Abstract
Cellular division is an ordered, tightly regulated process involving multiple checkpoints that assess extracellular growth signals, cell size and DNA integrity. Progression throughout the cell cycle is based on the activation of different CDK-cyclin complexes that prevent cells from entering into a new phase until thay have successfully complete the previous one. In addition, a series of cell cycle checkpoints are designed to preserve genome integrity and chromosomal stability. Neoplastic lung cells develop the ability to bypass several of these checkpoints, and tumor cell proliferation is frequently associated with genetic or epigenetic alterations in key regulators of the cell cycle. The goal of this review is to summarize the knowledge about the dysregulation of major cell cycle regulators in lung cancer pathogenesis and to discuss the use of these proteins as targets for therapeutic intervention.
Key words: lung cancer, cell cycle regulators, kinases, checkpoint, therapy
Precise regulation of the cell cycle is a fundamental requirement for the homeostasis of eukaryotic cells, and uncontrolled cell proliferation is an invariable characteristic of human cancers. Indeed, the proliferation of cancer cells is sustained in the absence of growth factors and is insensitive to growth-inhibitory signals.1 In non transformed lung epithelial cells, cellular division is an ordered, tightly regulated process involving multiple checkpoints that assess extracellular growth signals, cell size and DNA integrity. Among the many pathways altered in lung cancer malignancy, the most criticals involve disruption of the normal cell cycle regulation. The goal of this review is to summarize the knowledge about the dysregulation of major cell cycle regulators in lung cancer pathogenesis and to discuss the use of these proteins as targets for therapeutic intervention.
Overview of the Cell Cycle Regulation
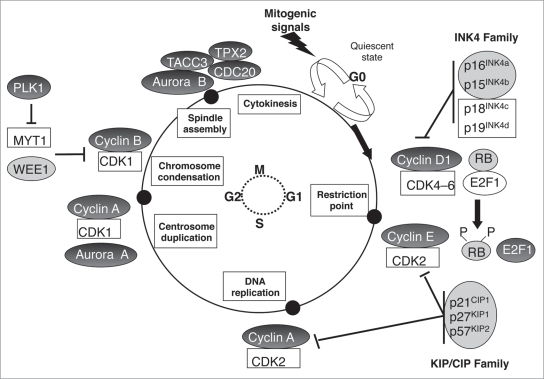
The mammalian cell cycle machinery is comprised of five sequential stages: G0, G1, S, G2 and M. During two of these phases, cells execute the two basic events in cell division: generation of a single and faithful copy of its genetic material (S phase) and partitioning of all the cellular components between two identical daughter cells (mitosis or M phase). The two other phases of the cell cycle, G1 and G2, represent “gap” periods during which cells prepare themselves for the successful completion of the S and M phases respectively. When cells cease proliferation, either due to specific antimitogenic signals or to the absence of proper mitogenic signalling, they exit the cycle and enter a non-dividing quiescent state known as G0. These last decades, the molecules controlling the successive phases of the cell cycle have been extensively characterized. The central players are the cyclin-dependent kinases (CDKs), a group of serine/threonine kinases whose activity is regulated by their arrangement in a multimeric complex with larger proteins called cyclins, owing to their cyclical expression and proteasomal degradation during the cell cycle. Throughout the cell cycle progression, different CDK-cyclin complexes are formed and are activated by sequential phosphorylation/dephosphorylation with a clear-cut timing, thereby preventing cells from entering into a new phase until they have successfully completed the previous one.2 In addition, all along the cell cycle, a series of surveillance pathways named cell cycle checkpoints ensure that cells pass accurate copies of their genome onto the next generation, in order to preserve genome integrity and chromosomal stability.
The Molecular Players of G1 and S Phases
When cells in the quiescent (G0) phase enter the cycle in response to extracellular signals such as mitogenic activation, intracellular levels of D-type cyclins (D1, D2, D3) increase, resulting in the formation and nuclear localization of cyclin D-CDK4 and cyclin D-CDK6 complexes that initiate phosphorylation of the retinoblastoma protein (RB1, also known as p105-RB) and possibly of other members of the “pocket” protein family.3,4 The RB pocket proteins (p105, p107 and p130) negatively modulate the G1 to S phase transition at least in part through binding and inactivation of the E2F transcription factors that promote transcription of genes required for DNA replication.5 Partial phosphorylation of RB proteins by cyclin D-CDK4 and cyclin D-CDK6 complexes in early G1 inactivates their function as transcriptional repressors and leads to the release of E2F transcription factors, enabling the expression of genes required for G1 to S phase transition and DNA synthesis. In late G1, levels of E-type cyclins accumulate and these cyclins associate with CDK2 to reinforce RB1 phosphorylation on additional sites, and to irreversibly initiate the gene expression programme of the S phase.6 This stage, called the restriction point, is crucial in cancer as alterations of the molecular players involved in the G1 to S phase transition allow cells to proliferate independently of mitogenic stimuli. It is therefore not surprising that CDK inhibitors (CKI) exist to control the timely activation of cyclin-CDK complexes.7 These proteins belong to two different families: the INK4 family of proteins that include INK4A (also known as p16INK4a), INK4B (also known as p15INK4b), INK4C (also known as p18INK4c), INK4D (also known as p19INK4d), as well as the kinase inhibitory protein (WAF/KIP) family. The four members of the INK4A family exert their inhibitory activity by binding to the CDK4 and CDK6 kinases and by preventing their association with D-type cyclins. The three members of the WAF/KIP family, WAF1 (also known as p21WAF1/CIP1), KIP1 (also known as p27KIP1) and KIP2 (also known as p57KIP2) can form heterotrimeric complexes with the G1/S CDKs. However, in stoechiometric amounts, they only inhibit the kinase activity of cyclin E-CDK2 complexes. Beyond the restriction point, RB1 is maintained in a hyperphosphorylated state through the sequential activities of cyclin A-CDK2, cyclin A-CDK1 and cyclin B-CDK1 complexes, thereby ensuring S phase completion.
The Molecular Players of the G2 and M Phases
In parallel with DNA replication, the centrosome cycle begins. Centrosomes duplicate during late S phase to early G2 phase, and separate to form the poles of the mitotic spindle at the beginning of mitosis. At these poles, each centrosome maturates to form its own aster of dynamic microtubules. The centrosome cycle and the formation of the mitotic spindle are controlled by mitotic kinases such as CDK1. During the G2/M transition, the cyclin A-CDK1 complex is activated to initiate mitosis through regulation of chromosome condensation and microtubule dynamics.8 Then, following destruction of the nuclear membrane, cyclin A is degraded and cyclin B1-CDK1 complexes are activated to allow the progression through the M phase by promoting chromosome condensation and spindle assembly.8 The complete separation of the two daughter cells occur when cyclin B1 is degraded by the anaphase-promoting complex or cyclosome (APC-C). Aurora and polo-like kinase (PLK) are also essential regulators of mitosis.9,10 Aurora A localizes to duplicated centrosomes and spindle poles during mitosis and has well-established roles in centrosome function and duplication, mitotic entry and bipolar spindle assembly.11 Aurora B is the catalytic component of the chromosomal passenger complex, which is composed of three additional noncatalytic subunits that direct its activity: survivin, INCENP and borealin. The chromosomal passenger complex orchestrates the accurate segregation of the chromatids, histone modification and cytokinesis.12 Aurora C does not seem to have a role in mitosis in the majority of normal cells, with expression essentially restricted to the testis.13,14 The best characterized member of the mammalian Polo-like family is PLK1. PLK1 specifically localizes to centrosomes, the spindle midzone and the post-mitotic bridge, and participates in both mitotic entry and mitotic progression.15,16
The Regulators of the Cell Cycle Checkpoints
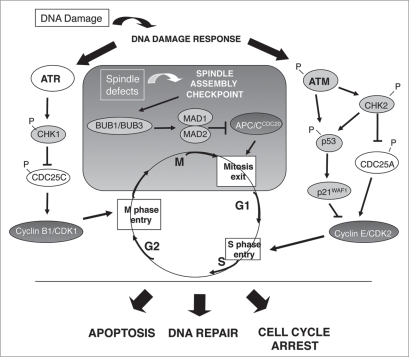
The cell cycle checkpoints are designed to preserve genome integrity and chromosomal stability in response to induced or spontaneous DNA lesions that are common events in the life of the cell, as well as upon abnormal chromosomal segregation. They constitute therefore a physiological barrier that guards against progression of tumors from early stages to malignant invasive lesions.17,18 Upon DNA damage, these checkpoints give the cell time to repair the DNA lesion by triggering cell cycle arrest in G1, S or G2 phase. If lesions are irreparable, the programmed cell death is induced. The crucial regulators of these pathways are the related kinases ataxia telangiectasia mutated (ATM), ataxia telangiectasia and RAD3-related protein (ATR) and their downstream effectors, the checkpoint kinases CHK1 and CHK2.19 The ATM/CHK2 pathway regulates mostly the G1 checkpoint, through activation of the tumor suppressor gene p53, leading to transcriptional activation of the CDKI p21WAF1/CIP1 that prevents cells from entering the S phase. Damaged cells that have already passed the transition from G1 to S phase can also be halted before entry into mitosis through activation of the ATR/CHK1 pathway that induces the cytoplasmic sequestration of the CDC25C phosphatase required for CDK1 activation. Lastly, the mitotic checkpoint, also known as the spindle assembly checkpoint (SAC) is activated when chromosomes are not properly attached to the mitotic spindle. This pathway involves the activation of signalling proteins including aurora B, mitotic arrest deficient proteins 1 and 2 (MAD1, MAD2), monopolar spindle 1 (MPS1), budding uninhibited by benzimidazole 1 (BUB1) and its homologous BUB3 and BUB1B. They act by inactivating APC-C, thereby preventing cyclin B1 proteolysis and cytokinesis.
