Abstract
The poor prognosis of most non small cell lung carcinomas is due to their ability to efficiently invade surrounding tissues and blood vessels, finally metastasizing to distant organs. Integrin mediated adhesive interaction with the surrounding extracellular matrix is a key limiting step in the regulation of the invasive properties of several cancer cell types. Here, we examine the rising evidences about the role that integrins can play in the physiopathology of non small cell lung carcinomas by regulating cell adhesion as well as the activation of growth factors and the traffic of their cognate receptors. Modulation of the signaling pathways controlled by integrins in lung cancer cells might offer the opportunity to design and develop new drugs that might be successfully combined with conventional chemotherapy and radiotherapy.
Key words: integrins, lung cancer, metastasis, fibronectin, traffic
Introduction
Colonization of distant organs by cancer cells moving out from primary tumors is the most life-threatening event in the oncogenic process and accounts for most human cancer deaths.1 In multicellular organisms both in physiological and pathological settings, e.g., tissue morphogenesis and metastatization, cells move by dynamically adhering on proteinaceous extracellular matrices (ECM) via heterodimeric αβ integrin receptors.2 In mammals, 18 α subunits and 8 β subunits of integrins assemble into 24 distinct receptors. On the cell surface, integrin heterodimers are present in either low or high affinity conformations depending on the balance between inhibiting and activating signal transduction pathways triggered by extracellular guidance cues, such as chemokines,3 growth factors4,5 and semaphorins.6 At the same time, the regulation in space and time of cell adhesion and migration on ECM proteins also depends on the modulation of the endoexocytic shuttling of integrins back and forth from the plasma membrane.7 Thus, alterations in integrin expression levels,8 conformational activation9 and traffic10,11 can endow cancer cells with the abnormal and advantageous ability to cross the physiological barriers of the tissue of origin, invade and alter the functionality of vital organs. In addition to mediating mechanical interactions of cells with the surrounding environment, integrins can promote cancer cell proliferation, survival and differentiation by activating latent growth factors,12 regulating the traffic10,13 and the downstream signaling8 of tyrosine kinase receptors.
Lung epithelial cells adhere to a basement membrane of which laminin 332 (aka laminin 5, nicein, kalinin or epiligrin) is the major component.14 α6β4 integrin is the main epithelial laminin receptor that localizes at the basal epithelial cell surface, where it associates with intermediate filaments and plays a key role in the formation and maintenance of multiprotein adhesion complexes known as hemidesmosomes.15 α3β1, which interacts with the actin cytoskeleton, is another laminin-binding integrin that, while not directly involved in the assembly of hemidesmosomes, exerts a crucial control in the deposition and organization of the laminin 332 containing basement membrane.16 Mice lacking the α3 integrin subunit die during the first twenty four hours after birth and display a severe decrease in bronchial branching as well as in the maturation of the distal bronchiolar epithelium.17 Other integrin heterodimers, such as α5β1, αvβ3 and αvβ6, bind to ECM ligands other than those normally present in the basement membrane, such as fibronectin (FN) and osteopontin (OPN), which are instead induced together with their receptors at sites of epithelial repair and tumor development. Because of its aggressive and highly metastatic potential, lung cancer is a major cause of cancer death worldwide.18 Here we will review experimental evidences supporting a role of integrins in lung cancer progression. In particular, we will focus on non-small cell lung cancer (NSCLC) since this is the histotype in which the potential role of integrin signaling has been better documented up to now.
Fibronectin and α5β1 Integrin Regulate Invasion and EGF Receptor Signaling
FN is a large disulfide-linked dimeric glycoprotein, implicated in cell adhesion, migration and differentiation.19 FN is deposited as an insoluble cross-linked multimeric fibrillar network by many cell types and exists as soluble plasma FN as well. Each FN subunit contains several homologous modules displaying binding sites for integrins and for other ECM proteins, such as type I collagen or proteoglycans.20 During normal wound healing damaged blood vessels transiently release fibrin and plasma FN that polymerize in a matrix scaffold allowing the migration of repair cells.21 Many cancers behave as wounds that do not heal, in which chronically leaky vessels cause the formation of a fibrin/FN fibrillar network around the tumoral lesion; moreover, cancer cells themselves secrete FN.22 Hence, to growth and invade neoplastic cells have to deal with a FN-containing matrix.
