Abstract
The MET tyrosine kinase signaling pathway is upregulated in many cancers, including lung cancer. The pathway normally promotes mitosis, cell motility and cell survival; but in cancer it can also promote cell proliferation, invasion, metastasis and angiogenesis. The activating ligand, hepatocyte growth factor (HGF) is normally secreted by fibroblasts and smooth muscle cells, but can also be produced by tumor cells. MET upregulation in lung cancer is caused by overexpression and mutation. These mutations can vary with ethnicity. MET signaling affects cytoskeletal proteins such as paxillin, which participates in cell adhesion, growth and motility. Therapeutic approaches that block MET signaling are being studied, and include the use of: small interference RNA, Geldanamycin, competitive HGF homologues, decoy receptors and direct MET inhibitors such as K252a, SU11274, PHA665752 and PF2341066. It is hoped that blocking MET signaling may one day become an effective treatment for some lung cancers.
Key words: lung cancer, MET, tyrosine kinase, paxillin, hepatocyte growth factor
Introduction
For centuries physicians have been pursuing a cure for cancer. Much has changed since one physician’s 1857 lament, “From the many and frequent failures in attempting to cure this disease, it is now supposed by most surgeons to be incurable…”.1 Since that time the treatment arsenal has improved, with patients no longer being instructed to ingest hemlock, mercury, cod-liver oil, or grey lizards by the hundreds.1 Indeed greater understanding of cancer’s molecular basis has yielded compounds, such as the small molecule inhibitor imatinib, with extraordinary effectiveness. The success of this tyrosine kinase inhibitor has fostered interest in other tyrosine kinase pathways active in cancer, such as the MET tyrosine kinase pathway. This review discusses the role of MET in oncogenesis—especially lung cancer. Attention is paid to the molecular biology of MET, its interactions with the cytoskeleton and potential therapies that may one day treat cancer by altering MET signaling.
Normal Physiology and Biochemistry of MET
Researchers discovered the MET oncogene in 1984 and soon recognized it to be a tyrosine kinase receptor located at 7q21-q31.2,3 The MET tyrosine kinase is activated by a single ligand termed either hepatocyte growth factor (HGF) or scatter factor (SF). This molecule is secreted by mesenchymal cells4 especially fibroblasts and smooth muscle cells5,6 and activates MET via paracrine mechanisms.7,8 Of interest to oncologists, HGF can also be produced by tumor cells; with moderate expression observed in 45% of lung cancer tumors according to one study.9 Among the many effects of HGF signaling are increased cell movement and blood vessel formation.10
MET biochemistry.
The MET gene contains 21 exons separated by 20 introns.10 The transcript produces a 150 kDa precursor protein,11 which is glycosylated and cleaved, yielding an extracellular 50 kDa alpha chain and a transmembrane 140 kDa beta chain.12 The two chains are linked with disulfide bonds.12
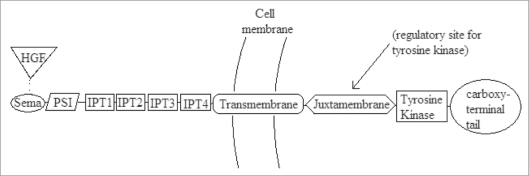
The beta chain is often divided into seven domains (Fig. 1), which have functional significance and homology with other cell signaling proteins. The semaphorin (or Sema) domain is the HGF binding site, and is related to the plexin receptor.8,12 The presence of a semaphorin places MET in a subfamily of tyrosine kinases that includes Ron and Sea.11 The PSI domain is so named because it is found in plexins, semaphorins and integrins.12 The four IPT repeats are so named because they are found in immunoglobulins, plexins and transcription factors.12 Like all tyrosine kinases, the transmembrane domain contains a single alpha helix.8 There is also a juxtamembrane domain; a tyrosine kinase domain that shares homology with insulin growth factor I receptors and the Tyro 3 family of immunoregulatory molecules8 and a carboxy-terminal tail region.
Figure 1.
Schematic of the MET beta chain (140 kDa) containing SEMA domain (HGF binding site), PSI domain, 4 IPT repeats, transmembrane domain, juxtamembrane domain, tyrosine kinase and carboxyterminal tail.
Molecular signaling.