These last decades a growing number of studies have demonstrated the constant invalidation of some of these cell cycle regulators (Fig. 1) and checkpoint proteins (Fig. 2) in human lung tumors. In the second part of this review, we will summarize the principal points of deregulation according to each phase of the cell cycle.
Figure 1.
Abnormalities of cell cycle regulators in human lung tumors. Alterations of cell cycle regulators in lung tumors occur all along the different phases of the cell cycle. Their sequential accumulation contributes to uncontrolled cellular proliferation, as well as to genomic instability. Pale grey circles indicate loss of expression of the corresponding protein and dark grey circles indicate overexpression. In all other cases, abnormalities are very unfrequent or have not been studied.
Figure 2.
Alterations in components of the cell cycle checkpoints in human lung tumors. In normal lung epithelial cells, cellular checkpoints preserve genomic integrity and prevent neoplastic transformation. Genotoxic stresses induce the activation of a DNA Damage Response (DDR) that is mediated through ATM- and/or ATR-dependent cell cycle checkpoints. The Spindle Assembly Checkpoint (SAC) is another checkpoint that controls the proper distribution of chromosomes at the metaphase-to-anaphase transition. This scheme depicts some of the DDR and SAC checkpoint proteins which expression is either downregulated (in pale grey) or upregulated (in dark grey) in human lung tumors, likely to abrogate apoptosis, DNA repair and/or cell cycle arrest and to trigger tumor progression.
Alteration of the Components of the G1 to S Phase Transition in Lung Cancers
The p16INK4A/cyclin D1/CDK4-CDK6/RB pathway.
The retinoblastoma gene, located on chromosome 13q14, was the first tumor suppressor identified in lung cancer.20,21 Loss of RB1 protein review is observed in a high proportion of SCLC tumors (>90%)22,23 as a result of loss of heterozygosity (LOH) at the RB locus20 and inactivation of the remaining allele by several mechanisms including point mutations or decreased mRNA expression.22 By contrast, loss of RB1 protein occurs in only 15% of NSCLC.24–26 RB2/p130 point mutations are also observed in primary NSCLC27 and undetectable levels of RB2/p130 protein are associated with the most aggressive tumor phenotypes, suggesting an independent role of this pocket member in the development and/or progression of NSCLC.28,29 In SCLC, dysregulation of p107 and p130 has been rarely reported.30
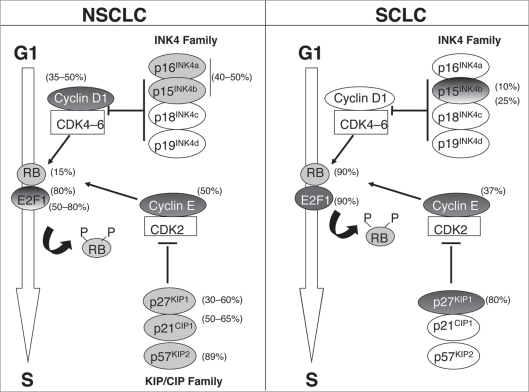
In contrast to neuroendocrine lung tumors in which RB protein is frequently lost, the majority of NSCLC exhibits RB inactivation through the deregulation of upstream regulators of RB pathway, such as p16INK4a and cyclin D1. These events lead “in fine” to RB1 hyperphosphorylation and loss of anti-proliferative control.31 Loss of p16INK4a protein is observed in 40–50% of NSCLC as a consequence of gene deletion, promoter hypermethylation or gene mutation.31–33 Homozygous deletions of the INK4a locus occurs in 30% of NSCLC tumors whereas inactivating mutations of INK4a gene are relatively rare. Hypermethylation of the INK4a promoter is observed in about 40% of the cases and is also detected in bronchial epithelium from chronic smokers, suggesting that inactivation of p16INK4a is an early event in lung tumorigenesis.32,34 Studies evaluating the effect of p16INK4a expression on prognosis have shown an improved survival of NSCLC patients with high p16INK4a levels, although not all studies have reached statistical significance.35 Amplification of the cyclin D1 locus is observed in 5–32% of tumors depending on the studies.36,37 High levels of the cyclin D1 protein are found in invasive NSCLC38 as well as in a significant fraction of non-invasive lesions of the bronchial epithelia,39,40 indicating that, like p16INK4a, increased cyclin D1 expression is an early event. Overexpression of cyclin D1 is associated with a more favorable clinical outcome in some studies, whereas others correlate the upregulation of cyclin D1 with a worse outcome or even do not find any association.38 In a study limited to stages I and II NSCLC in which cyclin D1 expression was associated with shorter survival, a combination of high cyclin D1 levels with loss of p16INK4a was observed in patients with the worst prognosis.41 In contrast to p16INK4a or cyclin D1 dysregulation, amplification of CDK4 gene leading to CDK4 overexpression is a rare event in NSCLC.42 As a whole, these studies demonstrate that inactivation of the RB pathway is a constant event in lung cancer. In NSCLC, p16INK4a loss and cyclin D1 overexpression are always inversely correlated with RB loss indicating that cyclin D1 and p16INK4a act only through RB pathway during lung carcinogenesis. This is also the case in SCLC in which RB is mostly lost and p16INK4a or cyclin D1 alterations are rare events.
The three other members of the CDKI INK4 family: p15INK4b, p18INK4c, p19INK4d.
