Abstract
EGFR, a critical regulator of oncogenic signaling during cancer progression, is capable of integrating multireceptor signaling pathways that promote metastasis. EGFR is subject to regulatory cues from the extracellular matrix (ECM), of which hyaluronan (HA) is a major component. In mammary tumors, HA is deposited in the ECM where it functions in biomechanical support and modulates intracellular signaling. We utilized a 3D collagen system in which HA is either polymerized in collagen matrix or provided soluble in the media (sHA). Here we report that collagen-embedded HA (eHA) inhibits EGFR activation, filopodia formation and cell spreading on a collagen matrix. These findings demonstrate a novel role for eHA as a protective molecule when encountered in the collagen matrix during cancer progression.
Keywords: HA, EGFR, collagen, extracellular matrix
Introduction
EGFR (also known as Her/ErbB1) is a member of the ErbB family of receptor tyrosine kinases responsible for regulating an intricate network of signaling pathways during cell growth, survival, adhesion and motility and is overexpressed in a variety of epithelial carcinomas, including neuronal, lung and breast.1 In the basal-like breast cancers, a subtype of breast carcinoma strongly associated with metastatic disease, EGFR is highly overexpressed, and overexpression of EGFR ligands can induce breast cancer in transgenic mouse models.2 In addition to cognate ligand binding, EGFR activity can be further modified by interactions and cross-talk with numerous cell-surface adhesion receptors, such as integrins and CD44, and in this way can integrate diverse signaling pathways initiated by components of the extracellular matrix.3–5 As a result of this cross-talk and signal integration, EGFR is subject to extensive regulatory cues from the extracellular matrix (ECM) that are crucial to EGFR-dependent metastasis. ECM-mediated EGFR signaling has been shown to regulate such metastatic phenotypes as cell shape and motility through activation of the Rho-family of small GTPases, which, in turn, can lead to filopodia formation, cell invasion and degradation of the ECM.6,7
The composition of the ECM is also critical to metastasis as changes occur not only within epithelial cells but also in the surrounding stroma to accommodate invasion.8–10 Hyaluronan (HA, also known as hyaluronic acid or hyaluronate) is an extracellular and cell-surface-associated glycosaminoglycan that is a major component of extracellular and pericellular matrices.11 HA is a high molecular mass polysaccharide that consists of repeating disaccharides of glucoronic acid and N-acetylglucosamine and is primarily synthesized by stromal fibroblasts.12–14 Once synthesized, HA is deposited in the ECM where it interacts with numerous ECM components such as proteoglycans, versicans and collagen.12,15–17 Associations between HA and components of the ECM define the structural qualities of a matrix, directly influencing ECM-mediated cell signaling during cancer progression.18 Thus, HA provides biomechanical support to the ECM, but also regulates cellular activities at the cell surface through interactions with it’s receptors, CD44 and RHAMM.11
Importantly, HA has been shown to regulate EGFR activity both during embryonic development and during cancer progression.19,20 To date, however, research has relied on the use of soluble HA added directly to cell culture media or deposition of HA by increased Has2 activity in epithelial cells. This is in contrast to the fibroblast-produced HA that is found intercalated within the collagen matrix of most ECM environments.15–17 We have shown that the loss of CD44 in the MMTV-PyV MT mouse model of breast cancer significantly increases metastasis indicating that CD44-HA interactions inhibit breast cancer metastasis.21 Importantly, examination of HA synthesis in mammary tumors from MMTV-PyV MT mice showed that HA production was restricted to the stromal compartments surrounding tumors. Distinct and separate HA-epithelial interactions recapitulated in vitro in a 3D collagen culture system demonstrated that HA embedded in a type I collagen matrix strongly inhibited invasion. This is a novel observation of HA function, as HA provided soluble in media promotes invasion.22 As HA/CD44 can also modulate EGFR, we set out to determine if these opposing effects on invasive phenotypes could be accounted for by an effect on EGFR activation.
