Abstract
Neuropilins are highly conserved single pass transmembrane proteins specific to vertebrates. They were originally identified as adhesion molecules in the nervous system, but were subsequently rediscovered as the ligand binding subunit of the class 3 semaphorin receptor in neurons and then as blood vessel receptors for the vascular endothelial growth factor VEGF. More recently they have also been implicated as mediators of the T-cell immune response and as key prognostic markers in several types of cancer. Because neuropilins bind multiple ligands and associate with several different types of co-receptors, they variably promote cell adhesion, repulsion or attraction. Which response they ultimately invoke is decided by the cellular and even subcellular context the neuropilins find themselves in. Here, we review how the developmental functions of the neuropilins are influenced by such different contexts.
Key words: neuropilin, semaphorin, VEGF, plexin, neuron, endothelial cell, blood vessel, angiogenesis, cell migration
Introduction
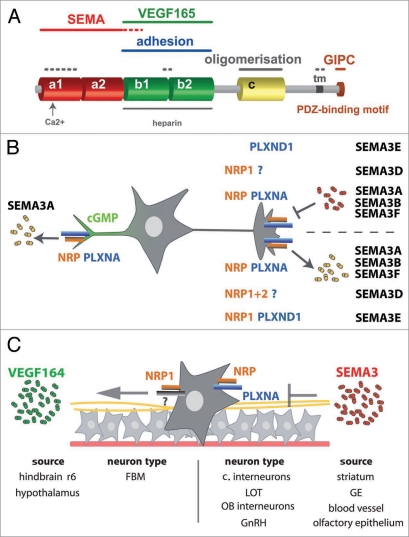
Neuropilins are single pass transmembrane proteins that are conserved in the vertebrate lineage. There are two neuropilins, neuropilin 1 (NRP1) and neuropilin 2 (NRP2), and they are 44% similar at the amino acid level.1 NRP1 and NRP2 have an identical domain structure, comprised of a large N-terminal extracellular domain (835 amino acid residues [aa] for NRP1, 844 aa for NRP2), a short membrane-spanning domain (23 aa for NRP1, 25 for NRP2) and a small cytoplasmic domain (44 aa for NRP1, 42 for NRP2). The extracellular domain contains two complement-binding homology domains, termed a1 and a2, two coagulation factor V/VIII homology domains, termed b1 and b2, and a meprin domain, termed c (Fig. 1A). The a- and b-domains are crucial for ligand binding, whilst the c domain and transmembrane domain promote receptor dimerisation; the intracellular domain contains a motif that binds PDZ-domain containing proteins (Fig. 1A). Owing to this complex domain structure, neuropilins can engage in many different types of signaling pathway to control cell migration.
Figure 1.
Neuropilin signalling in neuronal cell migration. (A) organisation and ligand binding. SEMA3-binding requires the a1/a2 domain and VEGF165-binding the b1/b2 domain; the b1/b2 domain also contains a binding site for heparan sulfate and the adhesion domain. The three last amino acid residues of the cytoplasmic domain (SEA) confer binding to the PDZ domain-containing protein GIPC1/synectin. (B) SEMA3 proteins bind to neuropilins, which interact with plexins to control axon and dendrite extension, except SEMA3E, which interacts directly with PLXND1 (right hand side). In most neurons, SEMA3 signals are repulsive (top right), but in some types of neurons, repulsion is converted to attraction (bottom right), for example when NRP2 interacts with the NRP1/PLXNA complex to mediate SEMA3B signalling, or when NRP1interacts with PLXND1 to mediate SEMA3e signalling. SEMA3D acts as a repulsive signal through NRP1 in association with an as yet unidentified co-receptor, but as an attractive signal when both and NRP2 are present. A high intracellular cGMP concentration in the dendrites of some types of neurons converts a repulsive SEMA3A response into an attractive one (left hand side). (C) Within the CNS, neuropilins guide tangentially migrating neurons or their progenitors along axons (yellow), other neurons (grey) or unknown substrates, perhaps blood vessels (red), which all have been implicated as sources of SEMA3 proteins. VEGF164 rather than SEMA3 attracts the NRP1-expressing cell bodies of FBM neurons. The neuron type and the source of the attractive or repulsive NRP ligand are shown below the schematic. Abbreviations: FBM, facial branchiomotor neurons; LOT, lateral olfactory tract neurons; GE, ganglionic eminence; OB, olfactory bulb.
