Abstract
The transmission of mechanical forces to cells is followed among all by biological signals related to changes in the assembly or disassembly of integrins associated linker proteins, such as vinculin. We applied for 3 hours 2% cyclic mechanical strain at the frequency of 1 Hz to human fibroblasts cultured on a deformable substrate; substrate deformation resulted to modify the number, length and area of vinculin positive focal adhesion contacts when compared to not stretched cells. The mechanism behind these morphological changes is related to Akt and RhoA roles in focal adhesion assembly. In the case of Akt and Rho inhibition, focal contacts disassembled only in presence of stretching mechanical stress, highlighting the role of mechanical stress on focal adhesion maturation in terms of multimolecolar assembly which from focal complexes leads to fibrillar adhesion.
Keywords: mechanical stress, vinculin, morphometry, Akt, Rho
Introduction
Tissue and cells participate to several physical interactions with the surrounding environment such as different mechanical stress as shear, compression or stretching forces. Mechanotransduction, known as the conversion process of the mechanical signals into intracellular biochemical changes, seems to be critical for normal tissue growth and function, as well as chemical environmental cues, e.g., growth factors.1
Several studies reporting cellular behavior in presence of different stress variations2,3 evidenced that cytoskeleton and other structural components have an established role in mechanotransduction, being able to transmit and modulate mechanical stimuli within the cell via focal adhesion sites, integrins, cellular junctions and the extracellular matrix.4,5 The cytoplasmic domain of integrins is functionally linked to various intracellular proteins that constitute the cytoskeleton and numerous kinases, forming a signalling interface between the extracellular matrix and the cell. Up to date, more than 50 proteins have been reported to be associated with focal contacts, including cytoskeletal proteins (e.g., vinculin, tensin, paxillin, α-actinin and talin), tyrosine kinases (e.g., Src, FAK, PYK2), serine/threonine kinases (e.g., ILK, PKC), modulator of small GTPases (e.g., ASAP1, Graf), tyrosine phosphatases (e.g., SHP-2) and other enzymes (e.g., PI3-kinase).6
Vinculin, that is required for stable cell adhesion, is indicated by electron microscopy as a globular head that contains binding sites for α-actinin, talin and for the vinculin tail, that can bind not only the vinculin head through a prolin-rich domain but also to paxillin, f-actin and phosphatidylinositol 4,5-biphosphate (PtdIns(4,5)P2).7 Transition from a “closed” to an “open” physical conformation of vinculin is induced by the binding of PtdIns(4,5)P2 to its tail. Thus, upon activation by PtdIns(4,5)P2, vinculin appears to facilitate the assembly of focal contacts by cross-linking and recruiting its various partners.6
The serine/threonine Akt regulates multiple biological processes, as cell survival, proliferation, differentiation.8 Akt was identified as a downstream component of survival signalling through PI3-kinase.9 In unstimulated cells, Akt protein exists in the cytoplasm, and the two regulatory phosphorylation sites at Thr308 and Ser473 are in an unphosphorylated state. After biochemical or mechanical stimulations, Akt is recruited to the plasma membrane. Fully activated Akt becomes available to phosphorylate its downstream substrate which translocate to various subcellular location.
RhoA, rac and Cdc42 are members of the Rho family of small GTPases, which contribute to coordinate cell behavior by modulating transcription and the actin cytoskeleton. In addition, all three GTPases regulate the formation of cell-matrix adhesion sites (focal contacts), which are intimately associated with the actin structures.10 Signalling through Rho GTPases can be initiated by activation of many different types of plasma membrane receptors11 and mechanical stretch stimulates RhoA via an unknown mechanism, initiating signalling pathways that enhance in some cases cell proliferation and contractility.12
Several works outlined how, by the way of focal adhesion complexes, cellular behavior is modified when mechanical stress is applied. First evidence is that cells subjected to deformations reorganize their actin cytoskeleton and align their long axis in the direction of the minimal substrate deformation, with a perpendicular orientation with respect to the force applied.13–16
Considering that vinculin plays a key role in the assembly and activation of focal contacts binding several proteins involved in the adhesion plaque, we have studied its rearrangement on cells during substrate deformation. Morphological and morphometrical analyses of vinculin positive focal contacts have been performed on fibroblasts cultured in a dynamic environment and data have been evaluated. Mechanism behind vinculin morphological changes, evaluating Akt and Rho activation following mechanical stress, has been studied.