The cell cycle inhibitor p15INK4b is frequently inactivated by homozygous deletions in NSCLC, always together with p16INK4a.43–45 In contrast, homozygous deletions of the p15INK4b gene is never observed in SCLC.45,46 Methylation of the 5′end of p15INK4b gene has also been reported in a few lung tumor cell lines,45,46 as well as in pulmonary squamous cell carcinoma,47 but its consequence on p15INK4b expression (mRNA or protein) remains poorly investigated. Mutations of the p15INK4b gene are rarely found.48,49 To investigate the possibility of a selective deregulation of p15INK4b in lung carcinogenesis, we have studied the status of p15INK4b in NE lung tumors in which deregulation of p16INK4a is rarely observed. We have shown that both p15 and p15.5 protein isoforms, that are generated from two alternative translation initiation codons, display a high heterogeneous pattern of expression (up or downregulation) in both normal and tumor tissues.50 In addition, aberrant methylation of p15INK4b was detected in 15% of the tumor cases but did not correlate with protein status. Together, these results indicate a complex deregulation of p15INK4b in lung tumors that is independent of p16INK4a. Very few studies have analyzed the status of p18INK4c in lung cancer cell lines and tumors, and neither rearrangements, nor deletions have been detected.48,51 However, it was recently shown in mouse models that p18INK4c collaborates with Men1 to constrain lung stem cell expansion and suppress non small cell lung cancers.52 These data reveal an unrecognized function of p18INK4c in lung tumor suppression. The status of p19INK4d has never been adressed in lung tumors.
The CIP/KIP family.
The CDK inhibitor p21WAF1/CIP1 inhibits the progression through the cell cycle via several mechanisms including inhibition of the cyclinD1/CDK4 and cyclin E/CDK2 complexes in early G1, and inhibition of the cyclinA/CDK2 complex prior to the S/G2 transition. In NSCLC, a positive expression of p21WAF1/CIP1 is detected more frequently in patients with stage I or II disease than in those with stage IIIa disease.53,54 In multivariate analyses, patients with tumors expressing p21WAF1/CIP1 survive longer than do those with tumors negative for p21WAF1/CIP1 expression.53,54 Therefore, positive expression of p21WAF1/CIP1 appears to be a significant factor for predicting a favorable prognosis. In addition, NSCLC patients who are negative for both p21WAF1/CIP1 and p16INK4a proteins have a significantly shorter overall survival,55 indicating that both CDKIs do not act on the same targets to inhibit lung tumorigenesis.
p27KIP1 is also a strong inhibitor of cell cycle progression through its ability to inhibit cyclin D/CDK4, cyclin D/CDK6, cyclin E/CDK2 and cyclinA/CDK2 complexes. Low levels of p27KIP1 are observed in NSCLC as compared to normal counterparts, and correlate with reduced cancer cell differentiation56,57 and high proliferative index.56 Downregulation of p27KIP1 associates with a poor outcome in NSCLC patients and is a significant prognostic factor in multivariate analyses.58–61 In contrast, SCLC exhibit increased p27KIP1 expression when compared to the normal lung epithelium.61 Together, these data suggest that p27KIP1 might play distinct biological roles in the pathogenesis of SCLC and NSCLC. In favor of such hypothesis, overexpression of p27KIP1 in SCLC cell lines have been reported to protect the cells from apoptosis in unfavourable microenvironments.62 In NSCLC, the low levels of p27KIP1 are associated with a high p27KIP1 proteolytic activity.63 p27KIP1 degradation is mediated at least in part by SKP2, an F-box-protein of the SCF complex. SKP2 has oncogenic properties and increased SKP2 protein levels have been observed in lung cancers of all histological types.64,65 Although low level of p27KIP1 do not always correlate with high SKP2 overexpression, high SKP2 protein levels have been associated with reduced p27KIP1 expression in NSCLC suggesting that dysregulation of SKP2 contributes to the altered expression of p27KIP1 in that case.66 In contrast, we showed that SKP2 and p27KIP1 are directly correlated in NE lung tumors65 confirming that both proteins might have distinct functions according to the histological types of lung tumors.
Only few studies have investigated the status of p57KIP2 in lung tumors. A significant reduced expression of p57KIP2 associated with a decrease of p27KIP1 has been reported in NSCLC as compared to normal counterparts, and was correlated with increased cellular proliferation.66 For both KIPs, SKP2-mediated proteolysis was the most important mechanism for downregulation although correlation between decreased level of p57KIP2 mRNA and promoter methylation, allelic loss or imprinting has been reported in some cases. Aberrant methylation of p57KIP2 promoter has been also observed in another study on lung cancer cell lines and tumors.67 As p57KIP2 expression could be restored in methylated cell lines following 5-aza-2′-deoxycytidine treatment, these data indicate that methylation contributes to p57KIP2 inactivation in lung cancer.
The transcription factors of E2F family.
It is currently admitted that loss of RB function contributes to uncontrolled cell proliferation by unleashing E2F transcription factors activity. In this respect, abnormal expression of the E2F1-E2F3 proteins is observed in a growing number of tumors.68 We previously showed that E2F1 is overexpressed in SCLC while it is undetectable in NSCLC as compared to corresponding normal lung, thereby identifying a differential pattern of E2F1 protein expression in lung tumors.69 By contrast, other studies have reported amplification of the E2F1 gene locus at 20q11.2,70 as well as increased E2F1 protein level in NSCLC,71–74 and have shown that E2F1 is an adverse prognostic factor in these tumors.71 The reasons of such discrepancies remain unknown. More recently, E2F1 expression was reported to correlate with expression of its transcriptional targets thymidilate synthase and survivin in NSCLC and to tumor proliferation.72 Amplification of the E2F2 gene locus at 1p36 has also been observed in SCLC75 and increased expression of E2F3 protein has been found in NSCLC and SCLC.74 Overall, these studies indicate that E2F factors likely contribute to lung carcinogenesis. However, as E2Fs and notably E2F1 have multiple biological functions, it remains to determine whether each protein contributes to lung tumorigenesis only through deregulation of cell cycle progression, or extands beyond the framework of RB and cell cycle control.
Cyclin E.
Deregulation of cyclin E is considered as a major actor of tumorigenesis. Indeed, increased cyclin E levels are associated with various malignancies, and its prolonged expression induces chromosomal instability.76 High levels of cyclin E are observed in lung tumors65,77,78 and transgenic cyclin E triggers dysplasia and lung carcinoma in mouse models.79 Increased expression of cyclin E is also detected in preinvasive lesions indicating that it is an early event during lung tumorigenesis.40 Cyclin E expression is consistently associated with shorter survival among stage I to IIIa NSCLC patients undergoing curative resection.35,78,80,81 High level of cyclin E is also a poor prognostic factor in lung adenocarcinoma patients when associated with p27KIP1 reduction.57 In high grade NE lung tumors, overexpression of cyclin E is directly associated with high E2F1 and SKP2 protein levels65 indicating that dysregulation of the three proteins may cooperate to the aggressive phenotype of these tumors. Altogether, these data are in favor of cyclin E being an important contributor to lung carcinogenesis.
In conclusion, all these data demonstrate that many components of the G1 phase are abnormally expressed in lung tumors, indicating that disruption of the G1/S transition is a crucial event during lung carcinogenesis. Furthermore, they show that the mechanisms of G1 escape is different between NSCLC and SCLC. In NSCLC, the lack of CDKi such as p16INK4a, p15INK4b, p21WAF1/CIP1 and p57KIP2 and the overexpression of cyclin D1 and cyclin E are some of the main mechanisms of G1 escape, whereas SCLC frequently display loss of RB and increase of E2F1, cyclin E and p27KIP1 (Fig. 3).
Figure 3.
Alterations of the G1/S transition regulators in NSCLC versus SCLC. Disruption of the G1/S transition is a crucial event during lung carcinogenesis. Striking differences are observed regarding the mechanisms of G1 escape in NSCLC versus SCLC. Pale grey circles indicate loss of expression of the corresponding protein and dark grey circles indicate overexpression. E2F1 and p15INK4b are depicted by gradual color in NSCLC and SCLC respectively, as controversial results have been reported regarding their status in these tumors. In all other cases, abnormalities are very unfrequent or have not been studied. The percentage of dysregulation is presented next to each molecule.