Here we present data that HA embedded in type I collagen can inhibit EGFR activation and consequently alter invasive cellular phenotypes. Taken together, these data demonstrate a differential role for soluble versus embedded HA in EGFR-mediated breast cancer metastasis.
Results
Type I collagen-embedded hyaluronan inhibits EGFR activation.
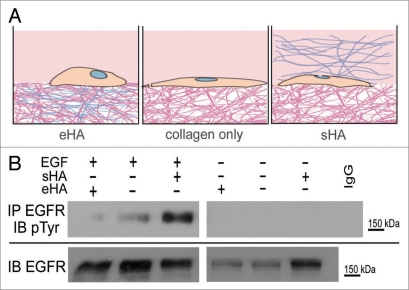
To examine the effect of a hyaluronan-containing matrix on EGFR activity, MDA-MB-231 cells were grown a on type I collagen gel matrix either in the presence of (A) HA embedded in the collagen gel matrix (eHA), (B) on a collagen matrix in the absence of exogenously added HA, or (C) HA provided in soluble form to the media (sHA)(Fig. 1A). All HA was high molecular weight (HMW) (avg. 2 mDa). Following overnight serum starvation, cells were stimulated with EGF and EGFR activation was analyzed.
Figure 1.
Type I collagen-embedded HA inhibits EGFR activation. (A) Diagram for experimental 3D culture system. Cells can either be grown on a collagen matrix that has been polymerized with HMW HA (eHA, left), on collagen alone with no exogenously added HA (middle) or in the presence of HMW HA added in soluble form to cell media (sHA, right). (B) Serum starved MDA-MB-231 cells grown in the presence of either collagen-embedded HA (eHA), collagen only or soluble HA (sHA) and were stimulated with EGF (10 ng/ml) for 2 hours (lanes 1–3) or as a control were untreated (lanes 3–6). Collagen gels were prepared as previously described21 and 0.125 mg/ml HMW HA was either polymerized into the collagen or added in soluble form to cell media. All HA used was high molecular weight (average 2 mDa). Protein lysates were collected and immunoprecipitated (IP) for EGFR and blotted (IB) for either EGFR (bottom) or phosphotyrosine (pTyr) (top). Molecular weight standards are shown at right.
We found that treatment with EGF stimulates tyrosine phosphorylation of EGFR in cells grown on collagen gels without HA and addition of sHA to cell media resulted in an increase in EGFR activity (Fig. 1B, lanes 2 and 3). Conversely, EGFR activity was inhibited when cells were grown on eHA (Fig. 1B, lane 1). Note that cells not treated with EGF have no EGFR tyrosine phosphorylation (Fig. 1B, lanes 3–6). These results demonstrate that eHA can suppress EGFR activation by EGF, indicating that ECM context may vastly alter EGFR function in cancer progression.
Collagen-embedded HA does not alter polymerization of a collagen matrix.
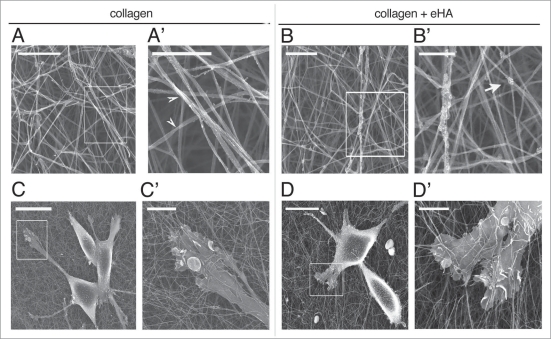
We have shown that embedding collagen with HA results in the suppression of receptor activation. We next investigated whether the addition of eHA altered the polymerization of collagen fibers and in this way was affecting receptor activity. To do this, we used scanning electron microscopy (SEM) to visualize collagen fiber polymerization in the presence and absence of eHA. We seeded MDA-MB-231 cells on collagen with or without eHA and then prepared the cells for SEM analysis. We found that collagen samples polymerized without eHA display a roping and polymerization pattern that is unchanged in the presence of eHA (Fig. 2 and compare A to B and A′ to B′). Collagen fibers in both cases display characteristic helical roping patterns visible at 20Kx (Fig. 2A′ and B′, arrowheads) regardless of the addition of HA. Visible remnants of dehydrated HA are visible on collagen polymerized with HA as an artifact of critical point dehydration (Fig. 2B′, arrow).
