Abstract
EMT is a complex process whereby cells lose cell-cell interactions and other epithelial properties whilst acquiring a migratory and mesenchymal phenotype. EMT is presently recognized as an important even for tumor invasion and metastasis. Functional E-cadherin loss is a hallmark of EMT and required for tumor invasion in the majority of carcinomas. Transcriptional downregulation is one of the major mechanisms for E-cadherin suppression in carcinomas. In the last decade several E-cadherin repressors, belonging to different transcriptional families, have been identified that, importantly, also act as potent EMT inducers. One of the last additions to EMT regulators are the class I bHLH factors E2-2 (also known as TCF4). However, the hierarchical and functional interrelations between the different EMT inducers are still poorly understood. Here, we comment on the new and so far unrecognized function of E2-2 factors in EMT and discuss on the potential interactions among various EMT inducers. Emerging evidence supporting the participation of TCF4 in human malignancies is also discussed. Thus, increasing understanding of EMT and its regulators is providing meaningful insights into the present knowledge on tumor progression.
Key words: bHLH factors, EMT, TCF4, E2-2, snail, twist, ZEB, tumour progression
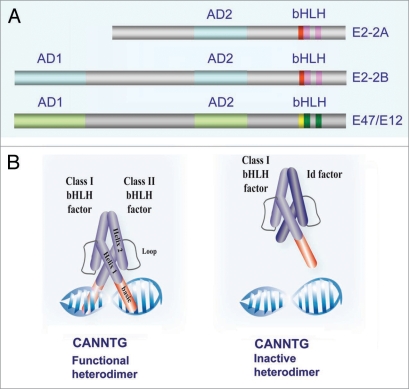
Helix-loop-helix (HLH) transcriptional regulators are broadly present in eukaryotic organisms from Saccaromyces cerevisiae to human and play important roles in many essential developmental processes, regulating cell growth and differentiation of distinct cell types. The HLH proteins have been classified into seven functional classes (I to VII).1 The conserved HLH domain mediates homo- or hetero-dimerization. In addition, most HLH factors contain a stretch of basic amino acid residues adjacent to the HLH domain, constituting a bHLH motif (Fig. 1A), through which they can bind to Ephrussi-box (E box) consensus binding site (CANNTG) (Fig. 1B). In mammals, there are three class I genes: E2A, HEB and E2-2, coding for several class I bHLH factors also known as E-proteins, which are widely expressed, but not ubiquitous.1 E-proteins have two conserved transactivation regions (Fig. 1A), AD1 and AD2, of which the N-terminal AD1 domain seems to be required both for activation and repressor activities in different cell types.2–4 E proteins form homodimers o heterodimers with class II family member; E proteins dimers usually act as transcriptional activators, but can also function as repressors depending on the bHLH partner and/or the co-regulators they are interacting with (Fig. 1B).5–8 The transcriptional activity of E-proteins can be negatively regulated by class V HLH factors, known as Id (Id1–Id4, inhibitor of differentiation) proteins, which lack the basic DNA binding domain, thus blocking promoter interactions of the corresponding E protein/Id heterodimers (Fig. 1B).9 The E2-2 gene (also known as ITF2, SEF-2 and TCF4) codes for two highly related isoforms, E2-2B and E2-2A (Fig. 1A). Is is important to remark here that E2-2/TCF4 factors should not be confused with T-cell factor 4, Tcf4 (recently renamed as Tcf7l2) involved in Wnt/β-catenin signalling.10 Both E2-2 isoforms differ in the N-terminal regions being E2-2A, the shorter isoform, devoid of the AD1 domain (Fig. 1A)2,11 and has been described as a transcriptional regulator with diminished or absent repressor capacity.2,12
Figure 1.
Class I HLH transcriptional factors. (A) Diagrammatic representation of the main functional domains of the E2-2A/B isoforms and E47/E12 factors; AD1, AD2: activation domain; bHLH: basic Helix-Loop-Helix domain (DNA binding domain). (B) Depiction of the binding of active heterodimer (class bHLH/class II bHLH) (left) and inactive heterodimer (class I bHLH/class V HLH/Id) to the E-box (CANNTG) element.