Alteration of the Components of the S Phase in Lung Tumors
The progression through S phase is principally regulated by the cyclin A/CDK2 complex. Elevated levels of cyclin A are observed in primary NSCLC, in lymph node metastasis and in some bronchial precursor lesions as compared to normal bronchial epithelium.82–85 Several studies have reported that overexpression of cyclin A is consistently associated with an unfavourable outcome in patients with NSCLC.83–85 Moreover, elevated levels of the licensing factors hCdt1 and hCdc6 that are key elements for DNA replication, are observed in NSCLC and correlate to increased tumor growth and aneuploidy in p53-defective tumors.73
Alteration of the Components of G2 and M Phases in Lung Tumors
CyclinB1/CDK1 is the classic M phase-promoting factor that drives entry into mitosis. High levels of cyclin B1 are observed in NSCLC, especially in stage I squamous cell cancers.86–88 Increased expression of cyclin B1 is also detected in some precursor lesions.83 High cyclin B1 expression correlates with differentiation, invasion and high proliferative index.83,86,88 Cyclin B1 has also been reported as a significant prognostic factor in NSCLC in multivariate analysis.86 In other studies, cyclin B1 was not found to be an independent prognostic parameter although its overexpression seems to be an adverse prognostic factor.87,88 Elevated levels of cyclin B1 have been associated with poor outcome in patients with early stage squamous cell carcinoma of the lung,88 suggesting that cyclin B1 expression may be a prognostic marker for these patients. Although the role of cyclin B1 in lung tumor development has been the subject of many studies, the implication of CDK1 has not been properly evaluated. However, in a genome profiling study high levels of CDK1 have been reported in early stage lung adenocarcinoma.89 CDK1 activity is controlled by phosphorylation, a process finely regulated by the WEE1 and PLK1 kinases. WEE1 delays mitosis by suppressing the activity of the cyclinB1/CDK1 complex whereas PLK1 favors mitosis by allowing the activation of cyclinB1/CDK1 complex. Downregulation of WEE1 expression has been reported in lung tumors. Patients with lack of WEE1 have a higher recurrence rate and a poorer prognosis and WEE1 expression is a significant prognostic factor in multivariate analysis.86 Therefore, the loss of WEE1 may have a potential role in promoting tumor progression and may be a significant prognostic indicator in NSCLC. By contrast, elevated levels of PLK1 are observed in NSCLC and overexpression of PLK1 is a negative prognostic factor in NSCLC patients.90 Interestingly, mice heterozygous for PLK4, another member of PLK family involved in centrosome separation and mitotic fidelity, develop lung tumors due to high frequency of mitotic errors.91 Although the status of PLK4 has not been yet studied in lung tumors, these data suggest that that PLKs might have opposite roles in lung tumorigenesis.
As defects in mitosis can lead to genomic instability, deregulation of the expression and activity of Aurora kinase family members has been implicated in tumorigenesis. A certain number of studies indicate that dysregulation of Aurora kinases plays a key role during lung carcinogenesis. Overexpression of Aurora A transcript and protein has been reported in NSCLC as compared to normal lung tissue and was correlated with poor differentiation.92 Association between Aurora A polymorphisms and lung cancer risk has also been described.93 Moreover, a general upregulation of Aurora B mRNA levels has been observed in NSCLC compared with normal epithelium, and was correlated with the level of genetic instability of the tumors.94,95 Overexpression of Aurora B protein has also been found in NSCLC and was significantly correlated with expression of survivin, a component of the chromosomal passenger complex.96 In this study high Aurora B expression levels were significantly associated with squamous cell carcinoma histology, poor tumor differentiation and lymph node invasion and predicted shorter survival for the patients with adenocarcinoma histology.
Although less extensively studied than Aurora genes, other mitotic genes display lung cancer-associated altered expression. They include microtubule-associated proteins such as TPX2 and TACC3 which overexpression has been associated with poor clinical outcome.97,98 Another example is the APC/C activator, CDC20, which upregulation has been observed in early stage lung adenocarcinoma.89
Alteration of the Components of Cell Cycle Checkpoints in Lung Tumors
The cell cycle checkpoints are designed to preserve genome integrity. Therefore, abnormalities of components of these networks likely play a role in the development of tumors. For instance, mutations and methylation that reduce activity of CHFR, a mitotic checkpoint gene that delays chromosome condensation in response to microtubule poisons have been described in NSCLC.99,100 Somatic mutations of ATM that correlate with smoking history and the presence of DNA repair defects are also detected in lung adenocarcinoma101 and a significant association between ATM polymorphisms and lung cancer risk has been described.102 Moreover, the Thr68-phosphorylated activated form of the checkpoint kinase CHK2, the downstream effector of ATM, also accumulates in lung carcinoma.103–105 High levels of activated CHK2 are observed in pre-invasive lesions together with phospho-ATM, histone H2AX and p53.17,18,106 Based on these studies, it has been proposed that the DNA damage response acts as a barrier against cancer that must be abbrogated for cancer progression. Consistently, downregulation or absence of CHK2 expression has been reported in NSCLC, mainly due to hypermethylation of the CHK2 gene promoter.107 Methylation of CHK2 is more frequent in squamous carcinoma and higher in never-smokers.108 Somatic mutations of CHK2 have been found in a small subset of lung cancer and a shorter isoform of CHK1 mRNA lacking part of the catalytic domain has also been detected in a subset of SCLC.109 Further studies are required to assess whether such abnormalities affect the activity of these kinases.
Defects in the SAC can lead to premature separation of sister chromatids and could facilitate chromosomal instability, which may favors tumor progression. Somatic mutations of several SAC regulators such as Bub1,110 and Mad1,111 have been reported in lung tumors but the effect of these mutations on mitotic checkpoint signalling has not been examined. Genetically engineered mice have also provided evidence of the critical role of other cell cycle checkpoint regulators in lung tumorigenesis although the demonstration of the genetic or epigenetic alteration of these genes has not been yet done in lung tumors. As an example, mice heterozygous for Mad2 display an increased incidence of papillary lung adenocarcinoms when compared with control animals112 and Bub3 heterozygous mice are more susceptible to 7,12-dimethylbenzanthracene (DMBA)-induced lung adenocarcinomas than their wild-type littermates.113
Cell Cycle Regulators as Targets for Lung Cancer Therapy
Aberrations in cell cycle control are a hallmark of lung tumors. Therefore, modulation of cell cycle regulators may have an important use for the treatment of these cancers. In this last part, we discuss the strategies that are currently developped to target the cell cycle machinery in lung tumors (Table 1).
Table 1.
Selected inhibitors of cell cycle regulators used in clinical trials for lung cancer therapy
| Inhibitor | Main targets | Clinical trials |
| Inhibitors of cyclin-dependent kinases | ||
| Flavopiridol also known as alvocidib | CDK1, CDK2, CDK4, CDK6, CDK7 and CDK9 | Phase I: NSCLC in combination with paclitaxel and carboplatin (ref. 115) |
| Aminothiazole SNS-032 also known as BMS-387032 (Sunesis) | CDK2, CDK7 and CDK9 (CDK1 and CDK4) | Phase I: NSCLC Sensitized radioresistant NSCLC cells to ionizing radiations (ref. 116) |
| R-roscovitine also known as CYC202 and seliciclib (Cyclacel) | CDK1, CDK2, CDK5, CDK7 and CDK9 | Phase I–II: NSCLC (ref. 117) |
| Indisulam, also known as E7070 | Not Assigned | Phase I: lung cancer in combination with irinotecan |
| SCH 727965 | CDK1, CDK2, CDK5 and CDK9 | Phase II: NSCLC |
| Inhibitors of mitotic checkpoint kinases | ||
| VX-680, also known as MK-0457 | Pan-aurora | Phase I–II: NSCLC Trials discontinued owing to QT prolongation (ref. 118) |
| Inhibitors of DNA Damage Checkpoint kinases | ||
| 7-hydroxy-staurosporine, also known as UCN-01 | CHK1 and MARK3 (PKC, PDK1, GSK3β, CDK1, CDK2 and CHK2) | Phase II: SCLC (with topotecan) |
Inhibitors of cyclin-dependent kinases.
