Abstract
During the last few years, several studies have pointed to a surprising link between environmental pollutants cellular signaling and important cell functions such as plasticity, adhesion and migration. This unexpected link could be related to endogenous functions of pollutants receptors that may be disrupted by environmental factors, which is supported by observations in invertebrate species. It could also reveal novel toxic end-points and mechanisms of those pollutants, such as teratogenesis and cancer metastasis that are highly relevant from a public health point of view. In the present short article, we will review our recent observations on the aryl hydrocarbon receptor and its new molecular and cellular targets. We identified HEF1/NEDD9/CAS-L, a multifunctional protein involved in integrin-based signaling as a transcriptional target of the receptor, and showed that its induction was critical for cell plasticity mediated by environmental pollutants. We will put our studies in perspective with other observations made by several groups.
Key words: aryl hydrocarbon receptor, toxicity, environmental pollutants, dioxins, aromatic hydrocarbons, Hef1/Nedd9/Cas-L, tumor progression, cell migration, development
Populations of industrialized and third world countries are commonly exposed to numerous organic contaminants or pollutants. Several of them are persistent in the environment and accumulate in the adipose tissue of animals and humans;1 those Persistent Organic Pollutants (POP) are characterized by a long half-life and are slowly metabolized. They are known to elicit a variety of toxic effects that are related to their molecular structure and their mechanism of action.2 The influence of some pollutants on tumor initiation and promotion has been clearly demonstrated (ex: benzo(a)pyrene in tobacco smoke). Remarkably, few studies have been undertaken to establish a relationship between exposure to POPs and tumor progression, particularly metastasis development, which is responsible of 90% cancer deaths and poor end-of-life conditions (www.iarc.fr). While some environmental risk factors have been clearly identified as critical tumor initiators and promoters (UV, tobacco), little is known on the possible role of those factors on tumor progression and metastasis. Obviously, such findings would be highly relevant for public health and would improve our knowledge on the relationship between environment and cancer.
The AhR, a Xenobiotic Receptor
Several POPs as well as other pollutants bind and activate an intracellular receptor called the Aryl hydrocarbon Receptor (AhR). We have recently identified the AhR as a regulator of cellular processes reminiscent of epithelial-mesenchymal transition (EMT), a phenomenon, which has been linked to tumor metastasis.3 The AhR is a member of the bHLH/PAS (basic Helix Loop Helix/Per ARNT Sim) family,4 which has been identified as a receptor of environmental pollutants including polyaromatic aromatic hydrocarbons (PAH), polyhalogenated hydrocarbons like dioxins, furans and polychlorinated biphenyls (PCBs) and polyphenols. This wide ligand spectrum is abundantly represented in our ecosystems cigarette smoke, combustion products, contaminated food (dioxins, PAH, PCBs…), natural food products (polyphenols, metabolites derivatives…) and as a consequence, human populations are daily exposed to AhR ligands.5 Upon ligand binding, the cytosolic receptor behaves like a classical nuclear receptor and translocates into the nucleus, heterodimerizes with a partner named ARNT (AhR Nuclear Translocator) belonging to the same family (PAS) and binds to Xenobiotic Responsive Elements (or XRE) located in the promoters of target genes.6 Historically, xenobiotic metabolizing enzymes have been identified as primary AhR targets; this pathway functions as an adaptive system allowing the sensing of xenobiotics, induction of their metabolism and transport and leading to their elimination.7,8 This auto regulatory loop is clearly critical for the cell and organism interactions with their environment. It should be noted that, considering this loop and compared to most classical AhR ligands, dioxins are particular because in fact, they are not metabolized by xenobiotic metabolizing enzymes. This could partly explain their long elimination half-life.
