Abstract
In the present work, we demonstrate that microbial alkaloid staurosporine (STS) and Ro 31-8220, structurally related to STS protein kinase C inhibitor, caused development of membrane tubular extensions in human neutrophils upon adhesion to fibronectin-coated substrata. STS-induced tubular extensions interconnected neutrophils in a network and bound serum-opsonized bacteria Salmonella enterica serovar Typhimurium. The diameter of STS-induced extensions varied in the range 160–200 nm. The extensions were filled with cytoplasm and covered with membrane, as they included fluorescent cytoplasmic and lipid dyes. Neither protein kinase C inhibitors H-7 and bisindolylmaleimide VII, nor tyrosine protein kinase inhibitors tyrphostin AG 82 and genistein caused such extensions formation. Supposedly, STS induces membrane tubular extension formation promoting actin cytoskeleton depolymerization or affecting NO synthesis.
Key words: staurosporine, neutrophil, tubular or tublulovesicular extensions, membrane tethers, cytonemes, Salmonella enterica serovar typhimurium, actin cytoskeleton, cytochalasin D, protein kinase C, tyrosine protein kinase
Introduction
Long distance cellular adhesive interactions mediated by long tubular or tubulovesicular cellular protrusions—cytonemes, membrane tethers, nanotubes,—were firstly observed in various embryonic and blood cells1 and later were found in nerve and other cells.2 Study and measurement of these structures is strongly complicated by their small size, which is near the limit of resolution for optic microscopy. Recently, a number of long tubular and taper cellular protrusions varying twenty times in diameter (from 2,000 to 100 nm) are united as cytonemes and nanotubes.
We determine the neutrophil tubular or tubulovesicular extensions (cytonemes) as membrane tethers with strongly uniform diameter along the entire length. The diameter can vary in the range 150–240 nm. High rate of development (1 µm/min and more) and flexibility also characterize neutrophil cytonemes. In human neutrophil physical and chemical factors can cause formation of membrane tubular extensions. Pulling of long and thin membrane tethers from the cell bodies was observed upon neutrophil flowing over spread platelets or immobilized P-selectin at the physiological rate.3 Neutrophils attached to platelet P-selectin by P-selectin glycoprotein ligand-1 receptors located on the neutrophil microvillus tips. Following microvillus elongation under shear stress resulted in the membrane tethers formation.4,5 Similar membrane tethers can be pulled from the neutrophil bodies by a micropipette manipulation.6,7
Membrane tubulovesicular extensions resembling neutrophil membrane tethers in size and behaviour appeared on the neutrophil cell bodies upon adhesion to fibronectin-coated substrata in Na+-free extracellular medium or in the presence of actin-disrupting agents cytochalasin D or B, an alkylating agent 4-bromophenacyl bromide,8 inhibitors of glucose metabolism and inhibitors of vacuolar type ATPases, chloride channel inhibitors.9 Nitric oxide (NO), the physiological regulator of leukocyte adhesion to endothelium, appears to be a natural causative factor for TVE formation.10,11 NO-induced neutrophil tubulovesicular extensions connected neutrophils to substrata and to the other cells, and bound and aggregated pathogenic bacteria over a distance of several cell diameters.
The mechanism of membrane tubular extensions formation remains to be elucidated. Cell membranes undergo continuous curvature changes required for formation of tubular and vesicular carriers for intracellular membrane trafficking, exocytosis and endocytosis. It is shown recently that proteins containing BAR or F-BAR domain possess the ability to induce membrane invagination and tubulation due to changing of membrane curvature.12–15 The diameter of the tubules varies between 40 and 200 nm. GTPase dynamin and actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins: at that membrane tubulation is enhanced by disruption of actin cytoskeleton, whereas dynamin antagonized membrane tubulation promoting vesicles formation and fission, which require actin cytoskeleton integrity.16–18 Among the central cytoskeletal regulators are actin-depolymerizing factor (ADF)/cofilin, which depolymerizes actin filaments.19,20 Phosphorylation on single serine in cofilin can block this activity. In human neutrophils staurosporine specifically inhibits the constitutively active serine 3 cofilin kinase, thus promoting actin depolymerization.19,21 There is a close interplay between the mechanisms that control actin dynamics and those that mediate plasma membrane invagination and fission. Cofilin and cofilin kinase are shown to regulate actin filament population required for the dynamin-dependent apical vesicular carrier fission from Trans-Golgi network.22
In the present work, we demonstrate that STS, the natural alkaloid isolated from the bacterium Streptomyces staurosporeus, induces formation of nanometer-wide tubular extensions connecting human neutrophils in a network upon adhesion to fibronectin-coated substrata. STS possesses multiple biological activities ranging from antibiotic to antihypertensive and anti-cancer.23–25 One of the target points for STS is inhibition of protein kinases. STS prevents ATP binding to a kinase due to its stronger affinity to the ATP-binding site. To elucidate whether inhibition of protein kinases is a causative factor for development of neutrophil tubulovesicular extensions we studied the effect of protein kinase C and tyrosine kinase inhibitors of different structure on neutrophil spreading on fibronectin-coated substrata. We also compared STS-induced extensions with those that were induced by cytochalasin D and a NO donor, diethylamine NONOate in size and behaviour.