As discussed above, CDKs are often overactive in lung cancer resulting in loss of checkpoint integrity and uncontrolled proliferation. Therefore, selective inhibitors of CDKs may limit the progression of a tumor cell through the cell cycle and facilitate the induction of apoptotic pathways. The therapeutic value of small molecules CDK inhibitors that modulate CDK by competing with ATP binding is the subject of intense work.114 First generation compounds to be evaluated in clinical trials included the pan-CDK inhibitor Flavopiridol which induced partial responses or stable disease in patients with NSCLC in a phase I study, when using in combination with the cytotoxic agents paclitaxel and carboplatin.115 A second-generation CDK inhibitor, the aminothiazole SNS-032 which was recently shown to sensitize radiotherapy-resistant NSCLC cells to ionizing radiation,116 is currently in phase I clinical trials as an intravenous agent. R-roscovitine (CYC202, seliciclib) is another pan-CDK inhibitor which has antitumor activity against a broad range of cancer cell lines and human cancer xenografts including NSCLC. This molecule is currently undergoing phase I-II trials in patients with NSCLC.117 Other inhibitors undergoing clinical trials in advanced cancers including NSCLC are indisulam (E7070) which is in phase I trial in combination with irinotecan and SCH727965 which is in phase II trial. Of note Indisulam is not a direct CDK inhibitor but it cause a depletion of cyclin E which reduces CDK2 activity.
Inhibitors of mitotic checkpoint kinases.
Many patients with cancer receive antimitotic agents that act as microtubule toxins as first-line therapy. However, because of the side effects of these drugs, these last years much work has focused on the identification of new mitotic targets that could block spindle assembly without affecting microtubules. In this respect, aurora kinases and PLKs have received particular attention and a diverse array of inhibitors have been developped. Preliminary clinical data from phase I trials have largely been consistent with cytostatic effects, with disease stabilization as the best response achieved in solid tumors. As an example, the pan-aurora inhibitor MK-0457 (VX-680) blocks tumor xenograft growth and induces tumor regressions in preclinical models.118 In phase I-II trials, MK-0457 was given to patients with previously treated tumors and disease stabilization was observed in one patient with lung tumor. However, owing to QT prolongation in 1 in 100 patients, trials have been discontinued. Numerous other compounds targeting Aurora and PLK kinases are currently in clinical development.114 Their use in lung tumors therapy has not been yet evaluated.
Inhibitors of DNA damage checkpoint kinases.
Two small molecules ATM inhibitors have been described (KU55933 and CP466722) that target ATM by blocking its ATP-binding site and display high specificity. Both compounds prevent phosphorylation of ATM effectors and sensitize cells to drugs that induce DNA double-strand breaks.119 They also specifically and reversibly disrupt ATM-dependent cell cycle checkpoint in response to DNA damage induced by ionizing radiation.120 Although this has to be fully investigated “in vivo,” the “in vitro” effects of these novel lead chemotypes are promising. At present, no specific ATR inhibitors have been identified. However, several CHK1 and CHK2 inhibitors have been developped and are currently in clinical evaluation. One of them, 7-hydroxystaurosporine (UCN-01) is undergoing Phase II trials in SCLC patients in combination with topotecan cytotoxic agent. This compound efficiently abrogates DNA damage checkpoint in cancer cells that lack p53 and have been treated with DNA-damaging drugs, resulting in mitotic catastrophe.
Concluding Remarks
These last decades, numerous studies have been conducted to examine the status of cell cycle regulators in human lung tumors. They have led to the conclusion that all the signaling networks controlling cell cycle progression are deregulated in these cancers. Based on these studies, independent prognostic factors have been identified that may be important in predicting patient outcome as well as clinical response to therapy. In addition, potential therapeutic targets have been identified and several therapeutic approaches have been developped. In this setting, the search of synthetic inhibitors of cyclin-dependent kinase as anticancer drugs is currently a growing field of research, and new generation of CDK inhibitors with superior activity and specificity is the subject of intense exploration. Moreover, there have been recent advances in the development of drugs that target checkpoints and mitotic kinases, and several ATP-competitive inhibitors are currently in clinical evaluation. Current research goals also include combination of cell cycle kinases inhibitors with classical chemotherapy to enhance clinical efficacy. Indeed, in most human lung tumors, the function of the DNA damage checkpoint in G1 is impaired owing to the loss of p53/RB function. Treatment of these cells with chemotherapeutic agents often results in S or G2 checkpoint-mediated arrest. Therefore, abrogation of these DNA damage checkpoints is an attractive strategy currently being explored in chemotherapeutic-combined clinical trials as it could result in mitotic catastrophe and cell death. In addition, inhibitors targeting Aurora kinases have given very promising results and the knock-down of Aurora A by RNA interference have been found to sensitize lung cancer cells to chemotherapy. Further studies are needed to elucidate the mechanisms by which targeting Aurora kinase interacts with chemotherapy at the molecular and cell cycle levels but these results are very encouraging. Moreover, as cell cycle regulators are also involved in important molecular pathways with therapeutic cues in lung cancer (EGFR, Kras, Braf…), uncovering the potential usefulness of combined therapies would probably provide novel insights into lung cancer treatment.
Aknowledgments
Supported by the Ligue Nationale contre le Cancer (Equipe labellisée Ligue 2007), the Conseil Scientifique National d’AGIR à dom. and INCa (Programme National d’Excellence Spécialisé, 2005–2007).
Abbreviations
- APC/C
anaphase-promoting-complex or cyclosome
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad 3 related
- BUB1
budding uninhibited by benzimidazoles 1
- BUB1B
BUB1 homologue beta (also known as BUBR1)
- CDC20
cell division cycle 20 protein
- CDK
cyclin-dependent kinase
- CDKI or CKI
CDK inhibitors
- CHK
checkpoint kinases
- DDR
DNA damage response
- E2F
E2F transcription factor
- INCENP
inner centromere protein antigens 135/155 kDa
- MAD1
mitotic arrest deficient protein
- NE
neuroendocrine
- NSCLC
non small cell lung carcinoma
- PLK
polo-kinase
- RB
retinoblastoma
- SAC
spindle assembly checkpoint
- SCF
SKP1-cullin 1-F box protein
- SCLC
small cell lung carcinoma
- SKP2
S-phase associated kinase protein 2
- TACC3
transforming acidic coiled coil-containing protein 3
- TPX2
targeting protein for xenopus kinesin-like protein 2
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10977