Regulation of HEF1 Expression by the AhR
Knockout mouse AhR models have rapidly suggested that the AhR protein might have endogenous and alternative functions and this has been recently supported by studies in invertebrates models (fly, nematode) in which the AhR functions as a transcription factor but does not bind dioxin.9–17 Transcriptomics experiments in mammalian model systems including ours, have allowed the identification of new gene targets of dioxin and the AhR; among them, we identified several putative regulators of migration.18 Besides, we were able to show in the MCF-7 & HepG2 cell lines, that AhR activation (achieved by addition of ligands or using a cell line stably transfected with a constitutively activated AhR only expressed upon tetracycline withdrawal) leads to increased cell migration, Jun Kinases activation and E-Cadherin downregulation.18 These features (that we cautiously refer to as “cellular plasticity”) are reminiscent of EMT-related processes. EMT is defined as a phenotypic change (epithelial to mesenchymal) allowing the cell to migrate from its initial anchorage site to other niches; this is suspected to play a critical role in metastasis formation especially for epithelial cancers.19,20 In that initial study by Diry et al. we did not provide a mechanism to explain the effects of the AhR ligands. Recently, we and others implicated the metastasis marker HEF1/NEDD9/CAS-L as an essential node in AhR-regulated cell plasticity,21,22 (Fig. 1). HEF1/NEDD9/CAS-L is a multifunctional docking protein involved in integrin-based signaling that notably affects cell motility and oncogenic transformation.23,24 Moreover, it has been implicated in cilia stability and centrosome regulation.25,26 HEF1 interacts with focal adhesion kinase (FAK) and the Src family of tyrosine kinases, two critical regulators of focal adhesion.27 As a result, HEF1/NEDD9/CAS-L regulates migratory processes as demonstrated in a melanoma cell line;28 moreover, in several human cancers such as melanoma,28 glioblastoma29 and lung tumors,30 increased HEF1/NEDD9/CAS-L expression was found to correlate with the metastasis potential of those tumors. On the other hand, other studies have also suggested a negative role for HEF1 in tumor progression.31,32 In our recent study, we show that several AhR ligands including dioxins and various common environmental pollutants (polycyclic aromatic pollutants) increase HEF1/NEDD9/CAS-L mRNA and protein expressionin the hepatocarcinoma cell line HepG2; the mechanism is transcriptional and implicates AhR binding to HEF1/NEDD9/CAS-L promoter as stated by ChIP experiments. Moreover, when RNA interference was used to block HEF1 upregulation, the cellular plasticity phenotype elicited by dioxin, including JNK activation, E-Cadherin downregulation, focal adhesion sites remodeling and activation of cell migration, was inhibited,18,22 (Fig. 1). To our knowledge, this is the first observation revealing a link between common POPs exposure and increased expression of a metastatic marker.
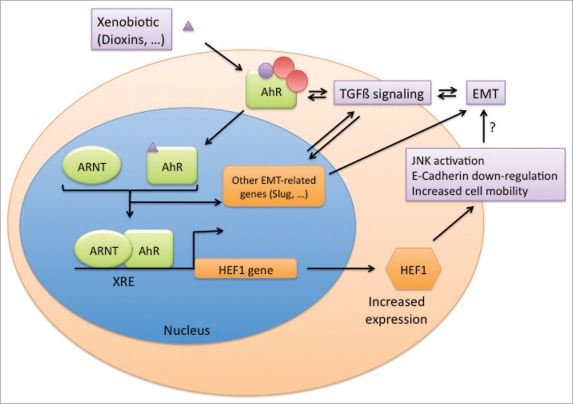
Figure 1.
The AhR is a xenobiotic receptor, which forms a complex with chaperones in the cytoplasm. Upon ligand binding, it translocates to the nucleus and forms a transcription factor with its nuclear partner, ARNT. The heterodimer binds xenobiotic responsive elements (XRE) in the promoters of target genes. We identified HEF1/NEDD9/CAS-L as an AhR target. HEF1 induction is responsible for dioxin-mediated cell plasticity including E-Cadherin downregulation, JNK activation and increased cellular motility. Thus, HEF1 induction might be responsible of EMT (Epithelial Mesenchymal Transition)-associated processes. Other AhR-related regulatory pathways might be involved in those phenomena including regulation of Slug or TGFbeta signaling pathways.