E2-2 is expressed in different tissues such as brain, muscle, liver, lung, testicle, trophoblasts and mammary gland.13,14 Homozygous TCF4 deletion in mice lead to early lethality of unknown reason,15 suggesting an essential role for E2-2 factors in development. In addition, genetic studies have recently demonstrated that loss of one copy of TCF4 in human causes the Pitt-Hopkins syndrome,16–18 a neurodevelopment disease characterized by mental retardation, seizures and hyperventilation,19 supporting an essential role for E2-2 factors in human nervous system development. In fact, it has been observed that E2-2 forms functional heterodimers with the bHLH factors HASH-1 and MATH1, involved in development of specific parts of the central and peripheral nerves.20,21 Nevertheless, the specific cellular roles of E2-2 factors remain elusive.
E2-2 factors have been also implicated in T-lymphocyte and B-cell differentiation,13,14 in the transition from proliferation to differentiation in the mammary gland of pregnant mice22 and more recently, as specific regulators of Pasmacytoid dendritic cell (PDC) development (a dendritic cell type specialized in anti-viral response) and the PDC-dependent interferon response in mice and human.23
In pathologies such as cancer, TCF4 expression has been found upregulated in human cancers with β-catenin defects helping to promote growth and/or survival of cancer cells.24 More recently, we have shown that expression of E2-2A/B in epithelial cells represses E-cadherin expression and drive a full epithelial-to-mesenchymal transition (EMT).25
EMT is a complex process whereby cells lose cell-cell interactions and other epithelial properties whilst acquiring a migratory and mesenchymal phenotype.26 EMT occurs in different biological situations involving cell migration or invasion, such as in normal embryonic development and wound healing, but also in pathologies like cancer and organ fibrosis.26–29 A hallmark of EMT is the functional down-regulation of the cell-cell adhesion protein E-cadherin.26,30 Diminished E-cadherin expression has been shown to correlate with increased invasiveness and metastatic potential28,31,32 and to be the rate limiting step in the transition from adenoma to carcinoma in pancreatic cancer models.33
Studies from our group and other laboratories have led to the identification of several E-cadherin transcriptional repressors and EMT-inducers, such as the zinc finger factors Snail1 (Snail) and Snail2 (Slug), the two-handed zinc finger and homeobox proteins SIP1/ZEB2 and δEF1/ZEB1, and the bHLH regulators E12/E47 and Twist (reviewed in ref. 32).
Here, we discuss the role of TCF4 products in the maintenance of the mesenchymal phenotype, their interplay with other E-cadherin repressors in the regulation of EMT and their implication in human malignancies.
E2-2 as a New Player in EMT and its Relation to Other EMT Inducers
Our recent data have provided evidence that E2-2 factors act as potent EMT inducers when overexpressed in MDCK cells. EMT-mediated by E2-2 holds all “essential” markers of EMT, mainly downregulation of E-cadherin and other epithelial markers (like plakoglobin), induction of mesenchymal markers, N-cadherin, vimentin and fibronectin and acquisition of a motile and highly invasive phenotype.25 Although the phenotype of MDCK-E2-2 cells is very similar to that previously reported for MDCK-E47 cells, expressing the closely related class I bHLH E-47,34 and for MDCK-Snail1 and MDCK-Snail2 cells,35,36 important differences were detected in the biological/tumorigenic behavior between the different cell lines. Thus, E2-2 expression does not induce a tumorigenic behavior,25 in contrast to the effect of Snail1, Snail2 and E47 in MDCK cells,34,35,37 whilts MDCK-E2-2 cells exhibit a stronger invasive behavior that MDCK-E47 cells.25,38 In agreement with these observations, genomewide expression analysis indicated important differences in the gene expression profile induced by both types of E-proteins and Snail factors.25,37 Interestingly, the gene profiling studies also showed that E2-2 is downstream of Snail1, Snail2 and E47 in the MDCK cell system,37 and further confirmed by RT-PCR analysis.25 On the other hand, although a complete suppression of E-cadherin promoter transcriptional activity is found in MDCK-E2-2 cells, this appears to be independent of E-boxes in the proximal promoter. Indeed, E2-2B is not bound to endogenous E-cadherin promoter, in contrast to Snail1 or E47 factors25 (Cubillo, Cano A, et al. manuscript in preparation), indicating that, at least, E2-2B is an indirect E-cadherin repressor.
