Abstract
The glia that reside at the midline of the Drosophila CNS are an important embryonic signaling center and also wrap the axons that cross the CNS. The development of the midline glia (MG) is characterized by migration, ensheathment, subdivision of axon commissures, apoptosis, and the extension of glial processes. All of these events are characterized by cell-cell contact between MG and adjacent neurons. Cell adhesion and signaling proteins that mediate different aspects of MG development and MG-neuron interactions have been identified. This provides a foundation for ultimately obtaining an integrated picture of how the MG assemble into a characteristic axonal support structure in the CNS.
Key words: axon, cell adhesion, CNS midline, drosophila, midline glia, neurexin IV, wrapper
Drosophila CNS Midline Cells and the Function of Midline Glia
The insect CNS consists of a brain and ventral nerve cord. Each ganglion within the ventral nerve cord has 400–500 lateral CNS neurons/hemiganglion separated by a distinct set of CNS midline cells (Fig. 1A). Most axons extending from the lateral neurons cross the midline via two axon bundles (the anterior commissure and posterior commissure) where they join the axon connectives that unite the CNS along its longitudinal axis (Fig. 1B). The embryonic midline cells consist of 22 neurons and glia/ganglion, yet are quite diverse, consisting of MG, interneurons, motorneurons and neurosecretory cells.1 In this commentary, I will focus on the molecules and mechanisms that allow MG to migrate and interact with neurons and axons to form a functional ventral nerve cord.
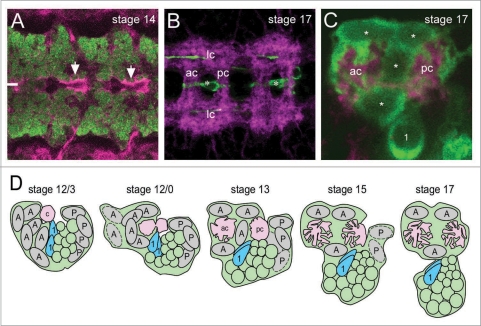
Figure 1.
(A) Horizontal view of a Drosophila stage 14 embryonic CNS showing neurons (anti-Elav; green) and MG (white line indicates midline) with Nrx-IV (magenta) localized at the MG-neuron interface (arrows). Two segments are shown. (B) Horizontal view of a stage 17 sim-Gal4 UAS-tau-GFP embryonic CNS showing the axon scaffold (MAb BP102, magenta) and location of MG (*; anti-GFP, green) ensheathing the axon commissures. Two segments are shown with longitudinal connectives (lc), anterior commissure (ac) and posterior commissure (pc) indicated. The prominent GFP+ axons (green) within the longitudinal connectives are from the Midline Precursor 1 (MP1) neurons. (C) Sagittal view of a stage 17 embryo showing 4 MG (*; anti-GFP, green) ensheathing the anterior (ac) and posterior (pc) commissures (axons stained with MAb BP102, magenta). Note the MG membrane projections that subdivide the commissures. MP1 neuron (1). (D) Sagittal view of MG development from stage 12/3 to stage 17, showing AMG (A), PMG (P), MP1 neurons (1, blue), additional midline neurons (green ovals), initial commissure (C, pink) at stage 12/3, and anterior commissures (ac, pink) and posterior commissures (pc, pink) at other stages. Cells depicted with dashed lines are apoptotic. Schematic depicts the migration of AMG around the commissures and their ensheathment and subdivision by MG processes. Figure is adapted from Wheeler et al. 2009, courtesy of the Company of Biologists Ltd.
