2.Asano N, Oseki K, Tomioka E, Kizu H, Matsui K. N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr Res. 1994;259(2):243–255. doi: 10.1016/0008-6215(94)84060-1. [DOI] [PubMed] [Google Scholar]
- 3.Asano N, Kato A, Miyauchi M, Kizu H, Kameda Y, Watson AA, Nash RJ, Fleet GW. Nitrogen-containing furanose and pyranose analogues from Hyacinthus orientalis . J Nat Prod. 1998;61(5):625–628. doi: 10.1021/np9705726. [DOI] [PubMed] [Google Scholar]
- 4.Asano N, Nishida M, Miyauchi M, Ikeda K, Yamamoto M, Kizu H, Kameda Y, Watson AA, Nash RJ, Fleet GW. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae) Phytochemistry. 2000;53(3):379–382. doi: 10.1016/S0031-9422(99)00555-5. [DOI] [PubMed] [Google Scholar]
- 5.Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y, Kata A, Nash RJ, Lee HS, Ryu KS. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.) J Agric Food Chem. 2001;49(9):4208–4213. doi: 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Inoue S, Isoda H. Effects of silkworm powder on glucose absorption by human intestinal epithelial cell line Caco-2. J Nat Med. 2007;61(4):387–390. doi: 10.1007/s11418-007-0164-5. [DOI] [Google Scholar]
- 7.Hughes AB, Rudge AJ. Deoxynojirimycin: synthesis and biological activity. Nat Prod Rep. 1994;11(2):135–162. doi: 10.1039/np9941100135. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Kim YH, Hong YS, Paek NS, Lee HS, Kim TH, Kim KW, Lee JJ. Alpha-glucosidase inhibitors from commelina communis. Planta Med. 1999;65(5):437–439. doi: 10.1055/s-2006-960803. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Kim SU, Lee HS, Kim I, Ahn MY, Ryu KS. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2003;1002(1-2):93–99. doi: 10.1016/S0021-9673(03)00728-3. [DOI] [PubMed] [Google Scholar]
- 10.Kimura T, Nakagawa K, Kubota H, Kojima Y, Yamaqishi K, Oita S, Oikawa S, Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007;55(14):5869–5874. doi: 10.1021/jf062680g. [DOI] [PubMed] [Google Scholar]
- 11.Ryu KS, Lee HS, Chung SH, Kang PD. An activity of lowering blood-glucose levels according to preparative condition of silkworm powder. Kor J Seric Sci. 1997;39(1):79–85. [Google Scholar]
- 12.Ryu KS, Lee HS, Kim SY. Pharmacodynamic study of silkworm powder in mice administered to maltose, sucrose and lactose. Kor J Seric Sci. 1999;41(1):9–13. [Google Scholar]
- 13.Yatsunami K, Ichida M, Onodera S. The relationship between 1-deoxynojirimycin content and alpha-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto. Jpn Nat Med (Tokyo) 2008;62(1):63–66. doi: 10.1007/s11418-007-0185-0. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikaki A, Hivonu M. The structure of moranoline, a piperidine alkaloid form Morus species. Nippon Nogei Kagaku Kaishi. 1976;50(11):571–572. [Google Scholar]
- 15.Yoshikuni Y. Inhibition of intestinal alpha-glucosidase activity and postprandial hyperglycemia by moranoline and its N-alkyl derivatives. Agric Biol Chem. 1988;52(1):121–128. doi: 10.1248/bpb1978.11.356. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikuni Y, Ezure Y, Aoyagi Y, Enomoto H. Inhibition of intestinal alpha-glucosidase and postprandial hyperglycemia by N-substituted moranoline derivatives. J Pharmacobiodyn. 1988;11(5):356–362. doi: 10.1248/bpb1978.11.356. [DOI] [PubMed] [Google Scholar]