Taken together, the above data indicate that different EMT inducers, belonging to E proteins and Snail factors, can play differential roles in the regulation of EMT and, more importantly, in the biological consequences of the process. Furthermore, they suggest the existence of epistatic and/or mechanistic links between different EMT regulators.32 In a search for the specific function of E2-2 factors and their relation to other EMT regulators, knockdown studies of TCF4 were performed in MDCK cells stably expressing Snail1, E47 or E2-2 factors. Surprisingly, specific silencing (up to 70–80%) of ectopic E2-2 in MDCK-E2-2 cells or endogenous E2-2 factors overexpressed in MDCK-Snail1 and MDCK-E47 cells did not led to upregulation of E-cadherin or to restoration of an epithelial-like phenotype,25 indicating that TCF4 expression is dispensable for the maintenance of the mesenchymal phenotype once EMT has been established by Snail1, E47 or E2-2 factors, at least in the MCDK system. This contrast to the effect of Snail1 or E47 knockdown in MDCK-Snail1,39 or MDCK-E47 cells (Cubillo, Cano A, et al. manuscript in preparation) which lead to a complete reversion of E-cadherin expression and the epithelial phenotype. This also raises the question of whether other downstream targets could be involved in the maintenance of the mesenchymal phenotype in E2-2 expressing cells. Interestingly, ZEB1 and Twist1 factors are found downstream of Snail1, E47 and E2-2 in MDCK cells and other cell systems.25,37,40
ZEB1 and Twist1 are also potent inducers of EMT,41,42 although the hierarchical relation between the different factors is still unclear. In addition, whether Twist1 is a direct repressor of E-cadherin has not been so far demonstrated.29,41 In Drosophila, Twist has been shown to be upstream of Snail43 and a recent report also supports a similar epistatic relation beween Twist1 and Snail1 in rat kidney cells;44 however, this epistatic relation has not been so far demonstrated in other systems. Indeed, our own results indicate that Twist1 is downstream of Snail1/Snail2, E47 and E2-2 factors,25,37 suggesting that the epistatic relation between Twist1 and other EMT inducers might be context dependent. The present evidence lead us to propose a model for the hierarchical relation between different EMT inducers, initially described in Peinado et al.32 and further extended in the scheme presented Figure 2A. The diagram shows the different interrelations between Snail and bHLH factors derived mainly from data obtained from the MDCK model system at mRNA level. Snail1, Snail2 and E47 upregulate E2-2 expression; E47 and Snail2 upregulate each other, while Snail1, E47 and E2-2 upregulate ZEB1 expression25,37,40 (Cubillo, Cano A, et al. unpublished data). On the other hand, Twist1 is upregulated by E47 and E2-2 and to a lower extent by Snail1 and Snail2.25,37 Whether Twist1 can also induce Snai1 factors in MCDK cells, as described in rat kidney cells,44 remains to be explored.
Figure 2.
Diagrammatic representation of the proposed hierarchical relations between different EMT inducers. (A) Model based in the analysis of gene expression profiles and RT-PCR in MDCK cells stably expressing Snail1, Snail2, E47 and E2-2 (solid arrows) (refs. 25 and 37); broken arrows indicate relations established at the mRNA level in other cell systems (refs. 40 and 46). (B) Model based in the analysis of the activity of the human Snail1, Snail2 and E2-2A promoters in MDCK cells after transient expression of Snail1, Snail2 or E2-2A factors.
Further evidence for a cross-talking regulation among Snail and bHLH factors is provided by promoter analysis. Our initial studies indicate that Snail1 and Snail2 upregulate E2-2A promoter by still undefined mechanisms, while E2-2 factors downregulate human Snail1 and Snail2 promoters as well as the E2-2A promoter (Sobrado, Cano A, et al. unpublished data), suggesting a delicate and intricate balance in the regulation of various EMT inducers (Fig. 2B). In contrast, E47 is apparently not able to regulate the E2-2A promoter, and E2-2 factors have a very faint effect on the E2A promoter. Interestingly, Snail1 has also been reported to downregulate its own promoter in different epithelial cell systems,45 further reinforcing the fine-tune regulation of various EMT regulators.