Results
Focal adhesions on dynamic cultures.
Vinculin positive focal adhesions present on the membrane of control cells were round-shaped and distributed on defined spot while on stressed cells focal contacts have a spindle shape and were distributed almost along all the membrane.
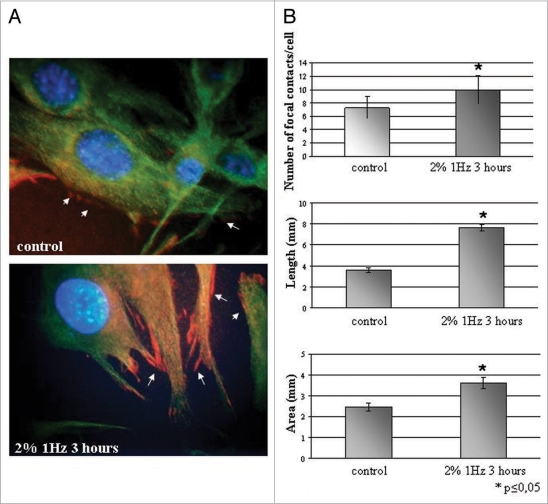
Images and graphs in Figure 1A and B showed results obtained from the analyses on vinculin positive focal contacts observed by fluorescence microscopy. Figure 1A reports representative images obtained after vinculin fluorescent staining on control and stressed cells. In details, Figure 1B reports the number of focal contacts per cell, the length of vinculin positive adhesion sites and focal contacts area measure.
Figure 1.
(A) Fluorescent images of vinculin-positive spot on cellular membrane on static and mechanical stretched samples. Pictures are representative of three different experiments Magnification (100x). (B) Morphometrical analyses of vinculin-positive focal adhesion sites. Number of focal adhesions/cell, length and area are measured on control cells cultures and cell cultures after applying stretching mechanical stress. *, indicates that results are statistically significant with respect to control.
Number of focal contacts/cell on cells subjected to mechanical stress was statistically higher respect to cells cultured on not stressed control substrates. Control has a mean of 7 ± 3 adhesion sites vinculin positive per cell; this number increased reaching a mean of focal adhesions per cell of 10 ± 4 in presence of cyclic uniaxial stress (2% substrate deformation at 1 Hz for 3 hours). The length of vinculin positive adhesion sites assessed by morphometric measures results higher with statistical relevance in cells cultured on mechanically deformed substrate (7.7 ± 0.3 µm) with respect to cells cultured on not deformed substrate (3.6 ± 0.2 µm). Morphometrical measures of vinculin positive focal contact areas evidenced increased values when cells were cultured on cyclically deformed substrate respect to control cells cultured on not deformed silicon substrate.
Vinculin synthesis and expression.