Figure 2.
Collagen-embedded HA does not alter polymerization of a collagen matrix. MDA-MB-231 cells were grown on type I collagen gels were prepared as described in Figure 1 for 2 hours and visualized by scanning electron microscopy. (A and B) Collagen gels were visualized by SEM in the presence of eHA (B and B′) or collagen alone (A and A′). Scale bars are 2 µm for (A and B) and 1 µm for (A′ and B′). (C and D) Cells growing on collagen alone (C and C′) or in the presence of eHA (D and D′) were visualized with SEM. Scale bars are 20 µm for (C and D) and 4 µm for (C′ and D′).
We next examined the ability of cells to interact with collagen fibers in the presence or absence of eHA (Fig. 2C and D). We found that eHA did not alter the cells’ interactions with the collagen fibers. On a collagen matrix, cells make numerous contacts with collagen fibers and use membrane extensions to burrow in collagen fibers (Fig. 2C and C′). On eHA, cells can also use membrane extensions for adherence and interactions with collagen fibers which is unaltered by the presence of eHA (Fig. 2D and D′). These data indicate that the addition of HA to collagen does not alter the structure of the matrix itself and thus changes to invasive phenotype are due to extracellular matrix-mediated signaling through the EGF receptor.
Hyaluronan-mediated inhibition of EGFR alters cell morphology.
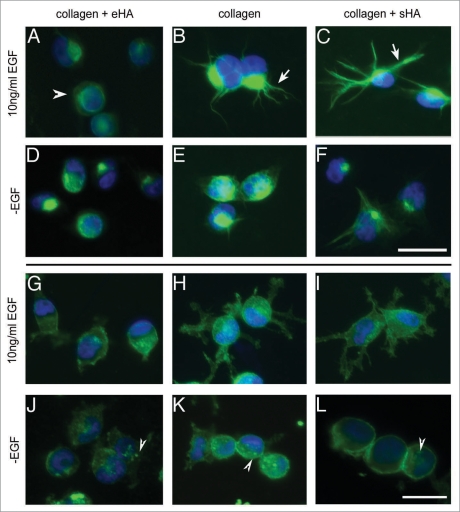
To determine the outcome of EGFR activity on cellular phenotype, we next examined the affect of HA deposition on cell morphology. To determine if downregulation of EGFR activation by eHA alters filopodia formation, MDA-MB-231 cells were seeded on collagen with eHA or treated with sHA and then stimulated with EGF to activate the receptor. To visualize filopodia formation, we examined vimentin expression by immunofluorescence (Fig. 3). Cells were seeded on collagen as described for 2 hours and treated with EGF to induce filopodia formation (Fig. 3A-C) or serum starved to allow cell attachment without filopodia formation (Fig. 3D-F). Notably, we found that EGF treatment of cells grown on eHA results in no visible filopodia formation although vimentin expression was still detected in these cells (Fig. 3A and arrowheads). Alternatively, EGF treatment of cells grown on collagen or sHA induced extensive filopodia formation (Fig. 3B and C, arrows).
Figure 3.
Type I collagen-embedded HA inhibits filopodia formation and alters membrane dynamics. MDA-MB-231 cells were serum starved overnight and then seeded on collagen gels for 2 hours in the presence of 10 ng/ml EGF or without EGF (-EGF). The cells were then fixed and visualized by immunofluorescence using anti-Vimetin and anti-CD44 antibodies. (A–F) Cells were visualized for vimentin. Cells were treated with EGF (A–C) or serum starved (D–F) and were grown on eHA (A and D), collagen alone (B and E) or sHA (C and F). (G–L) Cells visualized for CD44. Cells were treated with EGF (G–I) and grown on eHA (G and J), collagen alone (h and K) or sHA (I–L). Scale bars indicate 25 µm.