The AhR is Implicated in Regulation of Cell Migration
The study supports the new concept that AhR is essential to regulate cell migration: indeed, using immortalized cell lines from wild-type (AhR+/+) and mutant (AhR−/−) mouse mammary fibroblasts, the team of Dr. Pedro Fernandez-Salguero showed that AhR-deficient cells had a lower tendency to develop subcutaneous tumors in immunodeficient mice. In cell culture experiments, those cells also displayed reduced migration properties and lamellipodia formation. This could be related to the downregulation of the ERK-FAK-PKB/AKT-Rac-1 pathway in the AhR−/− cells.33,34 Interestingly, FAK activation status is also downregulated in mammary tumors arising in MMTV-polyoma virus middle T; Hef1 null mice.24 In another recent study, the same team demonstrated the involvement of Vav3, a guanosine diphosphate/guanosine triphosphate exchange factor (GEF) for Rho/Rac GTPases (identified as a transcriptional target of AhR) in the regulation of those processes. AhR deficient immortalized and mouse embryonic fibroblasts have reduced expression of Vav3 and subsequent reduced Rac1 activity and increased activation of the RhoA/Rho kinase (Rock) pathway. Interestingly, Vav3 is sensitive to the AhR status (+/+ versus −/−) of the cell but not to xenobiotic exposure (dioxin). The consequences of this imbalance are an increased cell area, increased F-actin stress fibers, depolarized focal adhesions, and enhanced spreading and adhesion.34 Interestingly, the AhR expression status does not lead to similar phenotypes in all cell models; indeed, the same team also showed that AhR deficiency increases keratinocyte migration and accelerates skin re-epithelialization probably because of a higher secretion of TGFbeta (transforming growth factor beta) by AhR null dermal fibroblasts.35 The regulation of cell migration by the AhR in the absence of xenobiotic exposure has also been observed in nematodes lacking the AhR ortholog, which display aberrant neuron migration, axon branching and axonal migration defects.12 While all of those experiments were performed using AhR-null models without stimulation by AhR ligands, other studies have reported an effect of dioxin or 3-methylcholanthrene (3MC, a polycyclic aromatic hydrocarbon) on cell migration: Peng et al. and Seifert et al. have reported increased gastric cancer cell invasiveness and MCF-7 cell migration upon TCDD addition and AhR activation.36,37 Moreover, Ikuta et al. also observed the induction of acritical regulator of epithelial-mesenchymal transition (EMT), Slug, upon AhR nuclear translocation (upon 3MC stimulation or low cell density).38 Again, this is not observed with all cell types as evidenced by a recent article showing that 3MC stimulation of HUVEC cells leads to reduced cell migration due to increased RhoA activity via suppression of a negative feedback pathway of FAK/p190RhoGAP.39
Perspectives and Conclusion
Several important points can be concluded from those studies: first, the regulation of cell migration by the AhR might be an ancestral function as clearly suggested by the invertebrates studies. Second, those articles and the ones reporting the use of AhR deficient cells, suggest that this process is not necessarily related to AhR stimulation by dioxins or aromatic hydrocarbons. Those studies do not rule out the possibility of AhR stimulation by endogenous ligands. Third, the involvement of the AhR in the regulation of migration appears to be clearly dependent on cell phenotypes (MEF, keratinocytes, cell lines). Interestingly, while we and others have suggested that AhR ligands might increase migration and invasion in various cell types, it is tempting to hypothesize that activation of the AhR during cancer promotion or progression reproduces developmental functions and might play a role in tumor progression. Finally, considering that HEF1 expression varies considerably among tissues (mostly abundant in vivo in polarized cell populations) and cell cycle (abundant in G2/M) and that its levels are critical to ensure proper functions (centrosome regulation, integrin signaling),40 exposure to AhR ligands might disrupt those pathways ultimately leading to cancer or abnormal development. It should also be noted that the only Drosophila CAS family member (DCas) is highly expressed in the embryonic nervous system (such as the AhR ortholog) and that its expression is critical for axon guidance; indeed, DCas defect or overexpression lead to similar abnormal phenotypes.41 Moreover, a balanced HEF1 expression level is important for neuronal cell fate in mouse primary culture cells42 or adhesion properties of chick neural crest cells.43 Interestingly, those studies show that HEF1 expression is controlled by all-trans retinoic acid (atRA) and TGFbeta pathways.42,43 Those connections between HEF1 expression and TGFbeta or atRA signaling have been also reported by other laboratories44–47 and are particularly important regarding the AhR field. Indeed, the metabolism of atRA is clearly disrupted by expression of AhR-regulated cytochromes P450. Moreover, TGFbeta proteins regulate and are regulated by the AhR both in vitro and in vivo.48
The observations described above should be examined in light of the recent discoveries of common features between cancer and developmental pathways. While AhR knockout mice do not present a lethal phenotype at birth, they show several important developmental defects including liver fibrosis and cardiovascular abnormalities.9,11 Those studies as well as those performed with invertebrate models, suggest that the Aryl hydrocarbon receptor regulates developmental pathways such as cellular differentiation and migration in the absence of xenobiotic exposure. Interestingly, many of these pathways including cell migration, are critical for cancer growth and progression. We hypothesize that the disruption of AhR endogenous functions upon xenobiotic exposure might interfere with those pathways and ultimately lead, in a tumor cell, to the acquisition of a mesenchymal/metastatic phenotype. The concept that part of pollutants toxicity stems from the disruption of essential endogenous functions of the AhR has rejuvenated the AhR field lately and will have to be further characterized in the future.