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 3.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 5.Lam EW, La Thangue NB. DP and E2F proteins: coordinating transcription with cell cycle progression. Curr Opin Cell Biol. 1994;6:859–866. doi: 10.1016/0955-0674(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 6.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 8.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 10.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 11.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 12.Vader G, Medema RH, Lens SM. The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173:833–837. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang CJ, Lin CY, Tang TK. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290:398–410. doi: 10.1016/j.ydbio.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59:249–263. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- 15.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 16.Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 17.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 18.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 19.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 20.Yokota J, Mori N, Akiyama T, Shimosato Y, Sugimura T, Terada M. Multiple genetic alterations in small-cell lung carcinoma. Princess Takamatsu Symp. 1989;20:43–48. [PubMed] [Google Scholar]
- 21.Harbour JW, Lai SL, Whang-Peng J, Gazdar AF, Minna JD, Kaye FJ. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouyer V, Gazzeri S, Bolon I, Drevet C, Brambilla C, Brambilla E. Mechanism of retinoblastoma gene inactivation in the spectrum of neuroendocrine lung tumors. Am J Respir Cell Mol Biol. 1998;18:188–196. doi: 10.1165/ajrcmb.18.2.3008. [DOI] [PubMed] [Google Scholar]
- 23.Gouyer V, Gazzeri S, Brambilla E, Bolon I, Moro D, Perron P, et al. Loss of heterozygosity at the RB locus correlates with loss of RB protein in primary malignant neuro-endocrine lung carcinomas. Int J Cancer. 1994;58:818–824. doi: 10.1002/ijc.2910580612. [DOI] [PubMed] [Google Scholar]
- 24.Haga Y, Hiroshima K, Iyoda A, Shibuya K, Shimamura F, Iizasa T, et al. Ki-67 expression and prognosis for smokers with resected stage I non-small cell lung cancer. Ann Thorac Surg. 2003;75:1727–1732. doi: 10.1016/s0003-4975(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 25.Reissmann PT, Koga H, Takahashi R, Figlin RA, Holmes EC, Piantadosi S, et al. Inactivation of the retinoblastoma susceptibility gene in non-small-cell lung cancer. The Lung Cancer Study Group. Oncogene. 1993;8:1913–1919. [PubMed] [Google Scholar]
- 26.D’Amico TA, Massey M, Herndon JE, 2nd, Moore MB, Harpole DH., Jr A biologic risk model for stage I lung cancer: immunohistochemical analysis of 408 patients with the use of ten molecular markers. J Thorac Cardiovasc Surg. 1999;117:736–743. doi: 10.1016/s0022-5223(99)70294-1. [DOI] [PubMed] [Google Scholar]
- 27.Claudio PP, Howard CM, Pacilio C, Cinti C, Romano G, Minimo C, et al. Mutations in the retinoblastoma-related gene RB2/p130 in lung tumors and suppression of tumor growth in vivo by retrovirus-mediated gene transfer. Cancer Res. 2000;60:372–382. [PubMed] [Google Scholar]
- 28.Baldi A, Esposito V, De Luca A, Fu Y, Meoli I, Giordano GG, et al. Differential expression of Rb2/p130 and p107 in normal human tissues and in primary lung cancer. Clin Cancer Res. 1997;3:1691–1697. [PubMed] [Google Scholar]
- 29.Baldi A, Esposito V, De Luca A, Howard CM, Mazzarella G, Baldi F, et al. Differential expression of the retinoblastoma gene family members pRb/p105, p107 and pRb2/p130 in lung cancer. Clin Cancer Res. 1996;2:1239–1245. [PubMed] [Google Scholar]
- 30.Helin K, Holm K, Niebuhr A, Eiberg H, Tommerup N, Hougaard S, et al. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc Natl Acad Sci USA. 1997;94:6933–6938. doi: 10.1073/pnas.94.13.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro GI, Edwards CD, Kobzik L, Godleski J, Richards W, Sugarbaker DJ, et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 1995;55:505–509. [PubMed] [Google Scholar]
- 32.Gazzeri S, Gouyer V, Vour’ch C, Brambilla C, Brambilla E. Mechanisms of p16INK4A inactivation in non small-cell lung cancers. Oncogene. 1998;16:497–504. doi: 10.1038/sj.onc.1201559. [DOI] [PubMed] [Google Scholar]
- 33.Wiest JS, Franklin WA, Otstot JT, Forbey K, Varella-Garcia M, Rao K, et al. Identification of a novel region of homozygous deletion on chromosome 9p in squamous cell carcinoma of the lung: the location of a putative tumor suppressor gene. Cancer Res. 1997;57:1–6. [PubMed] [Google Scholar]
- 34.Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, et al. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA. 1998;95:11891–11896. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005;11:3974–3986. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Gaeta P, et al. Cyclin D1 and retinoblastoma susceptibility gene alterations in non-small cell lung cancer. Int J Cancer. 1998;75:187–192. doi: 10.1002/(sici)1097-0215(19980119)75:2<187::aid-ijc4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Betticher DC, Heighway J, Hasleton PS, Altermatt HJ, Ryder WD, Cerny T, et al. Prognostic significance of CCND1 (cyclin D1) overexpression in primary resected non-small-cell lung cancer. Br J Cancer. 1996;73:294–300. doi: 10.1038/bjc.1996.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer. 2007;55:1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Brambilla E, Gazzeri S, Moro D, Lantuejoul S, Veyrenc S, Brambilla C. Alterations of Rb pathway (Rb-p16INK4-cyclin D1) in preinvasive bronchial lesions. Clin Cancer Res. 1999;5:243–250. [PubMed] [Google Scholar]
- 40.Jeanmart M, Lantuejoul S, Fievet F, Moro D, Sturm N, Brambilla C, et al. Value of immunohistochemical markers in preinvasive bronchial lesions in risk assessment of lung cancer. Clin Cancer Res. 2003;9:2195–2203. [PubMed] [Google Scholar]
- 41.Jin M, Inoue S, Umemura T, Moriya J, Arakawa M, Nagashima K, et al. Cyclin D1, p16 and retinoblastoma gene product expression as a predictor for prognosis in non-small cell lung cancer at stages I and II. Lung Cancer. 2001;34:207–218. doi: 10.1016/s0169-5002(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 42.Wikman H, Nymark P, Vayrynen A, Jarmalaite S, Kallioniemi A, Salmenkivi K, et al. CDK4 is a probable target gene in a novel amplicon at 12q13.3-q14.1 in lung cancer. Genes Chromosomes Cancer. 2005;42:193–199. doi: 10.1002/gcc.20122. [DOI] [PubMed] [Google Scholar]
- 43.Packenham JP, Taylor JA, White CM, Anna CH, Barrett JC, Devereux TR. Homozygous deletions at chromosome 9p21 and mutation analysis of p16 and p15 in microdissected primary non-small cell lung cancers. Clin Cancer Res. 1995;1:687–690. [PubMed] [Google Scholar]
- 44.Xiao S, Li D, Corson JM, Vijg J, Fletcher JA. Codeletion of p15 and p16 genes in primary non-small cell lung carcinoma. Cancer Res. 1995;55:2968–2971. [PubMed] [Google Scholar]
- 45.Hamada K, Kohno T, Kawanishi M, Ohwada S, Yokota J. Association of CDKN2A(p16)/CDKN2B(p15) alterations and homozygous chromosome arm 9p deletions in human lung carcinoma. Genes Chromosomes Cancer. 1998;22:232–240. doi: 10.1002/(sici)1098-2264(199807)22:3<232::aid-gcc9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 46.Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56:722–727. [PubMed] [Google Scholar]
- 47.Furonaka O, Takeshima Y, Awaya H, Ishida H, Kohno N, Inai K. Aberrant methylation of p14(ARF), p15(INK4b) and p16(INK4a) genes and location of the primary site in pulmonary squamous cell carcinoma. Pathol Int. 2004;54:549–555. doi: 10.1111/j.1440-1827.2004.01663.x. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto A, Hussain SP, Hagiwara K, Spillare EA, Rusin MR, Demetrick DJ, et al. Mutations in the p16INK4/MTS1/CDKN2, p15INK4B/MTS2 and p18 genes in primary and metastatic lung cancer. Cancer Res. 1995;55:1448–1451. [PubMed] [Google Scholar]
- 49.Rusin MR, Okamoto A, Chorazy M, Czyzewski K, Harasim J, Spillare EA, et al. Intragenic mutations of the p16(INK4), p15(INK4B) and p18 genes in primary non-small-cell lung cancers. Int J Cancer. 1996;65:734–739. doi: 10.1002/(SICI)1097-0215(19960315)65:6<734::AID-IJC4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Chaussade L, Eymin B, Brambilla E, Gazzeri S. Expression of p15 and p15.5 products in neuroendocrine lung tumours: relationship with p15(INK4b) methylation status. Oncogene. 2001;20:6587–6596. doi: 10.1038/sj.onc.1204798. [DOI] [PubMed] [Google Scholar]