CD44 is the predominant cell surface receptor for HA and we next examined the localization of this receptor by immunofluorescence (Fig. 3G-L). In the absence of EGF, cells remain rounded without membrane extensions and CD44 is predominant on the cell surface and in distinct foci (Fig. 3J-L, arrowheads). When treated with EGF on collagen alone, CD44 is found throughout the cell membrane, concentrated in foci along the cell membrane and extensively covering membrane extensions (Fig. 3H). Similar CD44 staining is observed in the presence of sHA (Fig. 3I). When grown in eHA, however, EGF treatment does not stimulate extension of CD44-containing filopodia (Fig. 3G). These results demonstrate that not only is eHA capable of inhibiting EGFR activation, but this inhibition correlates with a loss of EGF-mediated filopodia formation. On eHA, cells that normally extend filopodia in response to EGF treatment instead retain a rounded epithelial morphology. These alterations to cell morphology and membrane dynamics are characteristic of a less invasive cell phenotype, concurrent with impaired EGFR function and with previous findings that CD44 inhibits invasion in MDA-MB-231 cells when presented with eHA.21
Collagen-embedded HA delays cell spreading and alters membrane dynamics.
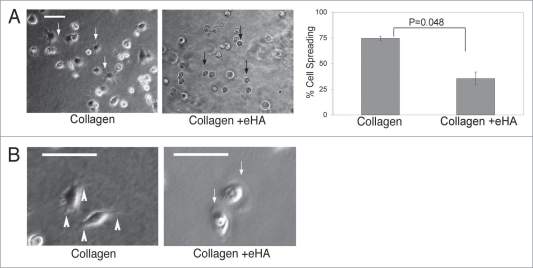
We have found that eHA alters EGF-dependent changes to cell morphology and membrane dynamics. Cell adhesion is a critical component of a cells ability to spread and utilize filopodia. To determine if eHA is also affecting adhesion dynamics of cells adhering to a matrix, MDA-MB-231 breast cancer cells were observed over a three hour period in the presence or absence of eHA. Cells were seeded on collagen and visually monitored for adhesion and spreading. While cells were able to adhere to the surface of the collagen matrix equally in the presence or absence of eHA (data not shown), cells that encountered eHA demonstrated a significant delay in spreading onto the gels (Fig. 4A, right, black arrows). In the absence of eHA, almost 75% of cells seeded onto the collagen matrix attached and spread by 45 minutes (Fig. 4A, left, white arrows). Alternatively, only 35% of cells spread on eHA collagen matrices (Fig. 4A, right, black arrows). Additionally, cells that did spread on eHA showed decreased levels of filopodia formation and were generally more rounded than cells seeded on gels cast without HA (Fig. 4B, white arrows). Note that eventually all cells spread on the collagen regardless of the presence of HA in the microenvironment.
Figure 4.
Cell morphology changes dependent on HA in collagen I gels. (A) MDA-MB-231 cells (5 × 104) were placed onto collagen matrix either immobilized with HMW HA or without HA in 20% FBS. Fourty-five minutes after placement of cells on matrix, cells were imaged and spreading was quantified. Cells were imaged at 60X magnification while all cells (>150) within a 10X field of view were enumerated. Statistics were calculated using an unpaired student’s t-test. The error bars represent s.d. from three separate experiments. (B) Time lapsed video microscopy was obtained from cells seeded as described in (A) over 1.5 hrs, revealing filopodia formation (1st panel, white arrowheads) when on collagen and lamellipodia formation (3rd panel, white arrow) when on collagen + eHA. Scale bar indicates 25 µm.
We next examined the dynamics of adhesion and migration for cells seeded on collagen I matrix in the presence or absence of HA. Using time lapsed brightfield microscopy, we monitored cells over 1.5 hours to examine the dynamics of cells migrating across these different substrates. When seeded on collagen alone, cells migrate using mainly filopodia and do not adhere to neighboring cells (Fig. 4B and 1st panel, white arrowheads and Suppl. movie 2). Alternatively, cells on eHA employed the use of lamel-lipodia to migrate across the matrix and formed adhesions to neighboring cells (Fig. 3B and 3rd panel, white arrows and Suppl. movie 1). Thus, matrix associated HA can alter the formation of invasive cellular phenotypes, influence membrane dynamics and promote cell-cell attachments.