Neutrophils appear to be professional phagocytes. STS was shown to inhibit internalization (phagocytosis) of bacteria or opsonized erythrocytes by phagocytes and other cells.26–29 At that it does not reduce but often increases adherence of objects for phagocytosis to the cells.28,30 We suggest that staurosporine facilitates extracellular binding of bacteria due to formation of long membrane tubular extensions strongly widening the area of the neutrophil contact interactions. To test whether these extensions play a role in binding of bacteria, we have studied neutrophil interaction with serum-opsonized S. typhimurium species in control conditions and in the presence of protein kinase C inhibitors H-7 and STS, and of protein kinase C activator phorbol 12-myristate 13-acetate (PMA).
Results and Discussion
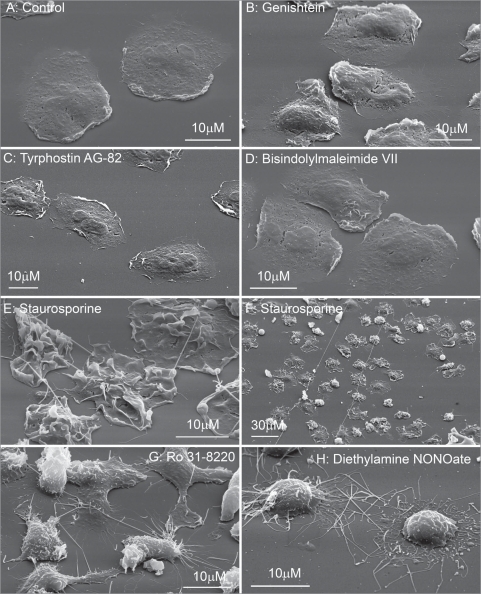
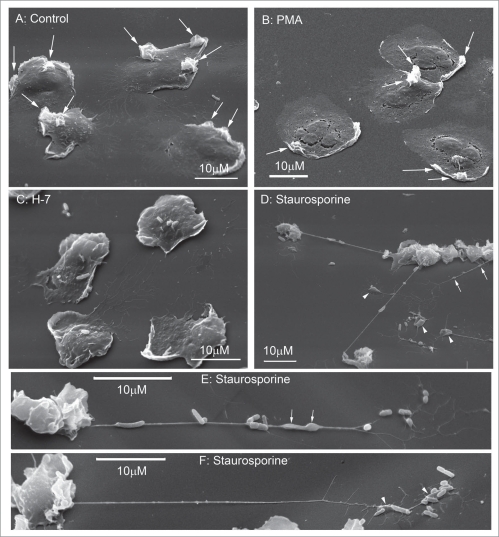
Human neutrophils have a round shape in suspension. Upon adhesion to fibronectin they attached and spread on fibronectin-coated substrata. The control cells plated at the density 106 cells/ml did not contact each other and appeared with a smooth surface (Fig. 1A). Neither tyrosine kinase inhibitors genistein and tyrphostin AG 82 (Fig. 1B and C), nor protein kinase C inhibitors bisindolylmaleimide VII (Fig. 1D) and H-7 (data not shown) altered significantly the neutrophil morphology when compared to the control cells (Fig. 1A). Neutrophils plated to fibronectin in the presence of STS (Fig. 1E and F) or RO 31-8220, a protein kinase C inhibitor structurally related to STS, had ruffled surface and were interconnected into network by thin tubular extensions (Fig. 1G). STS-induced extensions (Fig. 1E and F) like NO-induced neutrophil extensions (Fig. 1H) had definitely tubular shape and reached 60–120 µm (several cell diameters) in length during 20 min (Fig. 1F) but have fewer diameter (Table 1). These data indicate that to induce membrane tubular extension formation STS and RO 31-8220 possess other activities in addition to inhibition of protein kinase C or tyrosine kinase.