Tobacco is the major risk factor for lung cancer.18 Recently, genome wide association studies identified an association between single nucleotide polymorphism in nicotinic acetylcholine receptor subunit genes and susceptibility to lung cancer.23,24 Of note, the main tobacco alkaloid nicotine stimulates lung cancer cell growth by inducing FN synthesis.25 Indeed, through the α7 nicotinic acetylcholine receptor nicotine stimulates FN mRNA and protein synthesis. Moreover, silencing or functionally blocking α5β1 integrin, the major FN receptor, impairs the mitogenic effect of nicotine on lung cancer cells.25 Increased α5β1 levels significantly correlate with lymph node metastasis of NSCLCs.26,27 In addition, 40% of the NSCLC patients with a lymph node negative status die because of tumor recurrences.28 The 5-year survival rate of node-negative NSCLC patients that overexpress α5β1 integrin is significantly worse than that of individuals with NSCLC displaying normal α5β1 expression.26,27 Thus, it is conceivable that α5β1 integrin participates in promoting both NSCLC proliferation and metastatic dissemination.
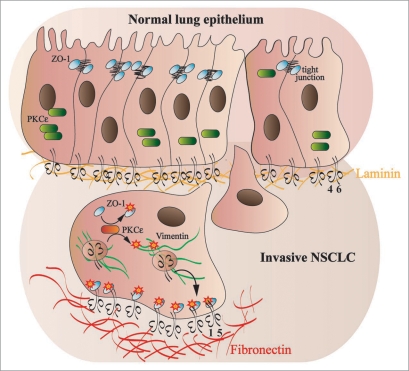
The natural history of the metastatic process implies that carcinoma cells need to disassemble the tight junctions that keep them firmly connected to neighboring epithelial cells and then exploit their integrin-mediated adhesion to migrate and colonize distant tissues and organs.29 In this respect, it is particularly relevant that the enzymatic activation of members of the protein kinase C (PKC) family can both trigger the disruption of tight junctions, e.g., by phosphorylating the zonula occludens-1 (ZO-1) protein,30 and promote cell directed motility through the control of integrin traffic.31–35 Moreover, a constitutively active version of PKCɛ has been detected in lung cancer cells.36 Notably, Tuomi and colleagues recently identified PKCɛ as a master regulator of a new molecular network controlling the migration of NSCL cells via ZO-1 and α5β1 integrin.37 Indeed, in motile NCI-H460 cells α5β1 integrin is required for the formation of the leading edge lamella, where it colocalizes with ZO-1 (Fig. 1). Here, the small GTPase Rac is known to signal the generation of new peripheral adhesive contacts (focal complexes), whereas Rho favors their maturation in focal adhesions localized under the cell body.38 Interestingly, ZO-1 silencing, while increasing Rac activation and the development of multiple protrusions containing focal complexes, significantly impairs the persistence, i.e., the straightness, of the migratory path of NSCL cells.37 In migrating NCIH460 cells, the PKCɛ-driven phosphorylation of ZO-1 on Ser168 allows its interaction with a noncanonical PSD-95-Dlg-ZO-1 (PDZ) motif in the α5 integrin cytoplasmic tail and localization at the lamella37 (Fig. 1). In addition, in situ proximity ligation revealed the presence of a ZO-1-α5β1 complex in histological samples of NSCLC patients with metastatic disease but not in carcinomas that had not metastasized. Therefore, the formation of the PKCɛ-α5β1-ZO-1 complex could play a crucial role in human NSCLC metastatization. In conclusion, in invading NSCL cells PKCɛ enzymatic activation would cause: (1) the disassembly of tight junctions; (2) the vimentin-mediated recycling of endocytosed β1 integrins; (3) the translocation of ZO-1 from tight junctions to the leading edge lamella (Fig. 1). The PKCɛ-dependent association of ZO-1 with α5β1 integrin would then stabilize the lamella, e.g., by signaling the inhibition of Rac GTPase outside of the leading edge (Fig. 1).
Figure 1.