Discussion
We have demonstrated that eHA can inhibit EGFR activation and reduce the formation of filopodia. Furthermore, cells growing on an eHA collagen matrix have a delay in cell adhesion to the matrix and migrate primarily using lamellipodia. In contrast, cells growing on sHA adhere easily to the collagen matrix and migrate using filopodia. Previous research has found that HA can activate erbB receptors and downstream pathways23,24 but to date, research examining HA-mediated signaling has relied on the use of soluble HA, added exogenously to cell media. We have demonstrated that polymerizing type I collagen with HA does not alter the polymerization of collagen suggesting that the effect of eHA on EGFR activity is due to cell-matrix interactions and demonstrates the significance of cell-matrix interactions on EGF-mediated signaling.
Importantly, HA is found deposited in the stromal compartments surrounding tumor cells and this research highlights the importance of examining HA-mediated biology in a context which accurately recapitulates epithelial-stromal interactions. Concurrent with a role for CD44 as protective molecule in cancer progression, HA functions to inhibit EGFR activation when embedded in the ECM. Research has shown, however, the HA catabolism increases with tumor progression and that epithelial cells upregulate Has2 expression (the primary hyaluronan synthase) thus increasing epithelial production of HA.11 Changes to HA production and catabolism may represent an extracellular matrix switch that accommodates metastatic invasion, while HA present in the ECM can act as a suppressor to tumor progression.
Importantly, these data demonstrate that EGFR activation is not only HA-dependent, but HA-context dependent, which suggests that therapeutic intervention with EGFR inhibitors may also be affected by the contextual presentation of extracellular matrix components. The role of matrix-embedded hyaluronan on EGFR-dependent signaling pathways and EGFR-inhibitors will be the subject of future work.
Materials and Methods
Cell lines and antibodies.
EGFR (1005), Vimentin antibody (V9), pTyr (PY99) and CD44 (Df1485) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). MDA-MB-231 cells (ATCC) were grown in RPMI (Cell Gro) with 10% FBS and 1% Penicillin Streptomycin. Cells were maintained in a humidified incubator at 37°C at 5% CO2.
Protein lysates and immunoblotting.
2 × 106 cells were seeded onto a collagen matrix [0.9 mg/mL type I rat tail collagen (BD Biosciences, San Jose, CA), 83% (v/v) M199 medium (Sigma), 0.18% NaHCO3], and incubated at 5% CO2 in a humidified chamber at 37°C for 1 hour. For collagen embedded with hyaluronan, 0.175 mg/ml high molecular weight HA (mean MW of 2.3 mDa) (Kraeber GmbH & Co., Ellerbek, Germany) was reconstituted in water and boiled to inactivate contaminates and added to collagen gel before polymerization. For soluble HA, 0.175 mg/ml HMW HA was added to cell media prior to seeding cells on collagen matrix. Cells were then lysed in Triton X-100 buffer [20 mmol/L HEPES (pH 8.0), 150 mmol/L NaCl, 1.0% Triton X-100, 2 mmol/L EDTA, 2 mmol/L sodium orthovanadate, 50 µmol/L ammonium molybdate, 10 mmol/L sodium fluoride, and Complete protease inhibitor (Sigma, St. Louis, MO)]. Equal protein concentrations were loaded onto SDS-PAGE gels and separated by electrophoresis. Proteins were transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membrane was blocked with either 5% nonfat milk in PBS (0.1% Tween20) or 3% BSA in TBS (0.1% Tween20) and immunoblotted. The membrane was then treated with Super Signal West Pico Chemiluminescent Substrate (Pierce), visualized on Imagetech-B film (American X-ray supply Inc., Louisville, TN, USA), and developed with a Konica SRX-101C.
Immunofluorescence.