The MG are a highly-specialized cell type that are molecularly, functionally and developmentally distinct from other insect glia. They carry-out a variety of important developmental and neuronal support roles. These include trophic support of commissural axons,2 cell signaling pathways that influence the formation and migration of nearby cell types,3–6 and production of Netrins and Slit that influence axon guidance.7,8 Most evident is their role in ensheathing the commissural axons that cross the CNS midline (Fig. 1C and D). The development of the MG-axon scaffold exemplifies coordinated development, since the axons are crossing the midline as the MG are migrating around and ensheathing them. During development, the MG initiate migration, move around the commissures, terminate migration and send projections into the commissures, which subdivides the crossing axons (Fig. 1D). The projection of MG membranes into the commissures may increase the likelihood that neurotrophic factors, such as Neurotrophin 1 (DNT1), Spätzle 5 (DNT2) and Spätzle, interact with crossing axons.2 In addition, commissural subdivision may facilitate correct pathway choices in the longitudinal connectives by helping to segregate axon fascicles. There are two distinct classes of MG; the anterior MG (AMG) and posterior MG (PMG) that reside in different segmental locations1,9 and differ in expression of several genes (most MG-expressed genes are expressed in both AMG and PMG).1,10 Only the AMG contribute to axonal ensheathment. The PMG all die during embryonic development. Their function is unknown, but one PMG cell remains in close apposition to posterior commissure axons and the others are in proximity to posterior midline neurons and the median neuroblast. Thus PMG could influence commissure formation and midline neuronal formation or differentiation. Key questions to be considered regarding MG-neuron interactions are: (1) how functionally similar are the midline cells of the insect ventral nerve cord and vertebrate spinal cord, (2) what are the molecules that control MG adhesion, migration, and ensheathment, and (3) how do MG communicate with axons and control axon guidance?
Similarities between Insect CNS Midline Cells and the Vertebrate Floorplate Cells
The vertebrate spinal cord and brain also have a specialized set of cells at the ventral midline, called the floorplate. There are good reasons to consider the insect and vertebrate midline cells as evolutionarily related. Both floorplate and insect midline cells consist of neurons and glia, and while the neurons have some intriguing similarities, it is the resemblance between the vertebrate non-neuronal floorplate cells and insect MG that is most convincing. Both cell types exist as a scaffold that ensheaths commissural axons,11,12 and both are important signaling centers. They are the source of attractive (Netrins) and repulsive (Slit) influences on midline-directed axon guidance.7,8 The floorplate is a source of Sonic hedgehog that patterns the spinal cord into distinct classes of neurons.13 The insect midline cells also influence the development of neurons in the lateral CNS,3,4 as well as the formation of the ventral epidermis14,15 and the dorsal median cells (these cells extend processes that act as motorneuron axon guidance substrata).5,6 Both morphologically and functionally, the insect CNS MG and vertebrate floorplate cells are similar; consequently, understanding the mechanisms that govern Drosophila MG development may inform understanding of floorplate development. In the case of human disease, defects in the establishment of the floorplate glial scaffold could result in abnormal development or maintenance of commissural axons, leading to neuropsychiatric or neurological disease.
Role of Cell Adhesion on Midline Glial Migration and Axonal Ensheathment
Cell adhesion is one of the key factors influencing MG migration and the interaction between MG with their surrounding neuronal and axonal environment. It is important to identify the adhesion molecules that mediate MG migration, and equally important to understand how adhesion is regulated, since modulation of adhesive interactions are presumably necessary for the migration and ensheathment events that occur. Recently, an important advance was made with the discovery that the Neurexin IV (Nrx-IV) and Wrapper proteins act as heterophilic adhesion proteins mediating MG-neuron adhesion and migration. Wrapper was reported in 1998 as a likely participant in MG-neuron adhesion.16 Wrapper is present in MG, and is a glycophosphatidylinositol-linked membrane protein containing three immunoglobulin superfamily domains and a single Fibronectin Type III domain. Analysis of wrapper mutant embryos revealed defects in MG migration and ensheathment of commissural axons. This phenotype was consistent with Wrapper playing a role in MG-neuron adhesion, but it took another 11 years for a neuronal-localized adhesion partner, Nrx-IV, to be reported.
Nrx-IV is a transmembrane protein containing 4 extracellular Laminin G domains, 2 EGF domains, and a Discoidin domain, and is present on the surface of neuronal cell bodies and axons.17,18 Nrx-IV mutants show identical MG migration and ensheathment defects as wrapper mutants. Strikingly, Nrx-IV protein accumulates at the interface between MG and neurons in wild-type embryos (Fig. 1A). This accumulation is dependent on the presence of Wrapper on the surface of MG, since Nrx-IV midline accumulation is absent in wrapper mutants. Furthermore, wrapper misexpression results in accumulation of Nrx-IV on the surface of adjacent cells, and elicits extension of MG along axon tracts over the Wrapper+ cells. Consistent with the embryonic experiments, when tissue culture cells transfected with wrapper and Nrx-IV were mixed, they adhered to one another and formed large aggregates. Nrx-IV is present as two splice variants that possess alternative Discoidin domains.17 One variant is present in epidermal cells, which form septate junctions (these structures contain Nrx-IV), and the other variant is present in neurons. Interestingly, the neuronal Nrx-IV variant interacts preferentially with Wrapper+ cells, indicating a specialization of Nrx-IV Discoidin domains for adhesive interactions. In summary, Nrx-IV and Wrapper act as heterophilic cell adhesion proteins and strongly influence MG migration and interactions of MG with neurons and axons.
