Abstract
Haematopoietic progenitor cells (HPC) traffic between the circulation and the bone marrow. Through contact with osteoblasts in the bone marrow niche, their survival, maintenance and proliferation is regulated. This review summarizes recent observations regarding the interaction between osteoblasts and HPCs, and the resulting downstream effects on signaling and niche maintenance. Using live imaging, amongst other techniques, HPCs were found to make prolonged contact with the osteoblast, via a specialized region of their membrane with high expression of prominin 1, CD63 and rhodamine PE. Following contact, portions of the HPC membrane expressing these molecules were phagocytosed by the osteoblast into SARA-positive signaling-endosomes. In response, Smad signaling was downregulated in the osteoblasts, leading to increased production of SDF-1; a chemokine involved in progenitor cell homing to the bone marrow, and thus regulating progenitor cell trafficking. The study summarised here presents important findings regarding progenitor cell trafficking, maintenance, proliferation and survival in the bone marrow and potentially other niche microenvironments, following signaling events initiated and propagated through single cell interactions.
Key words: osteoblast, haematopoietic progenitor cell, stem cell, endosome, live imaging
Maintenance and regulation of haematopoietic stem cell self-renewal and differentiation depends upon their specific microenvironment known as the ‘stem cell niche’.1 Haematopoietic stem-progenitor cells reside in the bone marrow, ultimately differentiating into blood and immune cells. Once their fate is determined, HPC move between the circulation and the bone marrow: a process regulated predominantly through osteoblasts, also residing within the bone marrow environment.2,3 Through contact with HPCs, osteoblasts are induced to secrete a number of cell signaling molecules that in turn regulate HPC trafficking, proliferation and survival. Until recently the molecular mechanisms determining these processes were unknown. This review summarizes recent findings by Gillette et al. (2009), published in Nature Cell Biology, in which sophisticated experiments have been performed to investigate different aspects of osteoblast and HPC interactions and the resulting downstream effects important for niche maintenance4 (Fig. 1). Their observations have significant importance in cell-cell communication in both the bone marrow and other cellular microenvironments.
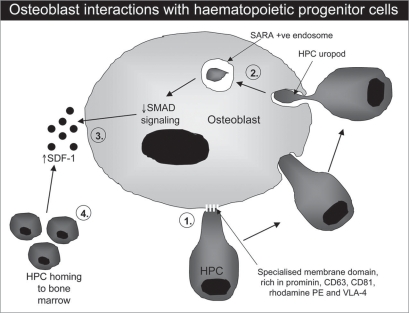
Figure 1.
Schematic representation of the events occurring during HPC-osteoblast interactions. HPCs interact with osteoblasts through specialized regions of the HPC membrane (uropod), expressing high levels of prominin, CD63, CD81, rhodamine PE and Vla-4 (1). Following contact, portions of the uropod membrane are actively phagocytosed by the osteoblast into Sara+ve endosomes (2). In response, osteoblast Smad signaling is reduced, leading to increased SDF-1 production (3) and ultimately increased HPC trafficking, proliferation and survival within the bone marrow (4).
The authors used a live-cell co-culture system, whereby HPCs (primary CD34+ cells or the KG1a progenitor cell line) were co-cultured with primary human osteoblasts or the human SaOS2 osteoblastic cell line, presenting live images with labeled cells to demonstrate their findings. HPCs were highly polarised, rapidly changing their morphology and migrating towards the osteoblasts, with a leading and lagging edge (termed the ‘uropod’).5 Further investigations of the distribution of HPC plasma membrane components identified asymmetric expression of the stem cell marker prominin 1 and the adhesion-signaling molecules CD63, CD68 and VLA-4. Rhodamine PE, a lipid that inserts within the membrane, also demonstrated this polarized pattern of expression (Fig. 1). Using cholesterol and actin depletion, the authors confirmed that both cholesterol and actin-based processes are involved the cell surface expression of these molecules in the polarized regions. Confocal and time-lapse imaging, scanning electron microscopy and quantum dot (QD)-labeling of the osteoblast-HPC interaction identified that the HPC uropod mediated the cell contact through microvilli-like projections, with highly polarized expression of prominin 1, rhodamine PE, VLA-4 and CD63 detected at the site of contact.
Gillette et al. next carried out further QD-labeling and confocal microscopy experiments to characterise the transfer of lipid and protein components from the HPC uropod membrane to the osteoblast.4 Using rhodamine-PE labeled cells, the authors observed that this transfer process was not observed at the same rate when HPCs were co-cultured with HeLa cells (i.e., cells that do not normally reside in the bone marrow), nor when a membrane separated the cell types, demonstrating that a cell-specific interaction takes place that requires direct contact between the two cell types. Chemical disruption of the uropod membrane also showed that this specialized part of the membrane is essential to mediate the intercellular transfer.
Using live imaging, the authors next identified that the transfer event involved dynamin-dependent phagocytosis, whereby osteoblasts uptake portions of the HPC membrane into endosomes, and did not involve fusion of the two cell membranes (Fig. 1). Staining fixed osteoblasts for Smad anchor for receptor activation (SARA), a FYVE-domain containing protein involved in signal transduction, showed that the signaling-endosomes (endosomes with activated receptors and downstream effectors6) were SARA-positive; following TGFβ receptor activation, translocation of Smads is induced, a process aided by SARA as a cofactor, ultimately leading to gene activation. In osteoblasts cultured alone, Smad2/3 were predominantly located within the nucleus, whereas in osteoblasts co-cultured with HPC, Smad2/3 were located within the cytoplasm, suggesting a reduction in activated Smad2/3 signaling and ultimately a reduction in TGFβ signaling. TGFβ signaling has previously been shown to downregulate osteoblast production of SDF-1, a chemokine involved with progenitor cell migration and adhesion, affecting HPC survival.7,8 Following co-culture with HPC, osteoblast expression of SDF-1 increased from 30 to 75%, and this was further shown to be associated with the intercellular transfer event and not simply due to prolonged cell contact.
In conclusion, this is the first paper to demonstrate downstream effects occurring following direct cell-cell contact of HPC with osteoblasts, and their potential importance in signaling and remodeling within the bone marrow niche. More specifically the authors used live imaging to demonstrate uptake of small portions of the HPC uropod membrane into SARA-positive signaling-endosomes within the osteoblast. Osteoblasts subsequently showed decreased Smad-signaling, and ultimately reduced SDF-1 production. As SDF-1 is involved with trafficking, survival and proliferation of HPC within the bone marrow environment, these cell contact events may play a vital role in HPC propagation. This work provides new insights into cell-cell communication and the resulting intercellular transfer and downstream events that are important in the bone marrow. Contact-dependent interactions between stem cells and organ-specific differentiated cells has been recognized for a few years now,9,10 but the complicated mechanisms underlying these processes has until now been largely unknown. Gillette et al. have elucidated one such mechanism.4 As adult stem cell niches reside in other microenvironments around the body, similar contact-dependent mechanisms may be employed to maintain and regulate the propagation and differentiation of such specific stem cell populations. Whether these are stable or dynamic situations, still needs to be identified.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10106
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 3.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 4.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–311. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev. 2009;10:353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 6.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Wright N, de Lera TL, Garcia-Moruja C, Lillo R, Garcia-Sanchez F, Caruz A, Teixido J. Transforming growth factor-beta1 downregulates expression of chemokine stromal cell-derived factor-1: functional consequences in cell migration and adhesion. Blood. 2003;102:1978–1984. doi: 10.1182/blood-2002-10-3190. [DOI] [PubMed] [Google Scholar]
- 9.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]