PKCɛ regulate the stabilization of the leading edge lamella of NSCLC cells via the α5β1-ZO-1 complex. In the normal lung epithelium, α6β4 integrin mediates the adhesion to the laminin-containing basal lamina (orange fibrils), PKCɛ is inactive (green rounded rectangle) and ZO-1 (light blue oval) localizes at tight junctions, where it participates to the stabilization of these adhesive structures. Upon transformation, PKCɛ is activated (red rounded rectangle) and phosphorylates ZO-1 on Ser168, causing the tight junctions dismantling that favor the migration of NSCLC cells through a fibronectin rich extracellular matrix (red fibrils). Active PKCɛ phosphorylates the vimentin cytoskeleton (green fibrils) and promotes the polarized recycling on internalized α5β1 integrins towards the cell leading edge (black curved arrow). Moreover, phosphorylated ZO-1 is able to bind via a noncanonical PDZ binding site the cytoplasmic domain of the α5 integrin subunit. The latter interaction stabilizes the localization of the α5β1-ZO-1 complex at the leading edge lamella and inhibits the formation of multiple protrusions, thus promoting an efficient directed cell migration of invasive lung cancer cells. Since the interaction between α5β1 and ZO-1 has been selectively detected in metastasizing human NSCLC samples, the formation of the PKCɛ α5β1-ZO-1 complex could play a crucial role in human NSCLC metastatization.
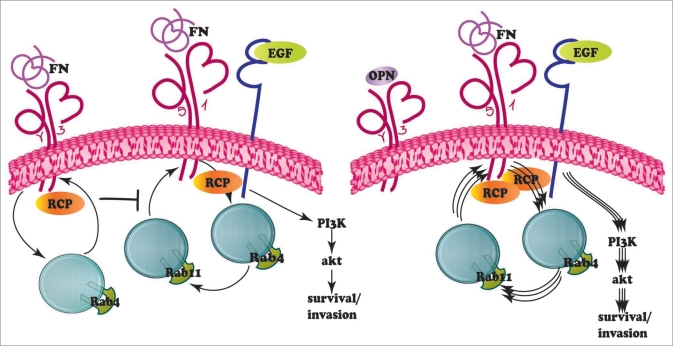
In epithelia ligand biding stimulates epidermal growth factor receptor (EGFR) tyrosine kinase activity that in turn triggers a series of signaling pathways regulating cell proliferation, survival, migration and vascularization.39 Constitutively activating EGFR mutations can occur early during human lung carcinogenesis and confer sensitivity to EGFR inhibitors.18,40 However, after the initial response the vast majority of NSCLCs acquire resistance to EGFR inhibition through still not fully characterized mechanisms.18,40 In this regard, it has been recently shown13 that in carcinoma cells the traffic of EGFR is coordinated with that of α5β1 integrin via the Rab coupling protein (RCP), a Rab11 effector (Fig. 2). In basal conditions, αvβ3 integrin, which competes with α5β1 for binding to RCP, lessen the Rab11-dependent recycling of α5β1 to the plasma membrane. Incubation of carcinoma cells with the αvβ3 soluble ligand OPN, which belongs to the small integrin-binding ligand N-linked glycoprotein (SIBLING) family,41 promotes the association of α5β1 with RCP and EGFR, thus enhancing EGFR recycling, auto-phosphorylation, and activation of the pro-invasive and pro-survival Akt signaling (Fig. 2). There is mounting evidence that SIBLINGs are soluble integrin ligands that regulate malignant progression. Indeed, OPN over-expression in tumors is associated with a poor clinical outcome of NSCLC patients.42 It is therefore possible that the OPN-activated αvβ3 integrin promotes lung cancer progression and metastatization by boosting the α5β1-EGFR signaling. Along this line, it is worth noting that one of the mechanisms by which NSCLCs become resistant to EGFR inhibition is the amplification of the hepatocyte growth factor (HGF) receptor gene encoded by the MET proto-oncogene43 and a major transcriptional target of the HGF/Met signaling is OPN.44 The value of OPN as an effective therapeutical target that could be combined with EGFR inhibitors has been already shown in preclinical studies. Indeed, silencing OPN expression or impairing its function with blocking antibodies was effective in counteracting cancer progression and metastatization in preclinical carcinoma models of esophagus, colon, liver and breast.41
Figure 2.