Acknowledgements
This work was supported by AFSSET (Agence Française de Sécurité Sanitaire de l’Environment et du Travail); ANR (Agence Nationale de la Recherche, 06SEST26, Oncopop); ARC (Association pour la Recherche sur le Cancer, 3927); Fondation pour la Recherche Medicale; Ligue contre le Cancer; Ministère de l’enseignement supérieur et de la recherche; Région Ile de France; Université Paris Descartes; Institut National de la Santé et de la Recherche Médicale.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10313
References
- 1.Mullerova D, Kopecky J. White adipose tissue: storage and effector site for environmental pollutants. Physiol Res. 2007;56:375–381. doi: 10.33549/physiolres.931022. [DOI] [PubMed] [Google Scholar]
- 2.Li QQ, Loganath A, Chong YS, Tan J, Obbard JP. Persistent organic pollutants and adverse health effects in humans. J Toxicol Environ Health A. 2006;69:1987–2005. doi: 10.1080/15287390600751447. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 4.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 6.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- 8.Tompkins LM, Wallace AD. Mechanisms of cytochrome P450 induction. J Biochem Mol Toxicol. 2007;21:176–181. doi: 10.1002/jbt.20180. [DOI] [PubMed] [Google Scholar]
- 9.Lahvis GP, Bradfield CA. Ahr null alleles: distinctive or different Biochem Pharmacol. 1998;56:781–787. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 10.Powell-Coffman JA, Bradfield CA, Wood WB. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc Natl Acad Sci USA. 1998;95:2844–2849. doi: 10.1073/pnas.95.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 12.Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270:64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Qin H, Zhai Z, Powell-Coffman JA. The Caenorhabditis elegans AHR-1 transcription complex controls expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior. Dev Biol. 2006;298:606–615. doi: 10.1016/j.ydbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Powell-Coffman JA, Jin Y. The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C. elegans. Development. 2004;131:819–828. doi: 10.1242/dev.00959. [DOI] [PubMed] [Google Scholar]
- 15.Crews ST, Brenman JE. Spineless provides a little backbone for dendritic morphogenesis. Genes Dev. 2006;20:2773–2778. doi: 10.1101/gad.1487706. [DOI] [PubMed] [Google Scholar]
- 16.Emmons RB, Duncan D, Estes PA, Kiefel P, Mosher JT, Sonnenfeld M, et al. The spineless-aristapedia and tango bHLH-PAS proteins interact to control antennal and tarsal development in Drosophila. Development. 1999;126:3937–3945. doi: 10.1242/dev.126.17.3937. [DOI] [PubMed] [Google Scholar]
- 17.Kim MD, Jan LY, Jan YN. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 2006;20:2806–2819. doi: 10.1101/gad.1459706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, Barouki R, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–5574. doi: 10.1038/sj.onc.1209553. [DOI] [PubMed] [Google Scholar]
- 19.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Frueh FW, Hayashibara KC, Brown PO, Whitlock JP., Jr Use of cDNA microarrays to analyze dioxin-induced changes in human liver gene expression. Toxicol Lett. 2001;122:189–203. doi: 10.1016/s0378-4274(01)00364-2. [DOI] [PubMed] [Google Scholar]
- 22.Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, et al. Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene. 2009;28:3642–3651. doi: 10.1038/onc.2009.224. [DOI] [PubMed] [Google Scholar]
- 23.Astier A, Manie SN, Law SF, Canty T, Haghayghi N, Druker BJ, et al. Association of the Cas-like molecule HEF1 with CrkL following integrin and antigen receptor signaling in human B-cells: potential relevance to neoplastic lymphohematopoietic cells. Leuk Lymphoma. 1997;28:65–72. doi: 10.3109/10428199709058332. [DOI] [PubMed] [Google Scholar]