Figure 1.
Scanning electron microscopy images of human neutrophils attached to fibronectin-coated substrata in control conditions and in the presence of inhibitors of protein kinases, and diethylamine NONOate. Cells were attached to fibronectin-coated substrata during 20 min at 37°C in control conditions (A) and in the presence of: tyrosine kinase inhibitors, 100 µM genistein (B) and 100 µM tyrphostin AG 82 (C); protein kinase C inhibitors, 10 µM bisindolylmaleimide VII (D), staurosporine 200 nM (E and F) and 10 µM Ro 31–8220 (G); 1 mM diethylamine NONOate (NO) (H). All pictures represent typical images observed in three analogous experiments.
Table 1.
Diameter of neutrophil tubulovesicular extensions induced by different agents, as obtained by scanning electron microscopy
| Object of measurements | Dmin | Dmax | D + SEM |
| Control: tubulovesicular extensions connecting neutrophils in the presence of staurosporine, 200 nM | 128 | 188 | 158 ± 10 |
| Tubulovesicular extensions connecting S. typhimirium to neutrophils in the presence of staurosporine, 200 nM | 155 | 246 | 197* ± 8 |
| Tubulovesicular extensions developed in the presence of Ro 31-8220, 10 µM | 80 | 195 | 135* ± 5 |
| Tubulovesicular extensions developed in the presence of diethylamine NONOate, 1 mM | 150 | 240 | 184* ± 10 |
| Tubulovesicular extension developed in the presence of cytochalasin D, 5 µg/ml | 115 | 217 | 153 ± 3 |
Human neutrophils were attached to fibronectin-coated substrata during 20 min in the presence of STS, Ro 31-8220 or diethylamine NONOate and for 10 min in the presence of cytochalasin D and fixed for electron microscopy; The cytoneme diameters were measured on scanning electron images; The data presented are the mean values of diameter (D ± SEM) obtained from the measurements of diameters of 15–25 tubular cellular extensions; Data of three independent analogous experiments were summarized; *p < 0.001 as compared to the control value.
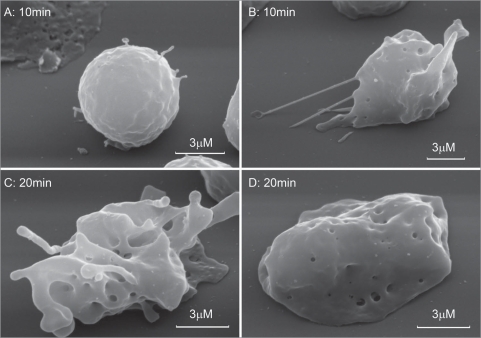
We suggest that staurosporine facilitate cytoneme formation through depolymerization of actin cytoskeleton. Depolymerization of actin filaments by cytochalasine D (Cyt D) induced cytonemes formation in neutrophils as revealed by phase contrast microscopy.8 Cyt D-induced extensions are characterized by rapid appearance and destruction due to swelling and lysis, which complicated fixation of extension for the electron microscopy study. This could be ascribed to the ability of Cyt D to stimulate secretion of lysosomal hydrolases desrupting cytonemes. Cyt D-induced extensions were partially conserved in neutrophils fixed during 10 min after addition of Cyt D (Fig. 2A and B) and resembled in size and behaviour STS-induced extensions (Table 1, Fig. 1E and F). Cells fixed 20 min after exposure to cyt D demonstrated either swelled fragments of extensions 300–450 nm in diameter, or no extensions were observed (Fig. 2D). Destruction of Cyt D-induced cytonemes was accompanied by appearance of specific round membrane invaginations on the neutrophil surface (Fig. 2B–D). These invaginations could represent exits for cytonemes or locuses of compensatory endocytosis upon cytonemes extrusion. Similar invaginations we observed on the neutrophil surface after shedding of NO-induced cytonemes as a result of interaction with bacteria.11
Figure 2.
Scanning electron microscopy images of human neutrophils attached to fibronectin-coated substrata in the presence of cytochalasin D. Neutrophils were attached to fibronectin-coated substrata during 10 min (A and B) and 20 min (C and D) at 37°C in the presence of 5 µg/ml cytochalasin D.