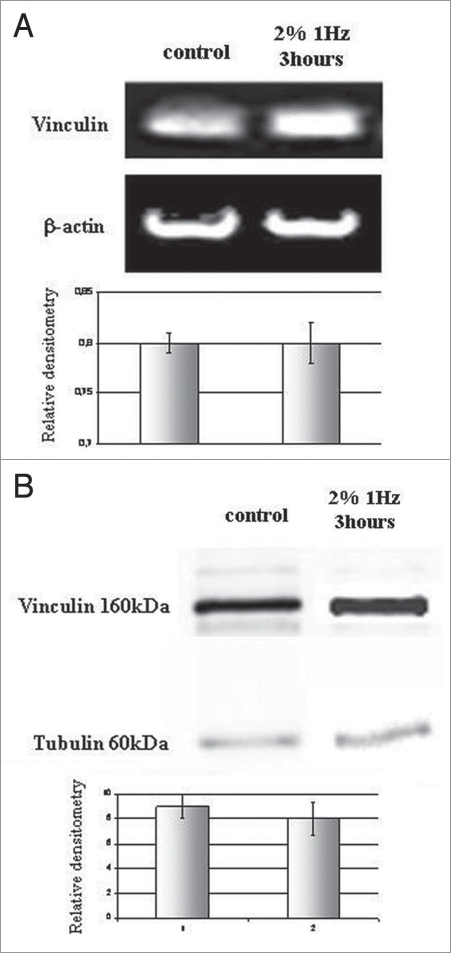
In order to evaluate the hypothesis that the different morphology of focal contacts was due to the presence of neo-synthesized vinculin after applying a mechanical stress, RT-PCR and western blot analyses were performed. As shown in Figure 2A and B, synthesis and expression of vinculin were not perturbed after stressing cells, as vinculin detected on control and on stressed cells had the same signal intensity and densitometry, performed normalizing vinculin bands with tubulin and β-actin level. Thus, after three hours, mechanical stress seemed not to interfere with vinculin synthesis and expression.
Figure 2.
(A and B) in order to verify eventual perturbations on vinculin synthesis and expression, RT-PCR and western blot analyses with relative densitometries were performed. Tests were repeated in triplicate.
PI3K pathway.
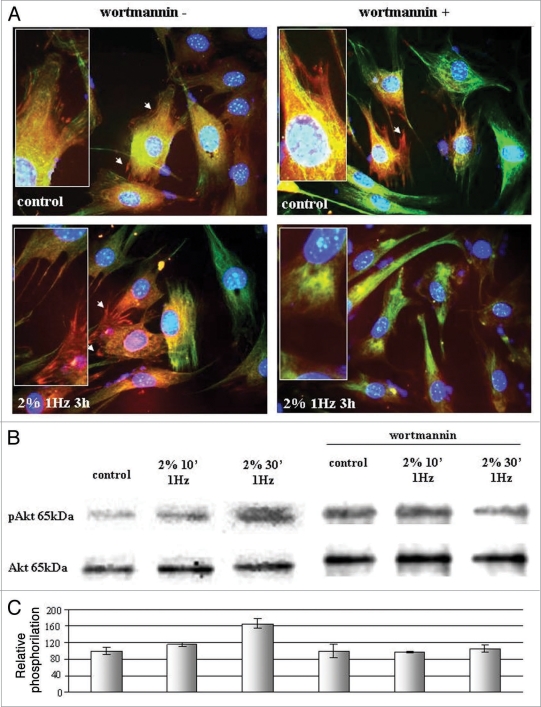
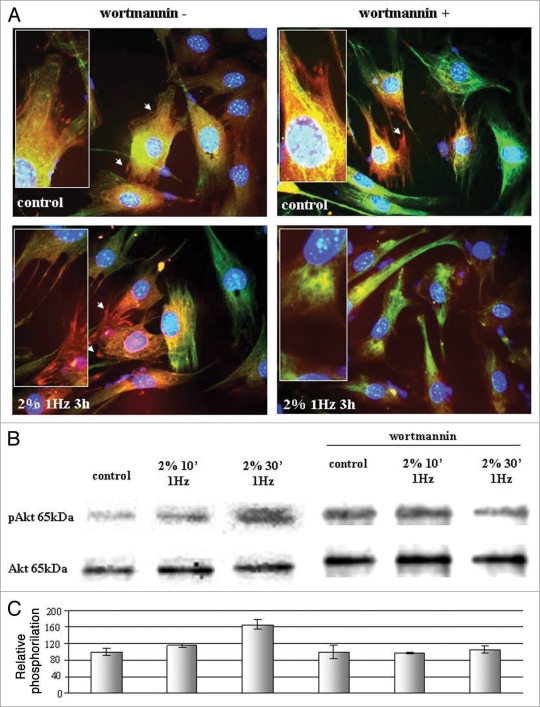
Concerning focal adhesion presence and morphology controlled by Akt and Rho involvement, results obtained (Figs. 3A and 4A) evidenced that in presence of Akt and Rho inhibitor vinculin-positive focal contacts disaggregate only if these conditions were associated with mechanical stress. In fact, focal contacts resulted not visualized on the membrane but eventually as a diffuse positivity on cells analyzed at fluorescent microscopy.