Neuropilins Associate with Plexins to Mediate Semaphorin Signaling
There are presently seven class 3 semaphorins known, termed SEMA3A-G.2 Amongst the different neuropilin functions, the mechanism of SEMA3-mediated repulsion of axonal growth has been studied in most detail.1 Thus, growth cone collapse is initiated when SEMA3 proteins bind to neuropilin that is in a complex with a signal-transducing transmembrane protein of the A-type plexin family (Fig. 1B). Whereas the A-type plexins (PLXNA) recruit a neuropilin as the ligand binding subunit for SEMA3 proteins, other plexins such as plexin D1 (PXND1) bind semaphorins directly.2 Plexins are able to activate several different intracellular signal transduction pathways that control cytoskeletal dynamics; for example, they act via RHO and RAS to modulate actin dynamics and via CDK5 to inhibit the interaction of CRMP with tubulin, and they can also activate intracellular kinases, such as SRC, PI3K and MAPK.2–4 Adding a further level of complexity, several tyrosine kinases can phosphorylate plexins to regulate intracellular signaling downstream of semaphorins.5 Finally, the adhesion molecule L1-CAM cooperates with neuropilin/plexin signaling in some types of neurons to activate the focal adhesion kinase FAK and therefore promote focal adhesion disassembly.6
Neuropilins and Plexins Cooperate to Confer Specificity to Semaphorin Signaling
SEMA3C can bind both neuropilins,7 but other SEMA3 family members preferentially bind NRP1 or NRP2. The best-studied examples of preferential neuropilin binding are provided by SEMA3A and SEMA3F during axon guidance. Thus, axons expressing NRP1 primarily respond to SEMA3A, whereas those expressing NRP2 preferentially react to SEMA3F.8–12 Importantly, both neuropilin pathways often cooperate to control the behavior of axons. For example, SEMA3A/NRP1 controls the timing of axon invasion into the limb as well as fasciculation, whilst SEMA3F/NRP2 guides a subset of motor axons within the ventral limb.13 Just as SEMA3 proteins prefer to bind specific neuropilins, the neuropilins preferentially associate with specific plexins. In particular, PLXNA4 appears to be the preferred co-receptor for NRP1 to mediate SEMA3A responses, and PLXNA3 the preferred NRP2 co-receptor for SEMA3F signaling.14–16 For example, axon pruning in the infrapyramidal tract is mediated by SEMA3F, NRP2 and PLXNA3 whilst the visceromotor branch of the facial nerve (FVM) relies on SEMA3A, NRP1 and PLXNA4, to organize its axons.10–12,17,18 The axons of the branchiomotor component of the facial nerve (FBM) are similarly affected when either SEMA3A/NRP1/PLXNA4 or SEMA3F/NRP2/PLXNA3 are lost. Because the FBM axons are most disorganized in mutants lacking semaphorin signaling through both neuropilins or both plexins, it is likely that the SEMA3A and SEMA3F pathways operate in parallel in this nerve.17,23 Despite the usual preference of NRP1 for PLXNA4 and NRP2 for PLXNA3, these pairings are not obligatory: Even though trochlear axons are guided by SEMA3F/NRP2, and not SEMA3A/NRP1 signaling,10,11 PLXNA4 can compensate for loss of PLXNA3; the loss of both plexins is therefore required to phenocopy the defect caused by loss of SEMA3F.14
Repulsion versus Attraction
Whilst SEMA3 proteins are best known for their role as axon repellents, they act as chemoattractants in other circumstances (Fig. 1B). This was first shown for cortical pyramidal neurons, in which SEMA3A is a chemoattractant for apical dendrites, but a chemorepellent for axons.19 SEMA3F can also be attractive, as demonstrated for cerebellar granule cell axons.20 In both types of positive guidance events, raised intracellular cyclic GMP levels are thought to switch the repellent semaphorin signal into an attractive one (Fig. 1B). In the case of cortical apical dendrites, elevated cyclic GMP levels were attributed to the asymmetric localisation of soluble guanylate cyclase in dendrites versus axons.19