- 51.Kawamata N, Miller CW, Koeffler HP. Molecular analysis of a family of cyclin-dependent kinase inhibitor genes (p15/MTS2/INK4b and p18/INK4c) in non-small cell lung cancers. Mol Carcinog. 1995;14:263–268. doi: 10.1002/mc.2940140406. [DOI] [PubMed] [Google Scholar]
- 52.Pei XH, Bai F, Smith MD, Xiong Y. p18Ink4c collaborates with Men1 to constrain lung stem cell expansion and suppress non-small-cell lung cancers. Cancer Res. 2007;67:3162–3170. doi: 10.1158/0008-5472.CAN-06-4517. [DOI] [PubMed] [Google Scholar]
- 53.Komiya T, Hosono Y, Hirashima T, Masuda N, Yasumitsu T, Nakagawa K, et al. p21 expression as a predictor for favorable prognosis in squamous cell carcinoma of the lung. Clin Cancer Res. 1997;3:1831–1835. [PubMed] [Google Scholar]
- 54.Shoji T, Tanaka F, Takata T, Yanagihara K, Otake Y, Hanaoka N, et al. Clinical significance of p21 expression in non-small-cell lung cancer. J Clin Oncol. 2002;20:3865–3871. doi: 10.1200/JCO.2002.09.147. [DOI] [PubMed] [Google Scholar]
- 55.Esposito V, Baldi A, Tonini G, Vincenzi B, Santini M, Ambrogi V, et al. Analysis of cell cycle regulator proteins in non-small cell lung cancer. J Clin Pathol. 2004;57:58–63. doi: 10.1136/jcp.57.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catzavelos C, Tsao MS, DeBoer G, Bhattacharya N, Shepherd FA, Slingerland JM. Reduced expression of the cell cycle inhibitor p27Kip1 in non-small cell lung carcinoma: a prognostic factor independent of Ras. Cancer Res. 1999;59:684–688. [PubMed] [Google Scholar]
- 57.Hayashi H, Ogawa N, Ishiwa N, Yazawa T, Inayama Y, Ito T, et al. High cyclin E and low p27/Kip1 expressions are potentially poor prognostic factors in lung adenocarcinoma patients. Lung Cancer. 2001;34:59–65. doi: 10.1016/s0169-5002(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S, Kamata Y, Tamo W, Koyanagi M, Hatanaka R, Yamada Y, et al. Relationship between postoperative recurrence and expression of cyclin E, p27 and Ki-67 in non-small cell lung cancer without lymph node metastases. Int J Clin Oncol. 2002;7:349–355. doi: 10.1007/s101470200053. [DOI] [PubMed] [Google Scholar]
- 59.Tsukamoto S, Sugio K, Sakada T, Ushijima C, Yamazaki K, Sugimachi K. Reduced expression of cell cycle regulator p27(Kip1) correlates with a shortened survival in non-small cell lung cancer. Lung Cancer. 2001;34:83–90. doi: 10.1016/s0169-5002(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 60.Ishihara S, Minato K, Hoshino H, Saito R, Hara F, Nakajima T, et al. The cyclin-dependent kinase inhibitor p27 as a prognostic factor in advanced non-small cell lung cancer: its immunohistochemical evaluation using biopsy specimens. Lung Cancer. 1999;26:187–194. doi: 10.1016/s0169-5002(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 61.Yatabe Y, Masuda A, Koshikawa T, Nakamura S, Kuroishi T, Osada H, et al. p27KIP1 in human lung cancers: differential changes in small cell and non-small cell carcinomas. Cancer Res. 1998;58:1042–1047. [PubMed] [Google Scholar]
- 62.Masuda A, Osada H, Yatabe Y, Kozaki K, Tatematsu Y, Takahashi T, et al. Protective function of p27(KIP1) against apoptosis in small cell lung cancer cells in unfavorable microenvironments. Am J Pathol. 2001;158:87–96. doi: 10.1016/s0002-9440(10)63947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, et al. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381–3385. [PubMed] [Google Scholar]
- 64.Yokoi S, Yasui K, Saito-Ohara F, Koshikawa K, Iizasa T, Fujisawa T, et al. A novel target gene, SKP2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am J Pathol. 2002;161:207–216. doi: 10.1016/S0002-9440(10)64172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salon C, Merdzhanova G, Brambilla C, Brambilla E, Gazzeri S, Eymin B. E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene. 2007;26:6927–6936. doi: 10.1038/sj.onc.1210499. [DOI] [PubMed] [Google Scholar]
- 66.Pateras IS, Apostolopoulou K, Koutsami M, Evangelou K, Tsantoulis P, Liloglou T, et al. Downregulation of the KIP family members p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer. 2006;119:2546–2556. doi: 10.1002/ijc.22214. [DOI] [PubMed] [Google Scholar]
- 67.Kobatake T, Yano M, Toyooka S, Tsukuda K, Dote H, Kikuchi T, et al. Aberrant methylation of p57KIP2 gene in lung and breast cancers and malignant mesotheliomas. Oncol Rep. 2004;12:1087–1092. [PubMed] [Google Scholar]
- 68.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eymin B, Gazzeri S, Brambilla C, Brambilla E. Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinoma. Oncogene. 2001;20:1678–1687. doi: 10.1038/sj.onc.1204242. [DOI] [PubMed] [Google Scholar]
- 70.Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, et al. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci USA. 2005;102:9625–9630. doi: 10.1073/pnas.0504126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorgoulis VG, Zacharatos P, Mariatos G, Kotsinas A, Bouda M, Kletsas D, et al. Transcription factor E2F-1 acts as a growth-promoting factor and is associated with adverse prognosis in non-small cell lung carcinomas. J Pathol. 2002;198:142–156. doi: 10.1002/path.1121. [DOI] [PubMed] [Google Scholar]
- 72.Huang CL, Liu D, Nakano J, Yokomise H, Ueno M, Kadota K, et al. E2F1 overexpression correlates with thymidylate synthase and survivin gene expressions and tumor proliferation in non small-cell lung cancer. Clin Cancer Res. 2007;13:6938–6946. doi: 10.1158/1078-0432.CCR-07-1539. [DOI] [PubMed] [Google Scholar]
- 73.Karakaidos P, Taraviras S, Vassiliou LV, Zacharatos P, Kastrinakis NG, Kougiou D, et al. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability—evidence of E2F-1 transcriptional control over hCdt1. Am J Pathol. 2004;165:1351–1365. doi: 10.1016/S0002-9440(10)63393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imai MA, Oda Y, Oda M, Nakanishi I, Kawahara E. Overexpression of E2F1 associated with LOH at RB locus and hyperphosphorylation of RB in non-small cell lung carcinoma. J Cancer Res Clin Oncol. 2004;130:320–3206. doi: 10.1007/s00432-003-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ried T, Petersen I, Holtgreve-Grez H, Speicher MR, Schrock E, du Manoir S, et al. Mapping of multiple DNA gains and losses in primary small cell lung carcinomas by comparative genomic hybridization. Cancer Res. 1994;54:1801–1806. [PubMed] [Google Scholar]
- 76.Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 77.Mishina T, Dosaka-Akita H, Hommura F, Nishi M, Kojima T, Ogura S, et al. Cyclin E expression, a potential prognostic marker for non-small cell lung cancers. Clin Cancer Res. 2000;6:11–16. [PubMed] [Google Scholar]
- 78.Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Prognostic significance of cyclin E overexpression in resected non-small cell lung cancer. Cancer Res. 2000;60:242–244. [PubMed] [Google Scholar]
- 79.Ma Y, Fiering S, Black C, Liu X, Yuan Z, Memoli VA, et al. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc Natl Acad Sci USA. 2007;104:4089–4094. doi: 10.1073/pnas.0606537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muller-Tidow C, Metzger R, Kugler K, Diederichs S, Idos G, Thomas M, et al. Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res. 2001;61:647–653. [PubMed] [Google Scholar]
- 81.Dosaka-Akita H, Hommura F, Mishina T, Ogura S, Shimizu M, Katoh H, et al. A risk-stratification model of non-small cell lung cancers using cyclin E, Ki-67 and ras p21: different roles of G1 cyclins in cell proliferation and prognosis. Cancer Res. 2001;61:2500–2504. [PubMed] [Google Scholar]