Figure 3.
(A) Fluorescence microscopy on vinculin-positive focal adhesions in presence of stretching mechanical stress (2% substrate deformation at 1 Hz for 3 hours) with and without PI3K inhibitor wortmannin (wt). Pictures are representative of three different experiments Magnification (100x). (B) Western blot analyses of Akt phosphorylation in presence of mechanical stress (2% substrate deformation at 1 Hz) after 10 and 30 minutes without and with PI3K inhibitor wortmannin (wt). (C) Densitometry obtained from three different experiments expressed as percentage with respect to controls.
Figure 3B shows western blot analyses on Akt activation after applying stretching mechanical stress on fibroblasts. Akt phosphorylation begins after 10 minutes of 2% of substrate stretching, reaching the ultimate activation after 30 minutes. Akt activation has been inhibited in presence of wortmannin (10 µM); the intensity of the protein phosphorylation do not increase even in presence of mechanical stress, maintaining comparable level to the not stressed control.
Western blot analyses on Rho activation in absence and in presence of Y27632 (Fig. 4B) shows the inhibition of Rho activity. Mechanical stress enhance Rho phosphorylation with respect to unstretched control, instead, in presence of Rho kinase inhibitor Y27632 mechanical stress do not increase Rho activation. Densitometry confirmed the statistical significance in all cases.
Figure 4.
(A) Fluorescence microscopy on vinculin-positive focal adhesions in presence of stretching mechanical stress (2% substrate deformation at 1 Hz for 3 hours) with and without Rho kinase inhibitor (Y27632). Pictures are representative of three different experiments (magnification 100x). (B) Western blot analyses of Rho activation in presence of mechanical stress (2% substrate deformation at 1 Hz) after 3 hours with and without Rho kinase inhibitor (Y27632). (C) Densitometry obtained from three different experiments expressed as percentage with respect to controls.
Discussion
Mechanical stresses are present on tissues and may help in a correct developing of tissues and organs, as well as maintaining their functionality. These forces, generated by external tension or by the cell’s type own contractile properties, can affect cell shape and alignment as a consequence of cytoplasmic reorganization that results by intracellular processes.
Focal adhesions resulted as highly dynamic structures influenced by external conditions, and the presence and area of focal adhesions are proportionally dependent on the local force applied by the cell.17 Thus, a structural change of the substrate promotes a deep modify in cell adhesion in term of focal contacts morphology by the action of the mechanotranduction process.
In this study, the increase in number of focal adhesions results in order to counteract the force applied by the substrate deformation; moreover, focal contacts morphology change after applying a mechanical stress, resulting modified in length and area.
Also a transition from an inactive (closed) to an active (open) conformation of vinculin can be produced by mechanical force in order to expose binding sites that can mediate new molecular interactions.18 Thus, the exposition of active vinculin binding sites for structural and adhesion proteins modifies focal adhesion aspect. We issued that the spindle shape obtained on stressed cells is due to a more extensive adhesion of active vinculin on actin filaments, following the stress fibers direction.
Mechanical stress involves a more extensive vinculin activation, exposing selective binding sites for f-actin and for other proteins involved in cellular adhesion. Results, supported by RT-PCR and western blot analyses, evidenced that focal contacts morphological modifications in number, length and area that follow mechanical stress were not related to an increase of vinculin synthesis or expression. Thus, mechanical stress could have a role in focal contact maturation. Rho and Akt pathways involvement have been demonstrated to be activated by mechanical stress and probably their involvement guides the focal adhesion assembly a disassembly, showing a key role in focal contacts maturation. These data support the hypothesis that the morphological change in focal adhesion is due to a conformational modification of vinculin in an activate state, able to bind more extensively actin filaments and not to a protein neo-synthesis. As a consequence, cells are able to modify the adhesion arrangement to strongly adhere to the substrate.