Different mechanisms for converting repulsive to attractive signals operate for other class 3 semaphorins (Fig. 1B). In zebrafish, NRP2 can convert NRP1-induced chemorepulsion into chemoattraction. Thus, SEMA3D repels NRP1-expressing axons of the medial longitudinal fasciculus, but attracts axons that form the anterior commissure, which express both NRP1 and NRP2.21 SEMA3D binds NRP1, but it is not yet known if SEMA3D also binds NRP2 independently of NRP1, or if NRP1 and NRP2 form repulsive heterodimers. SEMA3B also possesses dual functionality, as it repels commissural axons that connect the two olfactory bulbs in the mouse, but attracts commissural axons that connect the temporal lobes. Instead of recruiting a plexin into the receptor complex, NRP2 mediates this response by interacting with the cell adhesion molecule NrCAM, and the switch in attractive versus repulsive signaling by SEMA3B is mediated by FAK/SRC signaling.22 Finally, SEMA3E serves either as an attractive or repulsive cue for PLXND1-expressing axons depending on the presence of NRP1. Thus, Sema3E acts as a repellent for two types of axons that contain PLXND1, but not NRP1: the corticofugal axons, which connect the cortex and brainstem, and the striatonigral axons, which connect the striatum to the substantia nigra.23 This is possible, because SEMA3E is the only class 3 semaphorin that does not bind to a neuropilin, but directly to a plexin, PLXND1.24 However, NRP1 can convert SEMA3E/PLXND1-mediated repulsion into attraction, as in the case of subiculo-mammillary neuron axons, which project from the hippocampus to the hypothalamus.23
VEGF Isoforms in Neuropilin-Mediated Cell Migration
Alternative splicing of the mRNA encoding VEGF-A gives rise to a collection of isoforms, which differ by the presence or absence of specific domains that confer heparin sulfate and neuropilin binding in vitro. NRP1 binds VEGF165 and NRP2 binds both VEGF165 and the smaller VEGF145.25,26
VEGF121 is generally believed not to bind NRP1, but a recent report has contested this idea.27 The role of NRP1 in VEGF signaling was originally studied in porcine cells derived from aortic endothelium (PAE). These cells lack expression of both NRP1 and the main endothelial VEGF receptor, the tyrosine kinase VEGFR2 (also known as KDR or FLK1); they can therefore be engineered to selectively express NRP1, VEGFR2 or both together.25 In this model of blood vessel endothelium, NRP1 enhances VEGR2-mediated chemoattraction toward VEGF165, but NRP1 itself does not promote chemotaxis in PAE cells.25 In contrast, NRP1 may signal through its cytoplasmic tail in primary human umbilical vein endothelial cells.28
The precise mechanism of NRP1 action in vascular endothelium is not yet clear. Several hypotheses suggest that NRP1 interacts with VEGFR2 in cis to promote VEGF signaling. Thus, it has been suggested that NRP1 enhances the affinity of VEGF165 for VEGFR2,29 and that it enhances VEGF165 signaling by promoting VEGFR2 clustering30 or VEGFR2 endocytosis.31 Alternatively, or additionally, NRP1 may act on endothelium from a non-endothelial cell type in trans, as described for tumour cells30 and haematopoietic cells.32 In most cases, NRP1 has been implicated in enhancing VEGF-stimulated cell migration, rather than proliferation.25,33 However, when acting in trans, NRP1 may also induce endothelial cell proliferation.32
Observations on the patterning of FBM neurons have provided the first and so far only demonstration that NRP1 serves as a VEGF receptor to control cell migration in vivo. FBM neurons are born in the ventricular zone of a hindbrain segment termed rhombomere (r) 4, but move their cell bodies caudally towards the pial surface of r6 to form the paired facial motor nuclei. During their migration, FBM neurons first move tangentially, i.e., orthogonally to the brain surface. However, once they reach r6, they change their behavior to adopt a radial mode of migration towards the pial surface of the brain. FBM neurons express Nrp1 and Nrp2, and their semaphorin ligands are expressed in their environment.33 Analysis of knockout mice established that semaphorin signaling through NRP1 and NRP2 cooperatively controls the patterning of FBM axons (see above). Strikingly, VEGF164 (the murine equivalent of human VEGF165) and NRP1, rather than SEMA3 proteins, are important to guide the tangential migration of FBM cell bodies.33 During cell body guidance, VEGF164 appears to provide a chemoattractive signal for FBM cell bodies, as Vegfa is expressed in cells along the migration path and a stripe juxtaposed to the site of facial motor nucleus formation near the pial surface of r6; moreover, VEGF164-coated heparin beads attract FBM neurons in a hindbrain explant model (Fig. 1C).