Nrx-IV is well-conserved in vertebrates (Caspr/Paranodin), and there are interesting parallels between insect and vertebrate Nrx-IV.19 Caspr is localized to the surface of axons in the region adjacent to the node of Ranvier. These paranodal regions interact with loops of myelin that join to axons via septate-like junctions. Caspr mutants result in mice with defects at the paranodal junctions, including disorganization of the paranodal myelin loops and loss of the septate-like junctions.20 These defects are consistent with a role for Caspr in mediating glial-axon adhesion. The Caspr axon-glial contacts involve interactions with additional Ig superfamily proteins, including Neurofascin and Contactin.19 However, these proteins are weakly related to Wrapper, which has no clear ortholog in vertebrates. The molecular mechanisms that guide floorplate glial-axon interactions are unknown, but given the strong similarities between insect midline cells and vertebrate floorplate cells, it will be interesting to learn whether Caspr, Ig superfamily proteins, or other proteins described below are involved in floorplate development and glial-axon interactions.
Regulation of MG-Neuron Adhesion
One key issue concerns how MG-neuron adhesion is modulated during migration and ensheathment. MG-MG and MG-neuron interactions, including MG adhesion with the midline MP1 neurons and neurons adjacent to the midline,18 may be necessary to restrict MG migration to the midline. In addition, several signaling pathways likely play significant roles in MG-neuron interactions. These are reviewed below.
Egfr Signaling
Recent work suggests that signals emanating from neurons may directly modulate levels of Wrapper via the Spitz signaling pathway.21 Drosophila Spitz is an EGF-like ligand present on the membranes of neurons and axons that promotes MG survival by interacting with the EGF receptor (Egfr) present on the surface of MG.22 Mutant and misexpression experiments indicated that Spitz signaling upregulates wrapper transcription.21 The ability of ectopically-expressed spitz to activate wrapper expression suggests that spitz controls wrapper expression independently of spitz’s role in MG survival. This regulation may be direct since the wrapper MG enhancer has a site required for in vivo expression that resembles an ETS transcription factor binding site, and the Pointed ETS transcription factor mediates the transcriptional output of spitz signaling.23 Thus, Wrapper adhesion and Spitz signaling may function in a positive feedback loop to promote MG survival and migration: Wrapper-mediated adhesion is necessary for MG to receive a survival signal from axons, and Spitz in turn upregulates Wrapper levels to further promote adhesion and survival.
Pvr Signaling
The Pvf/Pvr pathway (homologous to the vertebrate PDGF and VEGF pathways) consists of the Pvr receptor and three ligands: Pvf1, 2, 3. Just as vertebrate PDGF influences oligodendrocyte migration and VEGF influences endothelial cell and macrophage migration, the Drosophila Pvr pathway is implicated in hemocyte24 and ovarian border cell migration.25 Pvr is present in MG and the ligands are differentially expressed in other midline-localized cell types, including midline neurons.26 Mutant and misexpression experiments of the receptor, Pvr, indicated that it is required for proper MG migration, number and morphology.26 It is also possible that Pvr may influence Wrapper levels. The Pvf ligands are present in different midline cells, and appear to play distinct roles in MG development. Misexpression of pvf2 outside the midline results in MG projections and migration towards the Pvf2 source, suggesting that Pvf2 is gliatropic. This is not observed with misexpression of pvf1, and mutants in pvf1 affect early MG migration, whereas a pvf2/3 double mutant affects later MG development, but not early migration. Thus, the Pvr pathway may play an instructive role in guiding the MG to their appropriate positions within the midline, as well as additional aspects of MG development.