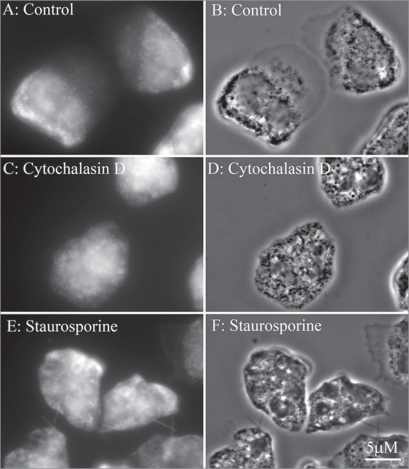
Assembly of neutrophil cytoskeleton undergoes strong reorganization upon adhesion to substrata and depends on substrata. Human neutrophils adherent to a polystyrene plastic surface are vigorously activated and exhibited an increase of cytoskeleton-accociated actin (F-actin) and a decrease of monomeric (G-actin) concentration when compared to suspended cells before plating. In contrast, fibronectin-adherent cells manifest only priming response and exhibit a decrease of F-actin and a rapid rise in G-actin cocentration.34–36 The peak F-actin depolymerization occurred in the first minutes of adhesion and is followed by partial F-actin remodelling. In our experiments neutrophils plated to fibronectin during 20 min in control conditions exhibited actin staining without profound actin filaments at less spead cell perifery (Fig. 3A and B), thus indicating adhesion-induced actin depolymerization. Cytochalasin D-treated cells exhibited diffusive staining of cytoplasm typical for G-actin (Fig. 3C and D). Staurosporine-treated cells (Fig. 3E and F) like cytochalasin-treated cells were difusively stained with FITC-phalloidin around the whole cell body including tubular extensions.
Figure 3.
Fluorescent (A, C and E) and phase contrast images (B, D and F) of neutrophils stained with FITC-phalloidin for actin cytoskeleton. Cells were attached to fibronectin-coated substrata in the control conditions (A and B) and in the presence of 5 µg/ml cytochalasin D (C and D) or 200 nM staurosporine during 20 min at 37°C.
Staurosporine could induce tubular extensions formation by blocking of actin remodelling in adherent neutrophils. Staurosporine specifically inhibits the neutrophil serine 3 cofilin protein kinase, thus keeping actin in the depolymerized state. The neutrophil serine 3 cofilin kinase is constitutively active and insensitive to a variety of selective antagonists of protein kinases (H-7, HA1004, ML-7, KN-62) but is blocked by STS.21 Another target point for STS in neutrophil cytoskeleton is inhibition of phosphorylation of L-plastin, a leukocyte-specific actin-bundling protein, which is phosphorylated on serine residue in response to adhesion or phagocytosis.37–39 L-plastin is a single protein, which interact with 4-bromophenacyl bromide,40 a drug capable of inducing cytoneme formation in more than 90% of cells. L-plastin is also one of the major S-nitrosation targets for NO, another potent inductor of cytoneme formation.41,42 STS43 and Ro-31-8220,44 but not other protein kinases inhibitors (H-7, H-8, herbimycin A) can inhibit L-plastin phosphorylation.
Mechanism of staurosporine-induced tubular membrane extension formation in neutrophils remains to be elucidated. It is not excluded that staurosporine causes extensions formation affecting NO availability and synthesis. Staurosporine has been shown to activate inducible45 and constitutive46,47 nitric oxide synthase isoforms expression and to inhibit production of superoxide48—a scavenger of NO.
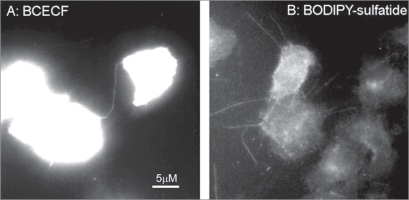
To demonstrate that STS-induced tubular extensions represent membrane tethers with cytoplasm inside, we used fluorescent cytoplasm dye BCECF and fluorescent lipid BODIPY-labelled sulfatide. STS-induced tubulovesicular extensions of BCECF-loaded neutrophils contained fluorescent dye along with neutrophil cytoplasm (Fig. 4A). Fluorescent analogue of natural lipid, BODIPY-sulfatide, permeated in the plasma membrane of STS-treated neutrophils and in STS-induced tubular extensions thus indicating their membranous structure (Fig. 4B).
Figure 4.
Fluorescent microscopy images of neutrophils attached to fibronectin-coated substrata in the presence of 200 nM staurosporine during 20 min at 37°C. Cells were preloaded with BCECF (A) or treated with BODIPY-sulfatide for 5 min at the end of experiment (B). Pictures represent typical images observed in two experiments.