VEGF164 versus SEMA3A in Neuropilin-Mediated Cell Migration
Based on findings in cultured PAE and neural progenitor cells, it was initially thought that SEMA3A competes with VEGF165 for binding to NRP1 and thereby inhibits cell migration.34,35 However, biochemical studies subsequently demonstrated that SEMA3A and VEGF165 associate with distinct domains of NRP1, and that they therefore do not compete with each other for binding to NRP1.36 Moreover, the analysis of FBM patterning suggested that NRP1 displays context-dependent ligand selectivity, as the cell bodies and axons of FBM neurons selectively respond to either VEGF164 or SEMA3A.33 The analysis of blood vessel formation and axon guidance in developing mouse limbs further supports the idea of ligand selectivity: Whereas VEGF164 is essential for blood vessel growth, SEMA3A controls axon behavior in the limb, but not vice versa.37 Accordingly, only the simultaneous loss of both VEGF164 and SEMA3A phenocopied the neurovascular limb defects caused by loss of NRP1.37 Whilst these findings suggest that NRP1-expressing cell types in vivo preferentially respond to either one or the other NRP1 ligand, it is presently not understood how this ligand selectivity is controlled. Possibilities include the differential expression of NRP1 co-receptors (i.e., plexins versus VEGF tyrosine kinase receptors) or a difference in extracellular co-factors (for example, different heparan sulfate proteoglycans).
Semaphorins in Neuropilin-Mediated Neuronal Migration
Even though VEGF164 is the important ligand in NRP1-mediated FBM cell body guidance, semaphorin signaling through neuropilins appears to contribute more commonly to the guidance of tangentially migrating neurons, as demonstrated for the precursors of GABAergic cortical interneurons and the lateral olfactory tract (LOT) neurons. Cortical interneuron progenitors are essential regulators of cortical activity. They are born in the ganglionic eminence (GE) of the subpallium, but leave their birthplace to migrate around the striatum into the cortex in a mechanism that is thought to require repulsive SEMA3 signaling through both neuropilins.38 In support of this idea, SEMA3A and SEMA3F are normally expressed in the developing striatum and repel cortical interneurons in vitro, and an excessive number of neurons enters the striatum in Nrp2-null mutants, because the neurons fail to respond to repulsive SEMA3F signals. However, Nrp1-null mice have not yet been examined for defects in cortical interneuron migration.
Semaphorin signaling has also been implicated in the positioning of interneurons within their correct neocortical layer.39 Thus, SEMA3F was proposed to guide NRP2-expressing interneurons into the intermediate zone, whilst SEMA3A is thought to guide NRP1-expressing interneurons into the subplate, cortical plate and marginal zone. However, the functional requirement for semaphorin/neuropilin signaling in this sorting process has not yet been established in vivo. It would also be interesting to investigate if neuropilin-binding VEGF isoforms contribute to interneuron patterning, as observed in the case of FBM neurons.
LOT neurons migrate in the opposite direction of cortical interneurons: they are born in the embryonic neocortex, but migrate tangentially along the brain surface towards the GE. When they reach the interface of the neocortex and GE, they change direction and spread laterally along the boundary to form guidepost cells for lateral olfactory tract axons. In Sema3f- and Nrp2-null mutants, many LOT neurons are found in their normal position, but others continue to migrate towards the GE.40 Because the mantle layer of the GE expresses SEMA3F, it seems likely that semaphorin signaling helps to create a repulsive border for NRP2-expressing neurons at the GE/neocortical boundary.40 A role for SEMA3A or NRP1 in LOT migration has not yet been described.
Neuropilin-mediated signaling also guides the migration of cortical neurons from their birthplace in the ventricular zone along radial glial fibers to the superficial cortical layers. Thus, SEMA3A acts as an essential chemoattractant for these neurons, and they therefore settle prematurely in deep cortical layers after knockdown of NRP1; PLXNA2 and PLXNA4 are thought to serve as co-receptors for NRP1 in this process.41 This radial migration defect precedes the previously discovered defect in apical dendrite extension from cortical neurons, but may be causally related (see above).41
Finally, neuronal progenitors born outside the central nervous system (CNS) also use semaphorin/neuropilin signaling to position themselves in strategic places, for example the gonadotropic releasing hormone (GnRH)-neurons that regulate reproductive behavior, and the neural crest cell (NCC) precursors of sensory and sympathetic neurons. GnRH neurons are born in the olfactory placode and migrate along olfactory nerves first into the forebrain and then into the hypothalamus, where they stimulate gonadotropin release from the pituitary gland into the circulation.42 Adult Nrp2-null mice have fewer GnRH neurons in the hypothalamus, and this is accompanied by infertility.43 Because Sema3f- and Nrp2-null mice have defasciculated olfactory nerves,44,45 the GnRH defect is at least in part due to abnormal cell migration on a defective axonal substrate. However, GnRH neurons themselves also express NRP2, and they are repelled by SEMA3F in vitro.43 This raises the possibility that SEMA3F/NRP2 signaling may play a second, more direct role in GnRH neuron guidance. Finally, GnRH neurons express NRP1 and respond to VEGF164 and SEMA3A in vitro,43 and loss of SEMA3A results in guidance errors of subsets of olfactory axons.46 However, the physiological significance of NRP1 signaling in GnRH patterning has not yet been investigated.