OPN relieves the αvβ3 inhibition on the traffic of the α5β1-EGFR complex. Integrins continuously undergo to an endo-exocytic cycle back and forth from the plasma membrane, which is regulated by members of the Rab family of small GTPases. Rab5 and Rab21 (not shown) promote integrin internalization, while Rab4 and Rab11 respectively control the fast or the slow recycling of internalized integrins. In carcinoma cells, in basal conditions Rab coupling protein (RCP) associates with both αvβ3 and α5β1 integrins. αvβ3 integrin signaling inhibits the Rab11-dependent recycling of α5β1 to the plasma membrane. The soluble αvβ3 ligand OPNreduces the amount of RCPbound to αvβ3 and promotes the association of α5β1 with RCPand EGFR, resulting in an enhanced EGFR recycling, auto-phosphorylation and Akt activation.
αvβ6 Integrin Controls TGFβ Activity
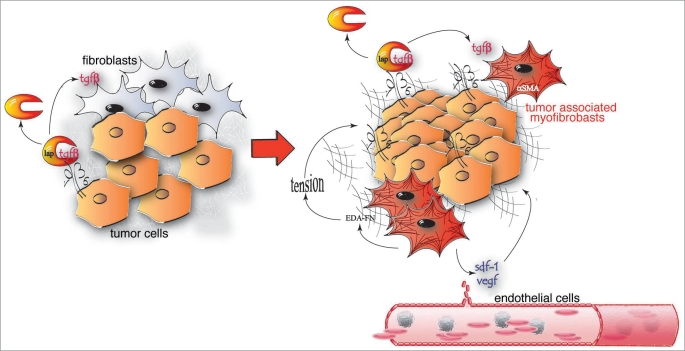
αvβ6 integrin, which is synthesized by epithelial cells mainly during development, in the adult organism is re-expressed together with its ligand FN during wound healing and inflammation.45 In addition, Kaplan-Meyer survival analysis indicates that the neo-synthesis of αvβ6 integrin in carcinoma cells is also a negative prognostic factor for the survival of NSCLC patients.46 Since this integrin is an efficient FN receptor, it is likely that expression of αvβ6, similarly to that of α5β1, endows lung tumor cells with an enhanced ability to adhere, migrate, and invade the FN-rich matrix that surrounds NSCLCs. However, an additional mechanism by which αvβ6 integrin can promote lung carcinoma progression and invasion is represented by its ability to activate the release of the ECM associated transforming growth factor β (TGFβ) in its bioactive form47,48 (Fig. 3).
Figure 3.
An αvβ6-dependent mechanical autocrine loop can support tumor growth and angiogenesis. To exert its activity on fibroblastic and tumor cells bioactive TGFβ needs to be released from the latency associated peptide (LAP). Lung cancer cells express high levels of αvβ6 integrin that binds and transmits the actomyosin contractile force to LAP, thus inducing a conformational change and the TGFβ release. TGFβ can promote the differentiation of peri-tumoral stromal fibroblasts into highly contractile αSMA-positive myofibroblasts, which secrete ED-A fibronectin (FN) and release soluble factors, such as SDF-1 and VEGF, which can support tumor growth and angiogenesis. The tension exerted by TGFβ-induced myofibroblast can in turn promote further release of bioactive TGFβ from LAP, giving rise to a mechanical autocrine loop.
TGFβ is synthesized as a large disulphide-linked homodimeric precursor that is then cleaved in the endoplasmic reticulum by furin proteases, giving rise to a small C-terminal dimer (bioactive TGFβ) and a large N-terminal latency associated peptide (LAP; Fig. 3). Bioactive TGFβ non-covalently associates with the LAP giving rise to the so called small latent complex (SLC).47 The latter is then covalently linked to the latent TGFβ-binding protein (LTBP)-1, -3 or -4 to form the large latent complex (LLC).12 Once secreted, LTBP allows the binding of LLC first to FN fibrils and then to microfibril scaffolds formed by the assembly of fibrillins on the FN network.12 The interaction of αvβ6 integrin with the microfibril-bound RGD motif of LAP allows the application of the cytoskeleton contractile force of lung epithelial cells to the LLC, likely causing its conformational modification, and the ensuing release of the sequestered TGFβ47 (Fig. 3).