- 24.Izumchenko E, Singh MK, Plotnikova OV, Tikhmyanova N, Little JL, Serebriiskii IG, et al. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer Res. 2009;69:7198–7206. doi: 10.1158/0008-5472.CAN-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Seventer GA, Salmen HJ, Law SF, O’Neill GM, Mullen MM, Franz AM, et al. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur J Immunol. 2001;31:1417–1427. doi: 10.1002/1521-4141(200105)31:5<1417::AID-IMMU1417>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, et al. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 30.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 31.Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 32.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulero-Navarro S, Pozo-Guisado E, Perez-Mancera PA, Alvarez-Barrientos A, Catalina-Fernandez I, Hernandez-Nieto E, et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem. 2005;280:28731–28741. doi: 10.1074/jbc.M504538200. [DOI] [PubMed] [Google Scholar]
- 34.Carvajal-Gonzalez JM, Mulero-Navarro S, Roman AC, Sauzeau V, Merino JM, Bustelo XR, et al. The dioxin receptor regulates the constitutive expression of the vav3 proto-oncogene and modulates cell shape and adhesion. Mol Biol Cell. 2009;20:1715–1727. doi: 10.1091/mbc.E08-05-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvajal-Gonzalez JM, Roman AC, Cerezo-Guisado MI, Rico-Leo EM, Martin-Partido G, Fernandez-Salguero PM. Loss of dioxin-receptor expression accelerates wound healing in vivo by a mechanism involving TGFbeta. J Cell Sci. 2009;122:1823–1833. doi: 10.1242/jcs.047274. [DOI] [PubMed] [Google Scholar]
- 36.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert A, Rau S, Kullertz G, Fischer B, Santos AN. TCDD induces cell migration via NFATc1/ATX-signaling in MCF-7 cells. Toxicol Lett. 2009;184:26–32. doi: 10.1016/j.toxlet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Ikuta T, Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp Cell Res. 2006;312:3585–3594. doi: 10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Chang CC, Tsai SY, Lin H, Li HF, Lee YH, Chou Y, et al. Aryl-hydrocarbon receptor-dependent alteration of FAK/RhoA in the inhibition of HUVEC motility by 3-methylcholanthrene. Cell Mol Life Sci. 2009;66:3193–3205. doi: 10.1007/s00018-009-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugacheva EN, Golemis EA. HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5:384–391. doi: 10.4161/cc.5.4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- 42.Vogel T, Ahrens S, Buttner N, Krieglstein K. Transforming Growth Factor {beta} Promotes Neuronal Cell Fate of Mouse Cortical and Hippocampal Progenitors In Vitro and In Vivo: Identification of Nedd9 as an Essential Signaling Component. Cereb Cortex. 2009;20:661–671. doi: 10.1093/cercor/bhp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aquino JB, Lallemend F, Marmigere F, Adameyko II, Golemis EA, Ernfors P. The retinoic acid inducible Cas-family signaling protein Nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience. 2009;162:1106–1119. doi: 10.1016/j.neuroscience.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inamoto S, Iwata S, Inamoto T, Nomura S, Sasaki T, Urasaki Y, et al. Crk-associated substrate lymphocyte type regulates transforming growth factor-beta signaling by inhibiting Smad6 and Smad7. Oncogene. 2007;26:893–904. doi: 10.1038/sj.onc.1209848. [DOI] [PubMed] [Google Scholar]
- 45.Knutson DC, Clagett-Dame M. atRA Regulation of NEDD9, a gene involved in neurite outgrowth and cell adhesion. Arch Biochem Biophys. 2008;477:163–174. doi: 10.1016/j.abb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J. 2000;19:6759–6769. doi: 10.1093/emboj/19.24.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng M, McKeown-Longo PJ. Regulation of HEF1 expression and phosphorylation by TGFbeta1 and cell adhesion. J Biol Chem. 2002;277:39599–39608. doi: 10.1074/jbc.M202263200. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Duran A, Carvajal-Gonzalez JM, Mulero-Navarro S, Santiago-Josefat B, Puga A, Fernandez-Salguero PM. Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFbeta signaling. Biochem Pharmacol. 2009;77:700–712. doi: 10.1016/j.bcp.2008.08.032. [DOI] [PubMed] [Google Scholar]