Breathless Signaling
Another potential regulator of MG development is the breathless (btl) pathway.27 Btl belongs to a class of receptors that bind FGF-like ligands. In Drosophila, btl was first studied in the developing trachea, where it is required for tracheal branch migration.27 Subsequently it was shown to bind to an FGF ligand, Branchless (Bnl).28 Btl is localized to a subset of midline cells, and mutants have MG migration defects.27 However, the defects seem to be in PMG migration, and not AMG. Thus, Btl signaling is influencing a distinct class of MG, likely due to restriction of the receptor on PMG and not AMG. Work in other cell types indicated that Bnl, and not other FGF ligands, is the relevant Btl ligand.29 However, the ligand responsible for Btl signaling in the midline is unknown.
Fear-Of-Intimacy
Another transmembrane protein expressed in MG is fear-of-intimacy (foi).30 Foi belongs to the ZIP family of zinc ion transporters. This gene affects migration of a variety of cell types, including CNS and peripheral glia. Analysis of foi mutant embryos indicated that MG migration was aberrant, and it was proposed that foi may be involved in terminating MG migration.30 Furthermore, it was suggested that Hedgehog may be the non-autonomous signal that is instructing Foi. If true, this would implicate another prominent signaling pathway in MG development. In the gonad, molecular studies demonstrated that Foi acts as a zinc transporter, and influences levels of the Shotgun (DE-cadherin) cell adhesion protein at both the transcriptional and post-transcriptional levels.31 It will be interesting to determine how Foi influences MG migration, and whether it is altering levels of Wrapper, Shotgun, or other molecules related to adhesion or migration.
MG Communication with Axons
The MG themselves are important sources of signaling to neurons. This includes Netrin and Slit signaling that controls axon guidance across the midline7,8 and Spitz signaling that controls the differentiation of lateral CNS neurons.3,4 The details of midline-directed axon guidance have been expertly reviewed,7,8 and here, only select cellular and molecular mechanisms that mediate aspects of MG to neuron signaling are considered.
Gliopodia
Live imaging of MG expressing UAS-gapGFP, which reveals actin-rich structures, demonstrated that MG have filopodia-like structures (referred to as gliopodia) that can extend away from MG up to 15 μm.32 This is a sufficient distance to interact with axons within the longitudinal connectives, as well as commissural axons. Gliopodia are dynamic and extend and retract at rates (up to 10 μm/min) similar to axonal filopodia. Slit protein is present on the surface of gliopodia, suggesting that gliopodia can present MG-generated Slit protein to CNS neurites, driving repulsion from the midline. When gliopodial number is reduced by expressing a dominant negative form of Drosophila Rac1, the axon scaffold showed a reduction in the distance of the longitudinal connectives from the midline, consistent with a reduction in Slit repulsion. Another potential function of MG gliopodia may be to position the terminal processes of sensory neuron axons within the neuropil using Slit and Semaphorin proteins.33–35 The role of gliopodia is analogous to that proposed for cytonemes, which mediate intercellular signaling in other cell types.36 In principle, gliopodia could also mediate communication from neurites to MG. Gliopodia are not restricted to MG, since both vertebrate glia and invertebrate non-midline glia have been observed to extend filopodial projections.37,38
Gliolectin
While Nrx-IV and Wrapper play a major role in MG-neuron interactions, additional molecules likely carry out specialized roles in axonogenesis. Gliolectin (Glec) is a heterophilic cell adhesion protein localized on the surface of MG that binds N-acetylglucosamine glycans on axons.39 Glec has a unique protein sequence that is not conserved outside of the Drosophila genus. Genetic analysis of glec mutant embryos indicated that commissural axons were not properly ensheathed by MG, but instead tended to bundle together.40 Thus, Glec may normally mediate MG-axon interactions, but when this interaction is genetically disrupted, axons instead adhere to themselves. Mutants also showed an effect on the longitudinal connectives that may result from a reduction in Slit signaling from the midline. Consequently, glec was proposed to mediate interactions between MG and axons; these interactions may be localized to gliopodia.