Literature reports interesting works concerning bio-chemically altered focal adhesion morphology, mainly focusing on GTPase family proteins Rho, Rac and Cdc42 as the major components involved in adhesion plaques formation.19,20 In particular, Rho seems to be the protein that connect different pathways involved in actin polymerization and focal adhesion assembly. Rho regulates phosphatidyl-inositol-4,5-kinase (PI4,5K) activity and consequently phosphatidylinositol-2-phosphate (PIP2) synthesis which is directly involved in vinculin activation by the way of a conformational change. Thus, the presence of PIP2 can lead to a more extensive vinculin activation even in terms of a more extensive binding of the protein with actin filaments due to the active (open) conformation of vinculin. This explain the spindle shape of the vinculin-positive spots after the application of mechanical stress, which follow the terminal fraction of actin filaments close to the plasma membrane where cells generate adhesion plaques.
An interesting finding is that actually the lack of focal adhesions was in presence of inhibitors only in occurrence of mechanical stress, which normally leads to Rho and Akt activation. In fact, even Akt activity is demonstrated to depend from PI3K.21 In this case, even Akt pathway inhibition and not only Rho inhibition has direct consequences on focal contacts maturation, inducing an extensive disassembly. Thus, stretching mechanical stress involves both Akt and Rho pathways inducing a crosstalk between the two molecular ways, with direct consequences on focal adhesion assembly and formation.
Materials and Methods
Cell culture.
Silicone sheets 0.1 mm thick have been obtained from Specialty Manufacturing Inc. (Saginaw, MI, USA) and have been used as deformable substrate. Silicone sheets have been cut in samples of 2 × 2 cm area and sterilized by autoclaving for 20 min at 121°C. Silicone samples have been coated with sterile fibronectin 10 µg/ml (Sigma, Milan, Italy) for 1 h at room temperature before cell seeding. Unless otherwise specified, all chemical reagents were purchased from Sigma (Milan, Italy). Culture media and fetal bovine serum (FBS) were from HyClone (CellBio, Milan). Human fibroblasts MRC5 (ATCC CRL 171) derived from normal lung tissue have been used at 15 × 103 cells/cm2. Cells have been cultured in DMEM enriched with 10% foetal bovine serum, glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 µg/ml).
Cells seeded on fibronectin-coated silicone samples have been maintained at 37°C in humidified atmosphere with 5% CO2 in static conditions for 24 hours before applying mechanical stress. For AKT and Rho activation studies, cells were starved, maintaining cell cultures in 1% fetal bovine serum for 12 hours before the application of mechanical test. For PI3K cascade inhibition tests, wortmannin 10 µM (wt-PI3K inhibitor) or Y27632 10 µM (Rho kinase inhibitor) has been added on starved cells cultures 30 minutes before mechanical stress has been applied.
Mechanical stress.
The Instron 5564 testing Instrument (Instron Corporation, Canton, MA, USA) has been used to apply 2% uniaxial deformation to silicon on which cells have been cultured in static conditions for 24 hours before applying mechanical stress. For vinculin studies, 2% substrate deformation has been applied for 3 hours at 1 Hz frequency and results were compared to cells cultured on not stretched controls. For AKT and RhoA activation studies stress has been applied for 10, 30 minutes and/or 3 hours at 2% substrate deformation at 1 Hz frequency to cells treated or not with wortmannin or Y27632. The device comprises an electronic control console and a loading frame capable of testing up to 2.5 N in tension; a drive system that operates tensing the samples until a predefined deformation and returning to the starting position in the case of cyclic stress. Samples have been soaked in a culture vertical chamber (Ugo Basile, Milan, Italy) filled with culture medium and maintained at 37°C with 5% CO2 in a closed bath.