E2-2 in Human Malignancies
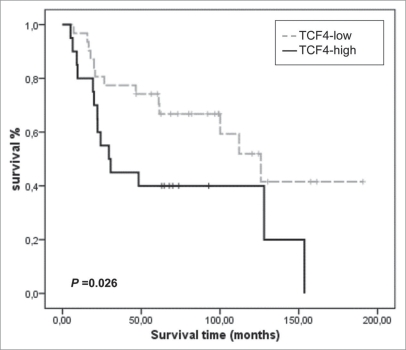
The above discussed data support a role for E2-2 factors in EMT regulation, and suggest their involvement in human malignancies. Scarce data on expression of E2-2/TCF4 in human tumors are already available. E2-2 was detected as a downstream target of β-catenin/Lef in human cancers with β-catenin defects.24 A recent report has detected E2-2/TCF4 as one of the four major upregulated genes in variant bone metastatic cells established from a human lung carcinoma cell line.46 Importantly, in this later study TCF4 was confirmed as one of three factors sufficient to confer bone metastatic ability to parental lung carcinoma cells,46 supporting an important role for E2-2 factors in bone metastasis. Whether this situation can be extended to other carcinoma and metastatic cell lines and tumors remains to be explored. More importantly, analysis of E2-2 proteins in human tumors (primary and metastasis lessions) should be performed once reliable antibodies are available. Meanwhile, we have interrogated public database of gene expression in lung squamous cell carcinomas47 for TCF4 mRNA expression and its potential relation with other EMT regulators. As shown in Figure 3, a statistical significant association was found between TCF4 expression and decreased overall survival. These data, together with those reported in lung carcinoma cells,46 support an important and so far unrecognized role for E2-2 factors in lung cancer. Interestingly, a previous similar survey of ZEB1 (TCF8) expression in public databases and correlation with clinico-pathological features showed an strong association of TCF8 expression with metastasis and low overall survival of N0 breast carcinomas, as well as a direct association with Snail2 expression.28 Together, these observations support the participation of the EMT inducers, E2-2 and ZEB1 factors, in human malignancies and support the hierarchical relation between both factors proposed from studies on cell systems (Fig. 2A).
Figure 3.
Kaplan-Meier analysis of TCF4/E2-2 expression in lung squamous cell carcinomas from public microarray dataset (n = 51; ref. 47). High (black) and low (grey) expression values of TCF4 mRNA were obtained from the average expression ratio. p value was derived from log-rank tests.
Further functional studies on dedicated cell model systems and development of transgenic cancer mouse models in which different EMT inducers are modified will be instrumental for establishing the functional role of E2-2 and other EMT factors and their interrelations in tumor progression. We also envision that these studies will provide new avenues and novel targets for anti-tumor and anti-metastatic strategies.
Acknowledgements
We thank G. Moreno-Bueno for analysis of public databases. Work at the A. C.’s lab is supported by grants from the Spanish Ministry of Education and Science (SAF2004-00361; SAF2007-63051; Consolider Ingenio 2010/26102) and the EU (MRTN 2004-005428).
Abbreviations
- EMT
epithelial-mesenchymal transition
- HLH
helix-loop-helix
- bHLH
basic helix-loop-helix
- Id
inhibitor of differentiation
- MDCK
madin darby canine kidney
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/9995
References
- 1.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eukaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerjanc IS, Truong J, Filion P, McBurney MW. A splice variant of the ITF-2 transcript encodes a transcription factor that inhibits MyoD activity. J Biol Chem. 1996;271:3555–3561. doi: 10.1074/jbc.271.7.3555. [DOI] [PubMed] [Google Scholar]
- 3.Petropoulos H, Skerjanc I. Analysis of the inhibition of MyoD activity by ITF-2B and full-lengh E12/E47. J Biol Chem. 2000;275:25095–25101. doi: 10.1074/jbc.M004251200. [DOI] [PubMed] [Google Scholar]
- 4.Bayly R, Chuen L, Currie RA, Hyndman BD, Casselman R, Blobel GA, et al. E2A-PBX1 interacts directly with the KIX domain of CBP/p300 in the induction of proliferation in primary hematopoietic cells. J Biol Chem. 2004;279:55362–55371. doi: 10.1074/jbc.M408654200. [DOI] [PubMed] [Google Scholar]
- 5.Lemercier C, To RQ, Carrasco RA, Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J. 1998;17:1412–1422. doi: 10.1093/emboj/17.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang A, Kim MS, Yanagisawa J, Takeyama KI, Kato S. Transrepression by liganded nuclear receptor via bHLH activator co-regulator switching. EMBO J. 2004;23:1598–1608. doi: 10.1038/sj.emboj.7600157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zhang J, Kalkum M, Yamamura S, Chait BT, Roeder RG. E protein silencing by the leukemogenic AML1-ETO fusion protein. Science. 2004;305:1286–1289. doi: 10.1126/science.1097937. [DOI] [PubMed] [Google Scholar]