Neuropilins in Neural Crest Cell Migration
SEMA3C does not appear to play an obvious role in axon guidance or neuronal migration. Rather, it patterns neural crest cells (NCCs) in the cardiac outflow tract, a cell population that does not give rise to neurons, but instead promotes outflow tract septation into an arterial and venous compartment.47,48 SEMA3C binds to either NRP1 or NRP2 in vitro.7 Accordingly, outflow tract development is normal in mice lacking semaphorin-signaling through either NRP1 or NRP2, but loss of semaphorin-signaling through both receptors severely disrupts outflow tract development with lethal consequence.49 It has been suggested that SEMA3C affects cardiac NCCs that express PLXNA2, PLXND1 and neuropilins.47,50 In contrast, others have reported that SEMA3C acts in outflow tract endothelium through a receptor composed of a neuropilin and PLXND1.51 As neuropilins may also complex with PLXNA2 to guide cardiac NCCs through the pharyngeal arches before they reach the outflow tract,47 the best current model from all available data is that neuropilins helps to pattern the great vessels by guiding NCC migration through the pharyngeal arches via PLXNA2, but act as a SEMA3C co-receptors for PLXND1 in the outflow tract endothelium. It is presently not clear if neuropilins mediate attractive or repulsive signals to pattern the great vessels. To add further complexity, endothelial NRP1 and its ligand VEGF164 are also essential to pattern the outflow tract.49
Whilst the specific requirement for VEGF164 or class 3 semaphorins other than SEMA3C in cardiac NCC guidance has not yet been investigated, several recent studies confirm that neuropilins play a general and essential role in NCC migration. Thus, the NCC precursors of sensory and sympathetic neurons use neuropilin signaling to position themselves in the embryonic head and trunk in places appropriate for neuronal differentiation. In the head, a subset of cranial NCC emigrating from r4 are misguided anteriorly in Sema3a- and Nrp1-null mice,52 and Sema3f- and Nrp2-null mutants exhibit mild migration defects in several cranial NCC populations.53 Moreover, mutants lacking both pathways show fusion of the trigeminal and facial ganglia, and this leads to an intermingling of their sensory axons.52 In the trunk, SEMA3A/NRP1 signaling controls the switch of NCC migration from an early path through the intersomitic furrows onto a major route through the anterior somites,54 whereas SEMA3F/NRP2 signaling helps to restrict NCC migration to the anterior half of each somite.53 Because SEMA3A/NRP1 and SEMA3F/NRP2 signaling control distinct aspects of NCC guidance, mutants deficient in both pathways display severe defects in NCC guidance that lead to fusion of the dorsal root ganglia.55,56 The signaling co-receptors for NRP1 and NRP2 in semaphorin-mediated NCC guidance have not yet been identified, but they are not PLXNA3 or PLXNA4.57 Rather, these plexins control sympathetic neuronal patterning, once NCCs have begun to differentiate into neuronal progenitors.57
Outlook
We know now that many different types of neurons, neuronal progenitors and NCCs use neuropilin signaling to position their cell bodies or their cellular processes in the developing embryo. We are also beginning to understand how the clash between opposing semaphorin and VEGF signals is controlled at the cellular level, and we are uncovering the mechanisms that govern co-receptor choice and the activation of downstream signaling pathways. Future focus will most likely be directed on the role of neuropilins as regulators of endocytosis for several different cell surface receptors. Thus, intriguing new data suggest that the cytoplasmic NRP1 domain binds the protein GIPC (also known as synectin) to link neuropilin-containing vesicles to the transport machinery via myosin 6.58 This pathway may control the intracellular distribution of at least two NRP1 co-receptors, VEGFR2 and PLXND1,31,59 but it additionally provides an interface to integrins.60 The idea that neuropilins control the function not only of L1-CAM, but also integrins has brought us full circle to the original discovery of neuropilin as an adhesion molecule. So neuropilin, let me know, how do you decide if a cell should stay or go?