Fluorescence microscopy.
To evaluate vinculin expression and localization, cells have been fixed in formaldehyde 3.7% for 30 minutes at 4°C and then labelled with mouse anti-human vinculin antibody 1:300 in PBS (Oncogene, Italy) followed by Texas red-conjugated horse anti-mouse secondary antibody 1:500 in PBS (Vector Laboratories, Inc., USA). For microtubule staining, cells have been incubated with mouse anti-tubulin antibody 1:20 in phosphate buffer solution (PBS) followed by fluorescein-conjugated horse anti-mouse secondary antibody 1:500 in PBS (Vector Laboratories, Inc., USA). DAPI staining for nuclei has been used.
The stained cells with vinculin positive focal contacts have been observed by fluorescence microscopy (Leica, DM 2500) at 40x magnification. Focal contacts have been examined quantitatively using ten images/sample in experiments performed in triplicate (n = 30). The analysis system is composed by a fluorescent microscope connected with a computer equipped with an image analysis software (Leica Q-Win) calibrated on 40x magnification. Various parameters including number of vinculin positive focal contacts/cell, mean length and mean area of vinculin positive adhesion sites have been measured. Results obtained from control cells cultured in static condition on silicon substrate have been compared to dynamic environments represented by cyclic uniaxial deformation (2% 1 Hz 3 h in presence or absence of inhibitors depending from the experimental conditions required).
Western blot.
To verify vinculin expression and AKT and Rho activation, western blot analyses have been performed. Cells have been lysed in RIPA buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 1% sodium deoxycolate, 1 mM Na3Vo3, 0.1% SDS, 50 mM sodium fluoride, 1% Triton, 0.5 M EDTA, 10 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 1 mM PMSF) 30 minutes on ice. Lysates, after sonication on ice, have been clarified by centrifugation at 14,000 rpm for 30 min. Protein concentration has been determined with bicinchoninic acid assay (Pierce, Rockford, IL) and 10 µg of proteins in sample buffer (62.5 mM Tris-HCl, pH 6.8, 20% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.5% bromophenol blue) have been used for SDS-PAGE electrophoresis (7.5% acrylamide gel) and transferred to nitrocellulose membrane (Amersham Biosciences, Buckinghamshire, England). Blotted proteins have been blocked 1 hour with 5% non-fat dried milk in PBS and incubated overnight with anti-human vinculin 1:500 (Oncogene, Italy), anti-pAkt 1:500 or anti-pRho 1:500 all in PBS (Calbiochem, Darmstadt, Germany). After washing three times with PBS 0.1% Tween 20, membranes have been incubated with secondary antibodies peroxidase-conjugates (Amersham Biosciences, Buckinghamshire, England) for 1 h at room temperature. After washing three times with PBS 0.1% Tween 20, bands have been visualized using ECL (Amersham Biosciences, Buckinghamshire, England) detection reagents at chemisensitive visualizer (VersaDoc, BioRad, Italy). To verify that the same amount of total proteins has been loaded and transferred on the blotting membrane we have reprobed the membranes after stripping with 100 mM 2-mercaptoethanol, 2% SDS and 62.5 mM Tris-HCl pH 6.7 for 30 minutes at 50°C. Then membranes have been incubated with 5% non fat dried milk pH 7.4, 1 hour at room temperature and incubated overnight with anti-tubulin, anti-Akt or anti-Rho antibody 1:500 in PBS (Calbiochem, Darmstadt, Germany). After washing three times with PBS 0.1% Tween 20, membranes have been incubated with secondary anti-mouse antibodies peroxidase-conjugates (Amersham Biosciences, Buckinghamshire, England) for 1 h at room temperature. After washing three times with PBS 0.1% Tween 20, bands have been visualized as previously described. Levels of vinculin, tubulin, pAkt, Akt, pRho and Rho have been quantified and normalized using a software QuantityOne (Bio-Rad Laboratories, CA, USA).
RT-PCR.