Once freed from the tumor ECM, bioactive TGFβ can diffuse and foster carcinoma progression by binding to surface receptors of either tumor cells themselves or host stroma fibroblasts. Indeed, on the one hand, by promoting epithelial-mesenchymal transition (EMT), TGFβ can favor the invasive behavior of carcinoma cells.49 On the other hand, the αvβ6-mediated released of TGFβ can drive the differentiation of peri-tumoral stromal fibroblasts into α-smooth muscle actin (αSMA)-containing myofibroblasts,48,50 aka carcinoma associated fibroblasts51 (Fig. 3). In turn, peri-tumoral myofibroblasts secrete chemokines, such as SDF-1,52 or growth factors, such as VEGF,53 that can stimulate tumor growth and angiogenesis (Fig. 3). Furthermore, de novo expression of αSMA significantly enhances myofibroblast contractility and ECM stiffness.48,54 In cancer cells, increased matrix rigidity can trigger the integrin-mediated activation of both Erk mitogenic signaling and Rho-mediated contractility.55 The latter, by further augmenting the tissue stiffness, can give rise to a mechanical positive feed-back loop that contributes to malignant progression.56 Furthermore, myofibroblast-generated biomechanical forces have been recently shown to cause the translocation of existing vascular loops into contracting tissues,57 a phenomenon very similar to the vessel co-option strategy adopted by cancers for being vascularized.58
The Integrin-Linked Kinase Signaling in Lung Cancer
Integrin-linked kinase (ILK) is a multifunctional protein, which participates in integrin biochemical signaling by acting both as an adaptor and a serine-threonine kinase enzyme.59 ILK consists of an N-terminal domain containing four ankyrin repeats, a central phosphatidylinositol three phosphate (PIP3) binding pleckstrin homology (PH) domain and a C-terminal kinase domain that interacts directly with several β subunit of integrins and the focal adhesion proteins paxillin and parvins.60
Integrin-mediated adhesion to the ECM stimulates phosphatidylinositol 3 kinase that through PIP3 activates ILK; by phosphorylation ILK then activates Akt and inactivates glycogen synthase kinase 3β (GSK-3β).61,62 Once activated by ILK, Akt promotes resistance to death of epithelial cells put in suspension (anoikis). Indeed, active Akt triggers anti-apoptotic pathways, such as the inactivation by phosphorylation of the Bcl-2/Bcl-XL-associated death promoter (BAD), a negative regulator of cell survival that binds and blocks the anti-apoptotic Bcl-2 family members.63 Inhibition of GSK-3β by ILK causes instead the accumulation of β-catenin and the consequent activation of the T cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors, which by stimulating the expression of cyclin D1 support cell cycle progression and proliferation. GSK-3β inhibition results in the upregulation NFκB-dependent of the transcriptional repressor Snail64 that suppresses E-cadherin expression and promotes EMT.65
Two independent histopathological studies demonstrated that a strong cytoplasmic staining of ILK is a poor prognostic factor in NSCLC,66,67 and increased phosphorylation in Ser 473 of the ILK effector Akt is an additional independent predictor of unfavorable prognosis as well.66 While the molecular mechanism responsible for ILK overexpression in several NSCLC is still undefined, it is conceivable that the activation of the integrin-ILK-Akt signaling pathway provides a significant advantage for NSCLC cell proliferation, survival, and invasion. Hence, the inhibition of ILK signaling could represent a new avenue to feed in NSCLC treatment. Up to now four generations of small molecule inhibitors of ILK have been developed and one of them, KP-392, was tested alone or in combination with cisplatin in a pre-clinical model of NSCLC.68 KP-392 was as effective as cisplatin in enhancing survival and the combination of the two drugs was significantly more effective than the single agents alone. Moreover, the combination KP-392/cisplatin inhibited NSCLC metastatization to kidney, bone and contralateral lung.68
The high mortality rate of NSCLC demands a likewise effective search for molecular mechanisms and new pharmacological targets to begin to alleviate the particular aggressiveness of this cancer histotype. The signaling pathways by which integrins and their ligands control the adhesion, migration, proliferation and survival of lung cancer cells might represent new therapeutical opportunities to develop drugs that might be successfully combined with chemotherapy and/or radiotherapy.