Abbreviations
- NRP
neuropilin
- SEMA3
class 3 semaphorin
- PLXN
plexin
- VEGF
vascular endothelial growth factor
- aa
amino acid residue
- NCC
neural crest cell
- CNS
central nervous system
- GE
ganglionic eminence
- LOT
lateral olfactory tract
- GnRH
gonadotropic releasing hormone
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10207
References
- 1.Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 9.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 10.Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- 11.Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–6680. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, et al. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 13.Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, et al. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48:949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45:513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25:3628–3637. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–263. doi: 10.1016/s0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz Q, Waimey KE, Golding M, Takamatsu H, Kumanogoh A, Fujisawa H, et al. Plexin A3 and plexin A4 convey semaphorin signals during facial nerve development. Dev Biol. 2008;324:1–9. doi: 10.1016/j.ydbio.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 19.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 20.Ding S, Luo JH, Yuan XB. Semaphorin-3F attracts the growth cone of cerebellar granule cells through cGMP signaling pathway. Biochem Biophys Res Commun. 2007;356:857–863. doi: 10.1016/j.bbrc.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 21.Wolman MA, Liu Y, Tawarayama H, Shoji W, Halloran MC. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci. 2004;24:8428–8435. doi: 10.1523/JNEUROSCI.2349-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 25.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 26.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected] J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, et al. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276:25520–25531. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 30.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 31.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, et al. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103:71–79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y, Oike Y, Ogawa H, Ito Y, Fujisawa H, Suda T, et al. Neuropilin-1 on hematopoietic cells as a source of vascular development. Blood. 2003;101:1801–1809. doi: 10.1182/blood-2002-01-0119. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, et al. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, et al. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 39.Tamamaki N, Fujimori K, Nojyo Y, Kaneko T, Takauji R. Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–248. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- 40.Ito K, Kawasaki T, Takashima S, Matsuda I, Aiba A, Hirata T. Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J Neurosci. 2008;28:4414–4422. doi: 10.1523/JNEUROSCI.0372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, et al. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 42.Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends Neurosci. 2007;30:638–644. doi: 10.1016/j.tins.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Cariboni A, Hickok J, Rakic S, Andrews W, Maggi R, Tischkau S, et al. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:2387–2395. doi: 10.1523/JNEUROSCI.5075-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walz A, Rodriguez I, Mombaerts P. Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci. 2002;22:4025–4035. doi: 10.1523/JNEUROSCI.22-10-04025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cloutier JF, Sahay A, Chang EC, Tessier-Lavigne M, Dulac C, Kolodkin AL, et al. Differential requirements for semaphorin 3F and Slit-1 in axonal targeting, fasciculation and segregation of olfactory sensory neuron projections. J Neurosci. 2004;24:9087–9096. doi: 10.1523/JNEUROSCI.2786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for guidance of olfactory axons in mice. J Neurosci. 2000;20:7691–7697. doi: 10.1523/JNEUROSCI.20-20-07691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 48.Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, et al. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 49.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Developmental Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, et al. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 51.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz Q, Vieira JM, Howard B, Eickholt BJ, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development. 2008;135:1605–1613. doi: 10.1242/dev.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz Q, Maden C, Vieira JM, Ruhrberg C. Neuropilin 1 signalling guides neural crest cells to coordinate pathway choice with cell specification. PNAS. 2009;106:6164–6169. doi: 10.1073/pnas.0811521106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz Q, Maden CH, Davidson K, Ruhrberg C. Neuropilin-mediated neural crest cell guidance is essential to organize sensory neurons into segmented dorsal root ganglia. Development. 2009;136:1785–1789. doi: 10.1242/dev.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development. 2009;136:1879–1888. doi: 10.1242/dev.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waimey KE, Huang PH, Chen M, Cheng HJ. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315:448–458. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci USA. 2006;103:12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prahst C, Heroult M, Lanahan AA, Uziel N, Kessler O, Shraga-Heled N, et al. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J Biol Chem. 2008;283:25110–25114. doi: 10.1074/jbc.C800137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]