Total RNA has been isolated from fibroblasts cultured on silicon in static condition or in dynamic environments (2% 1 Hz 3 h) and measured using the RediPlate™ 96 RiboGreen® RNA Quantitation Kit (Molecular Probes Inc., Eugene, OR). One microgram of RNA has been reverse transcribed into cDNA (Enhanced Avian RT First Strand Synthesis Kit), and amplified in 50 μl of PCR reaction. Primers for vinculin (sense, 5′-AAA CAC AGT TAC ACT TGT GCA CCC-3′; antisense, 5′-AAC AGA GGG AAG TGT CCC CT-3′), and β-actin (sense, 5′-ACA CTG TGC CCA TCTA CGA GGG G-3′; antisense, 5′-ATG ATG GAG TTG AAG GTA GTT TCG TGG AT-3′) have been used for amplification. PCR reactions have been run using PerkinElmer Thermal Cycler for 30 cycles as follows: denaturation (95°C-30″), annealing (60°C-30″) and extension (72°C-1′) following initial treatment (95°C-1′). Agarose gel electrophoresis has been used to separate the PCR products; PCR generated cDNA fragments (360 pb for β-actin and 125 pb for vinculin) have been recorded digitally using Bio-Rad Gel Doc1000 and densitometry, subtracted by background gel value in an area of 27 mm2 and determined using the QuantityOne software (BioRad).
Densitometry.
A semi-quantitative examination was carried out on data obtained from separate RT-PCR and western blot analyses. Levels of vinculin, pAkt, Akt, pRho and Rho have been quantified and normalized using a software QuantityOne (Bio-Rad Laboratories, CA, USA). Tests were performed in triplicate. Results were expressed as percentage ± standard deviation with respect to control for each experiment. *, indicates that results are statistically significant with respect to control.
Statistical analysis.
Results were expressed as mean ± standard deviation. Data have been analyzed using the SPSS for windows software. Statistical significance was determined by the t-student test. Differences were considered statistically significant at p < 0.05.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/9569
References
- 1.Geiger B, Bershadsky A. Exploring the neighbourhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 2.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Int Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang HQ, Zhao L. Impact of mechanical stress and tension-stress on angiogenesis in wound healing. Chin J Traumatol. 2006;9:118–124. [PubMed] [Google Scholar]
- 4.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JGN, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodelling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–49. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 7.Milam LM. Electron microscopy of rotary shadowed vinculin and vinculin complexes. J Mil Biol. 1985;184:543–545. doi: 10.1016/0022-2836(85)90301-8. [DOI] [PubMed] [Google Scholar]
- 8.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI3-kinase/Akt signalling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 10.Hall A. Rho GTPases and the control of cell behavior. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 11.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;23:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 12.Numaguchi K, Educhi S, Yamakama T, Motley ED, Inagami T. Mechanotrasduction of rat vascular smooth muscle cells requires RhoA and intact actin filaments. Cir Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- 13.Takemasa T, Yamaguchi T, Yamamoto Y, Sugimato K, Yamashita K. Oblique alignment of stress fibers in cells reduces the mechanical stress in cyclically deforming fields. Eur J Cell Biol. 1998;77:91–99. doi: 10.1016/S0171-9335(98)80076-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang JH, Grood ES, Florer J, Wenstrup R. Alignment and proliferation of MC3T3-E1 osteoblasts in microgrooved silicone substrata subjected to cyclic stretching. J Biomech. 2000;33:729–735. doi: 10.1016/s0021-9290(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 15.Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, et al. Mechano-chemical control of human endothelium orientation and size. J Cell Biol. 1989;109:331–339. doi: 10.1083/jcb.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neidlinger-Wilke C, Grood E, Claes L, Brand R. Fibroblast orientation to stretch begins within three hours. J Orthop Res. 2002;20:953–956. doi: 10.1016/S0736-0266(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 17.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 18.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 19.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 20.Nobes CD, Hall A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 21.Clark EA, King WG, Brugge JS, Symons S, Hynes RO. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]