Activation of the MET pathway involves a number of molecular events, yet because MET signaling affects many pathways, it is difficult to link specific signals to specific biological outcomes.12 Nevertheless, many of the molecular participants warrant comment.
When HGF binds to its receptor, MET autophosphorylates the tyrosine residues Y1230/1234/1235, which are located within the activating loop of the tyrosine kinase domain. This activates the intrinsic kinase activity of MET, causing downstream signaling molecules to be phosphorylated.13,14
Other phosphorylation sites have been identified as well. When Y1313 is phosphorylated it binds and activates phosphatidylinositol-3′kinase (PI3K), which likely promotes cell viability and motility. The Y1003 site, located in the juxtamembrane domain is a negative regulatory site for MET signaling that acts by recruiting c-CBL. Additionally, Y1365 regulates cell morphogenesis when phosphorylated.13
Sites Y1349 and Y1356 are located within the carboxy-terminal tail, and when phosphorylated/activated they become a multisubstrate signal-transducer site (Y1349VHVX3Y1356VNV).15 Many signaling and adaptor proteins are recruited and phosphorylated here: SH2 (Src homology-2), Src, Src kinase, SHP2 phosphatase, SHC, Gab 1, Grb2, Crk/CRKL, STAT3 (signal transducer and activator of transcription-3), and PLC-γ (phospholipase C-γ).11,12,15
Results of unregulated MET signaling.
Increased MET signaling has a number of oncogenic effects involving several pathways. For instance, MET activation of FAK promotes cell proliferation, survival and migration by activating the RAS, RAC, PI3K, ERK and CAS-CRK pathways.8 MET signaling also promotes invasion, where cells degrade or remodel the surrounding matrix and migrate through tissue boundaries, often using urokinase-type plasminogen activator (uPA),16 plasminogen activator inhibitor-1 (PAI-1)17 and matrix metalloproteases (MMPs).18 Distant metastasis is promoted by MET via activation of Grb2, PI3K or Shc pathways.8 Additionally, HGF and MET promote angiogenesis, which is crucial for tumor growth.19
MET in Lung Cancer
Many of MET’s oncogenic actions are extensions of its normal physiologic activities. For example, unregulated MET activity allows mitotic signals to produce uncontrolled growth; and signals that normally enhance cell migration instead trigger invasion and metastasis. Two important ways that MET dysregulation contributes to lung cancer pathogenesis are overexpression and mutation.
MET overexpression has been observed in both non-small cell and small cell lung cancers (Table 1). As early as 1996 researchers observed that, in a sample of 42 primary human non-small cell tumors with different histotypes, 25% of the samples showed a 2–10-fold increase in MET expression when compared with adjacent normal tissue. Of note, in the same study, HGF was overexpressed 10–100 fold.20 An even higher incidence of MET overexpression was observed in a study using tissues from 32 lung cancer patients. All samples expressed MET, with strong expression observed in 61% of non-small cell cancers (14 of 23), 60% of lung carcinoids (n = 5), and 25% of small-cell cancers (n = 4). In contrast, there was no significant MET staining in the normal lung tissues used as controls.21 In a parallel finding involving 10 small-cell cancer lines, MET was highly expressed in four of the lines, moderately expressed in two, and not expressed in the four remaining cell lines.14 Recent work suggests one possible mechanism for this overexpression. A study using formalin-fixed paraffin-embedded archival adenocarcinoma tumor tissue revealed a significant increase in the number of MET gene copies when compared with EGFR, PXN and HGF.22
Table 1.