The most impressive structures were formed during interaction of STS-treated neutrophils with bacteria. We compared human neutrophil interactions with serum-opsonized S. typhimurium bacteria in control conditions and in the presence of protein kinase C activator PMA and protein kinase C inhibitors H-7 and STS. In control conditions, neutrophils plated to fibronectin-coated substrata mainly ingested added S. typhimurium species. The specific membrane ruffles were left on the cell surface (Fig. 5A, large arrows). Such ruffles were shown to indicate the sites of S. typhimurium entering into the cells as a result of phagocytosis.49 In the presence of PMA bacteria were ingested by neutrophils in a similar way, leaving ruffles on the cell surface (Fig. 5B, large arrows).
Figure 5.
Scanning electron microscopy images of human neutrophils attached to fibronectin-coated substrata and exposed to serum-opsonized S. typhimurium bacteria. Cells were attached to fibronectin-coated substrata in control conditions (A), in the presence of 100 nM PMA (B), 100 µM H-7 (C) or 200 nM staurosporine (D–F) during 15 min at 37°C. Then bacteria (bacteria/cells ratio 20:1) were added, cells were further incubated for 5 min at 37°C, and fixed for electron microscopy. Arrows indicate bulges on tubular extensions. Arrowheads point to islands of neutrophil substances transported and shed by cytonemes. Pictures represent typical images observed in three experiments.
In the presence of protein kinase C inhibitors H-7, S. typhimurium species were attached to the neutrophil surface, while ruffles were practicularly absent (Fig. 5C). However, when bacteria were added to STS-treated cells, STS-induced tubular extensions of cells bound S. typhimurium species along with the cell surface (Fig. 5D–F). Interaction with bacteria resulted in a statistically significant increase in STS-induced extension diameters (Table 1) and appearance of multiple bulges along the extensions (Fig. 5D and E, arrows). The latter are supposed to transport neutrophil substances along the cytonemes. The interaction with bacteria caused shedding of cytonemes together with bound bacteria and bulges and formed a kind of adhesive for bacteria islands far from the cell surface (Fig. 5D and F, arrowheads).
We demonstrated that STS-induced extensions are membrane tubules with the average diameter 160 nm. They strongly differ from 15 nm-wide neutrophil “extracellular traps”—fibers consisted of granule proteins and chromatin without lipid membrane. These fibers are formed as a result of neutrophil cell death and can bind and kill bacteria extracellularly.50 STS-induced extensions also do not resemble long F-actin-containing filopodia (700–2,000 nm in diameter) of human macrophages, which bind bacteria and keep them untill bacteria undergo phagocytosis.51
STS-induced tubular membrane extensions creates a way for neutrophils to contact other cells and to capture bacteria over a distance from the cell surface. Transportation of neutrophil substances along cytonemes towards bound bacteria could deliver neutrophil bactericidal agents for destination without dilution and host tissues injury (Fig. 4). The binding of bacteria by cytonemes is not a prerequisite for phagocytosis of bacteria by neutrophils but is followed by shedding of tubular extensions together with bound bacteria. Following lysis of tubular extensions besides bound and aggregated bacteria could release neutrophil bactericides closely to bound pathogens. Such a mechanism could contribute to antibiotic activity of staurosporine.
Materials and Methods
Bicarbonate-free Hank’s solution, phorbol 12-myristate 13-acetate, staurosporine, H-7, bisindolylmaleimide VII, Ro31-8220 methanesulfonate salt, genistein, cytochalasin D, FITC labeled phalloidin were purchased from Sigma (Steinheim, Germany), tyrphostin AG 82 (A25) was from Alexis (Lausen, Switzeland). Ficoll-Paque was purchased from Pharmacia (Uppsala, Sweden). Fibronectin was from Calbiochem (La Jolla, USA). Diethylamine NONOate was from Cayman (Massy, France). Acetoxymethyl ester of 2′,7′-bis(2carboxyethyl)-5,(6)-carboxyfluorescein (BCECF) was obtained from Molecular Probes (Eugene, OR). BODIPY-sulfatide, 3-O-sulpho-D-galactosyl-β1-1′-N-[7-(4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene-8-yl)heptanoyl]-D-erythro-sphingosine, was prepared by a standard procedure used for such compounds,31 details of the synthesis will be published elsewhere.