Cells were seeded in serum-free RPMI or serum-free RPMI with 10 ng/ml EGF (Invitrogen) on collagen embedded with HA, on collagen alone or on collagen with soluble HA (as described above). Cells were allowed to invade into the gel for 2 hours at 37°C in 5% CO2 in a humidified chamber. The cells were then fixed in 4% paraformaldehyde for 10 minutes, washed in PBS, and permeabilized in (0.5% Triton X-100 in 10 mM Pipes (pH 6.8), 50 mM NaCl, 300 mM sucrose and 3 mM MgCl2) for 10 minutes at room temperature. Following permeabilization, cells are blocked in 3% NGS/0.025% Tween in PBS for 30 minutes at room temperature. The cells were incubated in primary antibody overnight at 4°C, and then following antibody incubation were washed every 10 minutes for two hours in PBS at room temperature. The cells were then incubated overnight at 4°C in Alexa fluor-conjugated secondary antibodies and then washed for two hours in PBS. Cells were then mounted with SlowFade Gold Antifade Reagent with DAPI (Invitrogen) and visualized using a Leico Compound Flourescent Microscope and LSM510 Software.
Scanning electron microscopy.
Type I collagen gels were prepared as described above. Gels were fixed for 1 hour in 4% glutaraldehyde in 0.1 M cacodylate buffer. Gels were postfixed in 1% osmium tetroxide for 1 hour, followed by 2% uranylacetate for 30 minutes. Samples were dehydrated through a standard ethanol series, critical point dried in CO2 (Polaron E31) and then mounted and coated with 10 nm platinum (Hummer 6.1). Scanning electron microscopy was performed on a SEM Hitachi S4800.
Video.
Cells were seeded in 20% FBS on a collagen gel matrix (described above) cast in the presence or absence of immobilized high molecular weight HA. Immediately following seeding onto gels, the cells are placed on 37°C on a warming stage and photographed every 20 seconds for 2 hours with a Q Imaging Retiga 2000R on a Leica DMIL inverted microscope. Image stacks were converted to AVI files using Image J Software.
Acknowledgements
We are grateful to Ben Bitler, Matt Hart, and Matt Callan for a critical reading of this manuscript, and to Dave Bentley with the University Spectroscopy and Imagine Facilities at the University of Arizona. This work was supported by grants from the Arizona Biomedical Research Commission (J.A.S.), National Institutes of Health (J.A.S.), the Congressionally Directed Medical Research Programs and Department of Defense (J.M.V.L., J.I.L.) and Arizona Cancer Center Support Grant (J.A.S.).
Abbreviations
- HA
hyaluronan
- ECM
extracellular matrix
- eHA
embedded HA
- sHA
soluble HA
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10252
Supplementary Material
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder JA, Lee DC. Transgenic mice reveal roles for TGFalpha and EGF receptor in mammary gland development and neoplasia. J Mammary Gland Biol Neoplasia. 1997;2:119–129. doi: 10.1023/a:1026347629876. [DOI] [PubMed] [Google Scholar]
- 3.Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- 6.Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, et al. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka I, Koizumi M, Baba T, Yamashita S, Suzuki T, Kudo R. Epidermal growth factor increased the expression of alpha2beta1-integrin and modulated integrin-mediated signaling in human cervical adenocarcinoma cells. Exp Cell Res. 2003;286:165–174. doi: 10.1016/s0014-4827(03)00065-x. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 10.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 11.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 12.Asplund T, Versnel MA, Laurent TC, Heldin P. Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res. 1993;53:388–392. [PubMed] [Google Scholar]
- 13.Bertrand P, Girard N, Delpech B, Duval C, d’Anjou J, Dauce JP. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer. 1992;52:1–6. doi: 10.1002/ijc.2910520102. [DOI] [PubMed] [Google Scholar]
- 14.Knudson W, Biswas C, Toole BP. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci USA. 1984;81:6767–6771. doi: 10.1073/pnas.81.21.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 16.Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 17.Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- 18.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 19.Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- 20.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 21.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 22.Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- 23.Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- 24.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.