- 8.Goardon N, Lambert JA, Rodriguez P, Nissaire P, Herblot S, Thibault P, et al. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 2006;25:357–366. doi: 10.1038/sj.emboj.7600934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- 10.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Henthorn P, Kiledjian M, Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa2 motif. Science. 1990;247:467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- 12.Furumura M, Potterf SB, Toyofuku K, Matsunaga J, Muller J, Hearing VJ. Involvement of ITF2 in the transcriptional regulation of melanogenic genes. J Biol Chem. 2001;276:28147–28154. doi: 10.1074/jbc.M101626200. [DOI] [PubMed] [Google Scholar]
- 13.Bergvist I, Eriksson M, Saarikettu J, Eriksson B, Corneliussen B, Grundstrom T, et al. The basic helix-loop-helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol. 2000;30:2857–2863. doi: 10.1002/1521-4141(200010)30:10<2857::AID-IMMU2857>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Wikstrom I, Forssell J, Goncalves M, Colucci F, Holmberg D. E2-2 regulates the expansion of pro-B cells and follicular versus marginal zone decisions. J Immunol. 2006;177:6723–6729. doi: 10.4049/jimmunol.177.10.6723. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2 and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockschmidt A, Todt U, Ryu S, Hoischen A, Landwehr C, Birnbaum S, et al. Severe mental retardation with breathing abnormalities (Pitt-Hopkins syndrome) is caused by haploinsufficiency of the neuronal bHLH transcription factor TCF4. Hum Mol Genet. 2007;12:1488–1494. doi: 10.1093/hmg/ddm099. [DOI] [PubMed] [Google Scholar]
- 17.Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Amer J Human Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt D, Hopkins I. A syndrome of mental retardation, wide mouth and intermittent overbreathing. Aust Paediatr J. 1978;14:182–184. doi: 10.1111/jpc.1978.14.3.182. [DOI] [PubMed] [Google Scholar]
- 20.Persson P, Jogi A, Grynfeld A, Pahlman S, Axelson H. HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex. Biochem Biophys Res Commun. 2000;274:22–31. doi: 10.1006/bbrc.2000.3090. [DOI] [PubMed] [Google Scholar]
- 21.Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc Natl Acad Sci USA. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itahana Y, Piens M, Fong S, Singh J, Sumida T, Desprez PY. Expression of Id and ITF-2 genes in the mammary gland during pregnancy. Biochem Biophys Res Commun. 2008;372:826–830. doi: 10.1016/j.bbrc.2008.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, et al. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with β-catenin defects and promotes neoplastic transformation. Cancer Cell. 2002;1:145–155. doi: 10.1016/s1535-6108(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 25.Sobrado VR, Moreno-Bueno G, Cubillo E, Holt LJ, Nieto MA, Portillo F, et al. The class I bHLH factors E2-2A and E2-2B regulate EMT. J Cell Sci. 2009;122:1014–1024. doi: 10.1242/jcs.028241. [DOI] [PubMed] [Google Scholar]
- 26.Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nature Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 27.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 33.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, et al. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276:27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- 35.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 36.Bolós V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 37.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 38.Peinado H, Marin F, Cubillo E, Stark HJ, Fusenig N, Nieto MA, et al. Snail and E47 repressors of E-cadherin induce distinct invasive and angiogenic properties in vivo. J Cell Sci. 2004;117:2827–2839. doi: 10.1242/jcs.01145. [DOI] [PubMed] [Google Scholar]
- 39.Olmeda D, Jordá M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumor growth and invasiveness. Oncogene. 2007;26:1862–1874. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- 40.Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–39216. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 43.Ip YT, Park RE, Kosman D, Bier E, Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992;6:1728–1739. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- 44.Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiró S, Escrivà M, Puig I, Barberò MJ, Dave N, Herranz N, et al. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–2084. doi: 10.1093/nar/gkl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicent S, Luis-Ravelo D, Antón I, García-Tuñon I, Borrás-Cuesta F, Dotor J, et al. A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res. 2008;68:2275–2285. doi: 10.1158/0008-5472.CAN-07-6493. [DOI] [PubMed] [Google Scholar]
- 47.Larsen JE, Pavey SJ, Passmore LH, Bowman R, Clarke BE, Hayward NK, et al. Expression profiling defines a recurrence signature in lung squamous cell carcinoma. Carcinogenesis. 2007;28:760–766. doi: 10.1093/carcin/bgl207. [DOI] [PubMed] [Google Scholar]