Blood of healthy volunteers with no pharmacological therapy in the 2 weeks preceding sampling was used for neutrophil preparation. Blood was taken via venous puncture as approved by Ministry of Public Health Service of Russian Federation. Blood experimental procedures were approved by the Institutional Ethics Committee of A.N. Belozersky Institute. Neutrophils were isolated from freshly drawn donor blood on a bilayer gradient of Ficoll-Paque (at densities 1.077 and 1.125 g/ml).32 Washed neutrophils were resuspended in bicarbonate-free Hank’s solution containing 10 mM HEPES, pH 7.35. Glass cover slips were incubated in Hank’s solution containing 5 µg/ml fibronectin for 2 h at room temperature and were thoroughly washed with phosphate-buffered saline. Neutrophils (106 cells/mL) were plated on protein coated cover slips in corresponding buffer and incubated for 20 min at 37°C. All test substances were dissolved in Hank’s solution, DMSO or ethanol, and were added to the cells before plating. Corresponding amounts of DMSO or ethanol (not exceeding 5 µl/ml) were added to the control cells.
Salmonella enteria serovar Typhimurium cells of virulent strain C53 were a kind gift of Prof. F. Norel.33 Bacteria were grown in Luria-Bertrani-broth and then washed twice using physiological solution with centrifugation at 2,000 g. Concentration of stock suspension was 2 × 108 CFU/ml.
Bacteria were opsonized with fresh serum from the same donor whose blood was used for neutrophil preparation. Serum was isolated by centrifugation of clotted blood. For opsonization, bacteria (4 × 108/ml) were incubated for 10 min in Dulbecco’s solution containing 10% serum. Then bacteria were washed by repeated sedimentation in Dulbecco’s medium. Neutrophils were plated onto fibronectin-coated slides during 15 min at 37°C, then bacteria were added (10:1 or 20:1 bacteria/cell) and cells were further incubated at 37°C for 5 min. After that cell were fixed for scanning electron microscopy.
Photomicroscope Opton III (Germany) was used for phase contrast and fluorescent microscopy of neutrophils. The pH-sensitive fluorescent dye BCECF and BODIPY-sufatide were used as a cytoplasmic marker, and a lipid fluorescent dye, respectively. For fluorescent microscopy, neutrophils after preparation were incubated with 5 µM BCECF (20 min at 37°C) and then were used in experiments. BODIPY-labelled sulfatide (5 µg/ml) was added to the attached cells (incubation for 5 min) at the end of experiments.
For actin cytoskeleton staining neutrophils were plated to fibronectin-coated substrata during 20 min in the presence of test drugs, fixed in 4% paraformaldehyde in Hank’s-HEPES solution without Ca+2 and Mg+2 and containing 5 mM EDTA (pH 7.3), permeabilized during 10 min with 0.1% Triton X-100 and stained with FITC-phalloidin.
For scanning electron microscopy, cells were fixed for 30 min in 2.5% glutaraldehyde, postfixed for 15 min with 1% osmium tetroxide in 0.1 M cacodylate (pH 7.3), dehydrated in an acetone series, then critical-point-dried with liquid CO2 as a transitional fluid in a Balzers apparatus, sputter-coated with gold-palladium and observed at 15 kV with a Camscan S-2 or JSM-6380 scanning electron microscope.
Tubular extension diameters were measured directly on highly magnified scanning electron images and calculated based on the respective bar’s value. The data expressed as mean ± standard error (D ± SEM). Student’s test for unpaired observations was applied. Values of p less than 0.05 were regarded as significant.