Acknowledgements
Research in the authors’ labs is supported by Telethon Italy (GGP04127 and GGP09175 to G. S.), Fondazione Guido Berlucchi (to G. S.); Associazione Augusto per la Vita (to G. Serini); Compagnia di San Paolo (to G. S.); Associazione Italiana per la Ricerca sul Cancro (to G. S. and F. B.); Ministero della Salute—Programma Ricerca Oncologica 2006 and Ricerca Finalizzata 2006 (to G. S. and F. B.); Regione Piemonte—Ricerca Sanitaria Finalizzata 2006 and 2008, Ricerca Scientifica Applicata 2004: grants D10 and A150, Ricerca industriale e sviluppo precompetitivo 2006: grants PRESTO and SPLASERBA, Piattaforme Tecnologiche per le Biotecnologie: grant Druidi (to G. S. and F. B.); Fondazione Cassa di Risparmio di Torino (to F. B.).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10976
References
- 1.Yilmaz M, Christofori G, Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535–541. doi: 10.1016/j.molmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Weber C, Kitayama J, Springer TA. Differential regulation of beta1 and beta2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 5.Trusolino L, Serini G, Cecchini G, Besati C, Ambesi-Impiombato FS, Marchisio PC, De Filippi R. Growth factor-dependent activation of alphavbeta3 integrin in normal epithelial cells: implications for tumor invasion. J Cell Biol. 1998;142:1145–1156. doi: 10.1083/jcb.142.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 7.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 9.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 10.Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol. 2006;294:271–279. doi: 10.1016/j.ydbio.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol. 2008;20:589–596. doi: 10.1016/j.ceb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha3beta1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 18.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 22.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 23.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 24.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol. 2007;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- 26.Adachi M, Taki T, Higashiyama M, Kohno N, Inufusa H, Miyake M. Significance of integrin alpha5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin Cancer Res. 2000;6:96–101. [PubMed] [Google Scholar]
- 27.Han JY, Kim HS, Lee SH, Park WS, Lee JY, Yoo NJ. Immunohistochemical expression of integrins and extracellular matrix proteins in non-small cell lung cancer: correlation with lymph node metastasis. Lung Cancer. 2003;41:65–70. doi: 10.1016/s0169-5002(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 28.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 30.Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol. 1992;117:169–178. doi: 10.1083/jcb.117.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivaska J, Whelan RD, Watson R, PJ P. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivaska J, Kermorgant S, Whelan R, Parsons M, Ng T, Parker PJ. Integrin-protein kinase C relationships. Biochem Soc Trans. 2003;31:90–93. doi: 10.1042/bst0310090. [DOI] [PubMed] [Google Scholar]
- 33.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxter G, Oto E, Daniel-Issakani S, Strulovici B. Constitutive presence of a catalytic fragment of protein kinase C epsilon in a small cell lung carcinoma cell line. J Biol Chem. 1992;267:1910–1917. [PubMed] [Google Scholar]
- 37.Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ, et al. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signal. 2009;2:32. doi: 10.1126/scisignal.2000135. [DOI] [PubMed] [Google Scholar]
- 38.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 39.Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19:124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 41.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrinbinding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang YS, Kim HJ, Chang J, Ahn CM, Kim SK. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007;57:373–380. doi: 10.1016/j.lungcan.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 44.Medico E, Gentile A, Lo Celso C, Williams TA, Gambarotta G, Trusolino L, Comoglio PM. Osteopontin is an autocrine mediator of hepatocyte growth factor-induced invasive growth. Cancer Res. 2001;61:5861–5868. [PubMed] [Google Scholar]
- 45.Sheppard D. Functions of pulmonary epithelial integrins: from development to disease. Physiol Rev. 2003;83:673–686. doi: 10.1152/physrev.00033.2002. [DOI] [PubMed] [Google Scholar]
- 46.Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, et al. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–5895. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 47.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol. 2004;16:552–557. doi: 10.1016/j.ceb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGFbeta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 51.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 52.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 53.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 54.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15:657–664. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 58.de Waal RM, Leenders WP. Sprouting angiogenesis versus co-option in tumor angiogenesis. EXS. 2005:65–76. doi: 10.1007/3-7643-7311-3_5. [DOI] [PubMed] [Google Scholar]
- 59.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 60.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 61.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18:8024–8032. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- 63.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27:53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 64.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 65.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 66.Okamura M, Yamaji S, Nagashima Y, Nishikawa M, Yoshimoto N, Kido Y, et al. Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer. Hum Pathol. 2007;38:1081–1091. doi: 10.1016/j.humpath.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Costello PC, Pham NA, Pintillie M, Jabali M, Sanghera J, et al. Integrin-linked kinase inhibitor KP-392 demonstrates clinical benefits in an orthotopic human non-small cell lung cancer model. J Thorac Oncol. 2006;1:771–779. [PubMed] [Google Scholar]