- 82.Kosacka M, Piesiak P, Porebska I, Korzeniewska A, Dyla T, Jankowska R. Cyclin A and Cyclin E expression in resected non-small cell lung cancer stage I-IIIA. In Vivo. 2009;23:519–525. [PubMed] [Google Scholar]
- 83.Cooper WA, Kohonen-Corish MR, McCaughan B, Kennedy C, Sutherland RL, Lee CS. Expression and prognostic significance of cyclin B1 and cyclin A in non-small cell lung cancer. Histopathology. 2009;55:28–36. doi: 10.1111/j.1365-2559.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 84.Dobashi Y, Shoji M, Jiang SX, Kobayashi M, Kawakubo Y, Kameya T. Active cyclin A-CDK2 complex, a possible critical factor for cell proliferation in human primary lung carcinomas. Am J Pathol. 1998;153:963–972. doi: 10.1016/S0002-9440(10)65638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volm M, Koomagi R, Mattern J, Stammler G. Cyclin A is associated with an unfavourable outcome in patients with non-small-cell lung carcinomas. Br J Cancer. 1997;75:1774–1778. doi: 10.1038/bjc.1997.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol. 2004;15:252–256. doi: 10.1093/annonc/mdh073. [DOI] [PubMed] [Google Scholar]
- 87.Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Kimura Y, et al. Clinical implication of cyclin B1 in non-small cell lung cancer. Oncol Rep. 2003;10:1381–1386. [PubMed] [Google Scholar]
- 88.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 89.Singhal S, Amin KM, Kruklitis R, DeLong P, Friscia ME, Litzky LA, et al. Alterations in cell cycle genes in early stage lung adenocarcinoma identified by expression profiling. Cancer Biol Ther. 2003;2:291–298. doi: 10.4161/cbt.2.3.399. [DOI] [PubMed] [Google Scholar]
- 90.Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 91.Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 92.Xu HT, Ma L, Qi FJ, Liu Y, Yu JH, Dai SD, et al. Expression of serine threonine kinase 15 is associated with poor differentiation in lung squamous cell carcinoma and adenocarcinoma. Pathol Int. 2006;56:375–380. doi: 10.1111/j.1440-1827.2006.01974.x. [DOI] [PubMed] [Google Scholar]
- 93.Gu J, Gong Y, Huang M, Lu C, Spitz MR, Wu X. Polymorphisms of STK15 (Aurora-A) gene and lung cancer risk in Caucasians. Carcinogenesis. 2007;28:350–355. doi: 10.1093/carcin/bgl149. [DOI] [PubMed] [Google Scholar]
- 94.Smith SL, Bowers NL, Betticher DC, Gautschi O, Ratschiller D, Hoban PR, et al. Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93:719–729. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heighway J, Knapp T, Boyce L, Brennand S, Field JK, Betticher DC, et al. Expression profiling of primary non-small cell lung cancer for target identification. Oncogene. 2002;21:7749–7763. doi: 10.1038/sj.onc.1205979. [DOI] [PubMed] [Google Scholar]
- 96.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 97.Jung CK, Jung JH, Park GS, Lee A, Kang CS, Lee KY. Expression of transforming acidic coiled-coil containing protein 3 is a novel independent prognostic marker in non-small cell lung cancer. Pathol Int. 2006;56:503–509. doi: 10.1111/j.1440-1827.2006.01998.x. [DOI] [PubMed] [Google Scholar]
- 98.Ma Y, Lin D, Sun W, Xiao T, Yuan J, Han N, et al. Expression of targeting protein for xklp2 associated with both malignant transformation of respiratory epithelium and progression of squamous cell lung cancer. Clin Cancer Res. 2006;12:1121–1127. doi: 10.1158/1078-0432.CCR-05-1766. [DOI] [PubMed] [Google Scholar]
- 99.Corn PG, Summers MK, Fogt F, Virmani AK, Gazdar AF, Halazonetis TD, et al. Frequent hypermethylation of the 5′ CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis. 2003;24:47–51. doi: 10.1093/carcin/24.1.47. [DOI] [PubMed] [Google Scholar]
- 100.Mariatos G, Bothos J, Zacharatos P, Summers MK, Scolnick DM, Kittas C, et al. Inactivating mutations targeting the chfr mitotic checkpoint gene in human lung cancer. Cancer Res. 2003;63:7185–7189. [PubMed] [Google Scholar]
- 101.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landi S, Gemignani F, Canzian F, Gaborieau V, Barale R, Landi D, et al. DNA repair and cell cycle control genes and the risk of young-onset lung cancer. Cancer Res. 2006;66:11062–11069. doi: 10.1158/0008-5472.CAN-06-1039. [DOI] [PubMed] [Google Scholar]
- 103.Eymin B, Claverie P, Salon C, Leduc C, Col E, Brambilla E, et al. p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol Cell Biol. 2006;26:4339–4350. doi: 10.1128/MCB.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raynaud CM, Mercier O, Commo F, Dartevelle P, Gomez-Roca C, de Montpreville V, et al. Telomere length, telomeric proteins and DNA damage repair proteins are differentially expressed between primary lung tumors and their adrenal metastases. Lung Cancer. 2009;65:144–149. doi: 10.1016/j.lungcan.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 105.DiTullio RA, Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol. 2002;4:998–1002. doi: 10.1038/ncb892. [DOI] [PubMed] [Google Scholar]
- 106.Nuciforo PG, Luise C, Capra M, Pelosi G, d’Adda di Fagagna F. Complex engagement of DNA damage response pathways in human cancer and in lung tumor progression. Carcinogenesis. 2007;28:2082–2088. doi: 10.1093/carcin/bgm108. [DOI] [PubMed] [Google Scholar]
- 107.Zhang P, Wang J, Gao W, Yuan BZ, Rogers J, Reed E. CHK2 kinase expression is downregulated due to promoter methylation in non-small cell lung cancer. Mol Cancer. 2004;3:14. doi: 10.1186/1476-4598-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim DS, Kim MJ, Lee JY, Lee SM, Choi JE, Lee SY, et al. Epigenetic inactivation of checkpoint kinase 2 gene in non-small cell lung cancer and its relationship with clinicopathological features. Lung Cancer. 2009;65:247–250. doi: 10.1016/j.lungcan.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Haruki N, Saito H, Tatematsu Y, Konishi H, Harano T, Masuda A, et al. Histological type-selective, tumor-predominant expression of a novel CHK1 isoform and infrequent in vivo somatic CHK2 mutation in small cell lung cancer. Cancer Res. 2000;60:4689–4692. [PubMed] [Google Scholar]
- 110.Gemma A, Seike M, Seike Y, Uematsu K, Hibino S, Kurimoto F, et al. Somatic mutation of the hBUB1 mitotic checkpoint gene in primary lung cancer. Genes Chromosomes Cancer. 2000;29:213–218. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1027>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 111.Nomoto S, Haruki N, Takahashi T, Masuda A, Koshikawa T, Fujii Y, et al. Search for in vivo somatic mutations in the mitotic checkpoint gene, hMAD1, in human lung cancers. Oncogene. 1999;18:7180–7183. doi: 10.1038/sj.onc.1203141. [DOI] [PubMed] [Google Scholar]
- 112.Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 113.Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, van Deursen JM. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–3453. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 115.George SL. Statistical issues in translational cancer research. Clin Cancer Res. 2008;14:5954–5958. doi: 10.1158/1078-0432.CCR-07-4537. [DOI] [PubMed] [Google Scholar]
- 116.Kodym E, Kodym R, Reis AE, Habib AA, Story MD, Saha D. The small-molecule CDK inhibitor, SNS-032, enhances cellular radiosensitivity in quiescent and hypoxic non-small cell lung cancer cells. Lung Cancer. 2009;66:37–47. doi: 10.1016/j.lungcan.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 117.Benson C, White J, De Bono J, O’Donnell A, Raynaud F, Cruickshank C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 119.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 120.Rainey MD, Charlton ME, Stanton RV, Kastan MB. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008;68:7466–7474. doi: 10.1158/0008-5472.CAN-08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]