Acknowledgements
This work was supported by Grants of Russian Foundation of Basic Research 09-04-00367, 07-04-00410, 09-04-00313.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10314
References
- 1.Galkina SI, Bogdanov AG, Davidovich GN, Sud’ina GF. Cytonemes as cell-cell channels in human blood cells. In: Baluska F, Volkmann D, Barlow PW, editors. Cell-cell channnels. Georgetown, New York: Landes Bioscience, Springer; 2006. pp. 236–244. [Google Scholar]
- 2.Gerdes HH. Tunneling nanotubes: membranous channels between anial cells. In: Baluska F, Volkmann D, Barlow PW, editors. Cell-cell channnels. Georgetown, New York: Landes Bioscience, Springer; 2006. pp. 200–207. [Google Scholar]
- 3.Schmidtke DW, Diamond SL. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J Cell Biol. 2000;149:719–730. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park EY, Smith MJ, Stropp ES, Snapp KR, DiVietro JA, Walker WF, et al. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters. Biophys J. 2002;82:1835–1847. doi: 10.1016/S0006-3495(02)75534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran V, Williams M, Yago T, Schmidtke DW, McEver RP. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. P Natl Acad Sci USA. 2004;101:13519–13524. doi: 10.1073/pnas.0403608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao JY, Ting-Beall HP, Hochmuth RM. Static and dynamic lengths of neutrophil microvilli. P Natl Acad Sci USA. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus WD, Hochmuth RM. Experimental studies of membrane tethers formed from human neutrophils. Ann Biomed Eng. 2002;30:1273–1280. doi: 10.1114/1.1528614. [DOI] [PubMed] [Google Scholar]
- 8.Galkina SI, Sud’ina GF, Ullrich V. Inhibition of neutrophil spreading during adhesion to fibronectin reveals formation of long tubulovesicular cell extensions (cytonemes) Exp Cell Res. 2001;266:222–228. doi: 10.1006/excr.2001.5227. [DOI] [PubMed] [Google Scholar]
- 9.Galkina SI, Sud’ina GF, Klein T. Metabolic regulation of neutrophil spreading, membrane tubulovesicular extensions (cytonemes) formation and intracellular pH upon adhesion to fibronectin. Exp Cell Res. 2006;312:2568–2579. doi: 10.1016/j.yexcr.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Galkina SI, Molotkovsky JG, Ullrich V, Sud’ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp Cell Res. 2005;304:620–629. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Galkina SI, Romanova JM, Stadnichuk VI, Molotkovsky JG, Sud’ina GF, Klein T. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Mic. 2009;56:162–171. doi: 10.1111/j.1574-695X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 12.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 13.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, et al. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 19.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Bi. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 20.Paavilainen VO, Oksanen E, Goldman A, Lappalainen P. Structure of the actin-depolymerizing factor homology domain in complex with actin. J Cell Biol. 2008;182:51–59. doi: 10.1083/jcb.200803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lian JP, Marks PG, Wang JY, Falls DL, Badwey JA. A protein kinase from neutrophils that specifically recognizes Ser-3 in cofilin. J Biol Chem. 2000;275:2869–2876. doi: 10.1074/jbc.275.4.2869. [DOI] [PubMed] [Google Scholar]
- 22.Salvarezza SB, Deborde S, Schreiner R, Campagne F, Kessels MM, Qualmann B, et al. LIM kinase 1 and cofilin regulate actin filament population required for dynamin-dependent apical carrier fission from the trans-Golgi network. Mol Biol Cell. 2009;20:438–451. doi: 10.1091/mbc.E08-08-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchya H, et al. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot. 1977;30:275–282. doi: 10.7164/antibiotics.30.275. [DOI] [PubMed] [Google Scholar]
- 24.Omura S, Sasaki Y, Iwai Y, Takeshima H. Staurosporine, a potentially important gift from a microorganism. J Antibiot. 1995;48:535–548. doi: 10.7164/antibiotics.48.535. [DOI] [PubMed] [Google Scholar]
- 25.Gescher A. Analogs of staurosporine: potential anticancer drugs? Gen Pharmacol. 1998;31:721–728. doi: 10.1016/s0306-3623(98)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Kogut MH, Genovese KJ, Lowry VK. Differential activation of signal transduction pathways mediating phagocytosis, oxidative burst and degranulation by chicken heterophils in response to stimulation with opsonized Salmonella enteritidis. Inflammation. 2001;25:7–15. doi: 10.1023/a:1007067426499. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakaran K, Harris EB, Randhawa B. Regulation by protein kinase of phagocytosis of Mycobacterium leprae by macrophages. J Med Microbiol. 2000;49:339–342. doi: 10.1099/0022-1317-49-4-339. [DOI] [PubMed] [Google Scholar]