Future Directions
The purpose of this commentary is to relate that the Drosophila MG are an excellent system to study glial migration and the function of glial-neuron interactions. Recent methodological advances allow MG migration and ensheathment to be studied with great precision. Employing confocal microcopy and other advanced imaging techniques such as live imaging, AMG and PMG can be identified throughout development by morphology, gene expression, and expression of fluorescently-tagged proteins. To date, a number of genes have been identified that appear to play distinct roles in MG development, and a large number of genes have been identified that are expressed in MG.1,10 Of the 54 MG-expressed genes listed on the Drosophila CNS Midline Gene Expression Database (MidExDB; www.unc.edu/~crews/MidExDB), 18 are annotated with function terms that include cytoskeleton, signaling and adhesion, and an additional seven have unknown function. These represent good candidates for genes involved in MG migration and ensheathment. Utilizing the tools available for studying Drosophila, it is now possible to gain a comprehensive view of how molecular pathways function in MG and neurons to govern important aspects of CNS development.
Acknowledgements
Our lab’s work on Drosophila midline cell development is funded by grants from the National Institutes of Health (HD25251, NS64264 and RR21055). The author would like to thank Steve Rogers and Scott Wheeler for helpful comments on the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10208
References
- 1.Wheeler SR, Kearney JB, Guardiola AR, Crews ST. Single-cell mapping of neural and glial gene expression in the developing Drosophila CNS midline cells. Dev Biol. 2006;294:33–35. doi: 10.1016/j.ydbio.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu B, Pennack JA, McQuilton P, Forero MG, Mizuguchi K, Sutcliffe B, et al. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008;6:284. doi: 10.1371/journal.pbio.0060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menne TV, Luer K, Technau GM, Klambt C. CNS midline cells in Drosophila induce the differentiation of lateral neural cells. Development. 1997;124:4949–4958. doi: 10.1242/dev.124.24.4949. [DOI] [PubMed] [Google Scholar]
- 4.Huh JY, Jeon SH, Kim SH. The CNS midline cells and Egfr signaling genes are required for establishment of the RP2 motoneuron lineage in the Drosophila central nervous system. Biochem Biophys Res Commun. 2009;380:729–735. doi: 10.1016/j.bbrc.2009.01.104. [DOI] [PubMed] [Google Scholar]
- 5.Luer K, Urban J, Klambt C, Technau GM. Induction of identified mesodermal cells by CNS midline progenitors in Drosophila. Development. 1997;124:2681–2690. doi: 10.1242/dev.124.14.2681. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Xiao H, Nambu JR. CNS midline to mesoderm signaling in Drosophila. Mech Dev. 1997;67:59–68. doi: 10.1016/s0925-4773(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 7.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 8.Garbe DS, Bashaw GJ. Axon guidance at the midline: from mutants to mechanisms. Crit Rev Biochem Mol Biol. 2004;39:319–341. doi: 10.1080/10409230490906797. [DOI] [PubMed] [Google Scholar]
- 9.Dong R, Jacobs JR. Origin and differentiation of supernumerary midline glia in Drosophila embryos deficient for apoptosis. Dev Biol. 1997;190:165–177. doi: 10.1006/dbio.1997.8688. [DOI] [PubMed] [Google Scholar]
- 10.Kearney JB, Wheeler SR, Estes P, Parente B, Crews ST. Gene expression profiling of the developing Drosophila CNS midline cells. Dev Biol. 2004;275:473–492. doi: 10.1016/j.ydbio.2004.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell RM, Peterson AC. Expression of a lacZ transgene reveals floor plate cell morphology and macromolecular transfer to commissural axons. Development. 1993;119:1217–1228. doi: 10.1242/dev.119.4.1217. [DOI] [PubMed] [Google Scholar]
- 12.Lane S, McDermott K, Dockery P, Fraher J. The developing cervical spinal ventral commissure of the rat: a highly controlled axon-glial system. J Neurocytol. 2004;33:489–501. doi: 10.1007/s11068-004-0512-x. [DOI] [PubMed] [Google Scholar]
- 13.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 14.Golembo M, Raz E, Shilo BZ. The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development. 1996;122:3363–3370. doi: 10.1242/dev.122.11.3363. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Crews ST. Influence of Drosophila ventral epidermal development by the CNS midline cells and spitz class genes. Development. 1993;118:893–901. doi: 10.1242/dev.118.3.893. [DOI] [PubMed] [Google Scholar]