- 28.Roubey RA, Ross GD, Merrill JT, Walton F, Reed W, Winchester RJ, Buyon JP. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18) J Immunol. 1991;146:3557–3562. [PubMed] [Google Scholar]
- 29.Rosenshine I, Duronio V, Finlay BB. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed KA, Booth TA, Hirst BH, Jepson MA. Promotion of Salmonella typhimurium adherence and membrane ruffling in MDCK epithelia by staurosporine. FEMS Microbiol Lett. 1996;145:233–238. doi: 10.1111/j.1574-6968.1996.tb08583.x. [DOI] [PubMed] [Google Scholar]
- 31.Koshy KM, Boggs JM. Partial synthesis and physical properties of cerebroside sulfate containing palmitic acid or alpha-hydroxy palmitic acid. Chem Phys Lipids. 1983;34:41–53. doi: 10.1016/0009-3084(83)90058-0. [DOI] [PubMed] [Google Scholar]
- 32.Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue antigens. 1974;4:269–274. [PubMed] [Google Scholar]
- 33.Kowarz L, Coynault C, Robbe-Saule V, Norel F. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence and regulation of spvR and spvABCD virulence plasmid genes. J Bacteriol. 1994;176:6852–6860. doi: 10.1128/jb.176.22.6852-6860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginis I, Zaner K, Wang JS, Pavlotsky N, Tauber AI. Comparison of actin changes and calcium metabolism in plastic- and fibronectin-adherent human neutrophils. J Immunol. 1992;149:1388–1394. [PubMed] [Google Scholar]
- 35.Wang JS, Pavlotsky N, Tauber AI, Zaner KS. Assembly dynamics of actin in adherent human neutrophils. Cell Motil Cytoskeleton. 1993;26:340–348. doi: 10.1002/cm.970260408. [DOI] [PubMed] [Google Scholar]
- 36.Wang JS, Coburn JP, Tauber AI, Zaner KS. Role of gelsolin in actin depolymerization of adherent human neutrophils. Mol Biol Cell. 1997;8:121–128. doi: 10.1091/mbc.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol Sin. 2005;26:769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones SL, Brown EJ. FcgammaRII-mediated adhesion and phagocytosis induce L-plastin phosphorylation in human neutrophils. J Biol Chem. 1996;271:14623–14630. doi: 10.1074/jbc.271.24.14623. [DOI] [PubMed] [Google Scholar]
- 39.Messier JM, Shaw LM, Chafel M, Matsudaira P, Mercurio AM. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil Cytoskel. 1993;25:223–233. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- 40.Rosales C, Jones SL, McCourt D, Brown EJ. Bromophenacyl bromide binding to the actin-bundling protein l-plastin inhibits inositol trisphosphate-independent increase in Ca2+ in human neutrophils. P Natl Acad Sci USA. 1994;91:3534–3538. doi: 10.1073/pnas.91.9.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao C, Guo H, Wei J, Mi Z, Wai PY, Kuo PC. Identification of S-nitrosylated proteins in endotoxin-stimulated RAW264.7 murine macrophages. Nitric Oxide-Biol Ch. 2005;12:121–126. doi: 10.1016/j.niox.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Keszler A, Broniowska KA, Hogg N. Characterization and application of the biotin-switch assay for the identification of S-nitrosated proteins. Free Radical Bio Med. 2005;38:874–881. doi: 10.1016/j.freeradbiomed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Shibata M, Ohoka T, Mizuno S, Suzuki K. Characterization of a 64-kd protein phosphorylated during chemotactic activation with IL-8 and fMLP of human polymorphonuclear leukocytes I. Phosphorylation of a 64-kd protein and other proteins. J Leuk Biol. 1993;54:1–9. doi: 10.1002/jlb.54.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Paclet MH, Davis C, Kotsonis P, Godovac-Zimmermann J, Segal AW, Dekker LV. N-Formyl peptide receptor subtypes in human neutrophils activate L-plastin phosphorylation through different signal transduction intermediates. Biochem J. 2004;377:469–477. doi: 10.1042/BJ20031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecker M, Preiss C, Schini-Kerth VB. Induction by staurosporine of nitric oxide synthase expression in vascular smooth muscle cells: role of NFkappaB, CREB and C/EBPbeta. Brit J Pharmacol. 1997;120:1067–1074. doi: 10.1038/sj.bjp.0701026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohara Y, Sayegh HS, Yamin JJ, Harrison DG. Regulation of endothelial constitutive nitric oxide synthase by protein kinase C. Hypertension. 1995;25:415–420. doi: 10.1161/01.hyp.25.3.415. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Forstermann U. Structure-activity relationship of staurosporine analogs in regulating expression of endothelial nitric-oxide synthase gene. Mol Pharmacol. 2000;57:427–435. doi: 10.1124/mol.57.3.427. [DOI] [PubMed] [Google Scholar]
- 48.Young LH, Ikeda Y, Lefer AM. Protein kinase inhibition exerts cardioprotective effects in myocardial ischemia/reperfusion via inhibition of superoxide release. Method Find Exp Clin. 2001;23:107–114. doi: 10.1358/mf.2001.23.3.627941. [DOI] [PubMed] [Google Scholar]
- 49.Bliska JB, Galan JE, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 50.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 51.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]