- 16.Noordermeer JN, Kopczynski CC, Fetter RD, Bland KS, Chen WY, Goodman CS. Wrapper, a novel member of the Ig superfamily, is expressed by midline glia and is required for them to ensheath commissural axons in Drosophila. Neuron. 1998;21:991–1001. doi: 10.1016/s0896-6273(00)80618-2. [DOI] [PubMed] [Google Scholar]
- 17.Stork T, Thomas S, Rodrigues F, Silies M, Naffin E, Wenderdel S, Klambt C. Drosophila Neurexin IV stabilizes neuron-glia interactions at the CNS midline by binding to Wrapper. Development. 2009;136:1251–1261. doi: 10.1242/dev.032847. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler SR, Banerjee S, Blauth K, Rogers SL, Bhat MA, Crews ST. Neurexin IV and wrapper interactions mediate Drosophila midline glial migration and axonal ensheathment. Development. 2009;136:1147–1157. doi: 10.1242/dev.030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- 20.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St. Martin M, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 21.Estes P, Fulkerson E, Zhang Y. Identification of motifs that are conserved in 12 Drosophila species and regulate midline glia vs. neuron expression. Genetics. 2008;178:787–799. doi: 10.1534/genetics.107.080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergmann A, Tugentman M, Shilo BZ, Steller H. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev Cell. 2002;2:159–170. doi: 10.1016/s1534-5807(02)00116-8. [DOI] [PubMed] [Google Scholar]
- 23.Klämbt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development. 1993;117:163–176. doi: 10.1242/dev.117.1.163. [DOI] [PubMed] [Google Scholar]
- 24.Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 25.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 26.Learte AR, Forero MG, Hidalgo A. Gliatrophic and gliatropic roles of PVF/PVR signaling during axon guidance. Glia. 2008;56:164–176. doi: 10.1002/glia.20601. [DOI] [PubMed] [Google Scholar]
- 27.Klambt C, Glazer L, Shilo B. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992;6:1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 29.Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–747. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pielage J, Kippert A, Zhu M, Klambt C. The Drosophila transmembrane protein Fear-of-intimacy controls glial cell migration. Dev Biol. 2004;275:245–257. doi: 10.1016/j.ydbio.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Mathews WR, Ong D, Milutinovich AB, Van Doren M. Zinc transport activity of Fear of Intimacy is essential for proper gonad morphogenesis and DE-cadherin expression. Development. 2006;133:1143–1153. doi: 10.1242/dev.02256. [DOI] [PubMed] [Google Scholar]
- 32.Vasenkova I, Luginbuhl D, Chiba A. Gliopodia extend the range of direct glia-neuron communication during the CNS development in Drosophila. Mol Cell Neurosci. 2006;31:123–130. doi: 10.1016/j.mcn.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]
- 34.Zlatic M, Li F, Strigini M, Grueber W, Bate M. Positional cues in the Drosophila nerve cord: semaphorins pattern the dorso-ventral axis. PLoS Biol. 2009;7:1000135. doi: 10.1371/journal.pbio.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furrer MP, Vasenkova I, Kamiyama D, Rosado Y, Chiba A. Slit and Robo control the development of dendrites in Drosophila CNS. Development. 2007;134:3795–3804. doi: 10.1242/dev.02882. [DOI] [PubMed] [Google Scholar]
- 36.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 37.Tucker ES, Tolbert LP. Reciprocal interactions between olfactory receptor axons and olfactory nerve glia cultured from the developing moth Manduca sexta. Dev Biol. 2003;260:9–30. doi: 10.1016/s0012-1606(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 38.Rumsby M, Afsari F, Stark M, Hughson E. Microfilament and microtubule organization and dynamics in process extension by central glia-4 oligodendrocytes: evidence for a microtubule organizing center. Glia. 2003;42:118–129. doi: 10.1002/glia.10211. [DOI] [PubMed] [Google Scholar]
- 39.Tiemeyer M, Goodman CS. Gliolectin is a novel carbohydrate-binding protein expressed by a subset of glia in the embryonic Drosophila nervous system. Development. 1996;122:925–936. doi: 10.1242/dev.122.3.925. [DOI] [PubMed] [Google Scholar]
- 40.Sharrow M, Tiemeyer M. Gliolectin-mediated carbohydrate binding at the Drosophila midline ensures the fidelity of axon pathfinding. Development. 2001;128:4585–4595. doi: 10.1242/dev.128.22.4585. [DOI] [PubMed] [Google Scholar]