MET mutations in lung cancer
| Observed in non-small cell lung cancer | Observed in small cell lung cancer | |
| MET overexpression | X | X |
| Increased MET activity | X | X |
| Sma missense MET mutations | ||
| E168D | X | |
| L229F | X | |
| S323G | X | |
| N375S | X | X |
| S178 | X | |
| L211W | X | |
| A347T | X | |
| E355K | X | |
| M362T | X | |
| Juxtamembrane missense MET mutations | ||
| R988C | X | X |
| T1010I | X | X |
| S1058P | X | |
| Tyrosine Kinase missense MET mutations | ||
| D1304 | X |
Along with this overexpression comes an increase in MET activity, which can be identified using immunohistochemical stains for activated MET (also called p-MET because it is phosphorylated, typically at sites Y1003 or Y1230/1234/1235). Investigators observed that 100% of their small-cell lung cancer samples showed phosphorylation at the Y1003 site, and 50% were phosphorylated at Y1230/1234/1235. Non-small cell samples also showed activation/phosphorylation at Y1003 and Y1230/1234/1235: adenocarcinoma (44 and 33% respectively), large cell (86 and 57%), squamous cell (71 and 0%), carcinoid (40 and 0%). Importantly, there was preferential expression of activated p-MET in tumor cells located at the invasive front of the non-small cell lung cancer tumor tissues.21
Mutations provide another mechanism for MET dysregulation. In one collection of small-cell lung cancer samples (ten cell lines and 32 paired tumor/normal tissue samples) there were several mutations identified. Most notably, two cell lines had a novel missense mutation (R988C) in the juxtamembrane domain (exon 14), another sample also had a novel juxtamembrane missense mutation (T1010I), and one more had a missense mutation (E168D) in the Sema domain (exon 2).14 Mutations at the juxtamembrane domain are significant, because this is an important regulatory site for the catalytic functions of tyrosine kinases.14 Likewise the Sema domain allows the kinase to bind HGF, dimerize and activate.21 Not surprisingly then, even before being identified in this study Sema mutations were postulated to play a role in small cell tumor progression.14
While the overall prevalence of mutations was low in these small-cell tumor samples, their potential for causing disease progression has proven to be significant. For instance, cells transfected with the juxtamembrane mutations R988C.Met and T1010I.Met showed transient growth factor independent proliferation (significant at 48 hours, but not sustained after 72 hours). When cultured, these cells had different cellular patterning, morphology, and cytoarchitecture (compared with the MET wild type). They had longer and more numerous neurite-like projections per cell; features associated with an invasive and metastatic phenotype. They also had less cell-spreading, less cell-adhesion, and a small but significant increase in anchorage-independent growth in a soft-agar colony-formation assay. Additionally, fibroblast cells transfected with this mutated MET had increased motility and migration. In short, the R988C and T1010I mutations caused increased tumorigenicity in vitro, likely because disruption at the juxtamembrane (regulatory) domain caused enhanced constitutive protein tyrosine phosphorylation.14
Mutations are found in non-small cell tumors also. In one study (four non-small cell lines and 127 lung adenocarcinoma tumor tissues) four missense mutations were found within the Sema domain: E168D, L229F, S323G, N375S. Notably, the N375S mutation was present in three different tissue samples. The R988C and T1010I mutations discussed previously were observed, as well as an additional juxtamembrane mutation (S1058P), and an alternative splice variant (missing the 47-amino acid exon 14) that also affected the juxtamembrane domain.21
MET mutations have been observed to vary along ethnic and racial lines, with the highest frequency occurring in East Asians, according to one recent study.23 Using tumor tissue genomic DNA from 141 East Asian, 76 Caucasian and 66 African American lung cancer patients, investigators found the mutation N375S to be the most frequent non-synonymous mutation, and found it occurred more frequently in East Asians than in Caucasians, and was absent from African American samples. Two synonymous mutations were also observed in the semaphorin domain, with 534C>T(S178) seen primarily in East Asians, and 1131C>T(1377) observed mainly in African Americans. Another finding was that the MET mutations in East Asians tended to be germline mutations. If ethnic differences correlate with the type and frequency of MET mutations in lung cancer, then greater knowledge of these differences could improve clinical understanding of lung cancer’s incidence, prognosis and optimal treatments.
Another connection between MET and lung cancer involves the somewhat controversial “progenitor cell” hypothesis. Progenitor cells (or lung cancer stem cells) are located at the bronchioalveolar duct junction and possess stem-cell like properties such as self-renewal and multipotent clonality. They also express both HGF and MET. Thus it has been theorized that the MET-HGF signaling axis drives the mobilization and dissemination of both normal and lung cancer stem cells. Supporting this theory, researchers have identified a distinct cell population that overexpresses MET and Stem Cell Factor (SCF), and is located at damaged/inflamed bronchiolar and alveolar junctions.9
MET, paxillin and the cytoskeleton.
The cytoskeleton plays a central role in oncogenesis by virtue of its involvement in cell division, growth, adhesion, motility and invasion. Thus, in a variety of cancers oncogenes have been reported to interact with the cytoskeleton during oncogenesis (e.g., v-src, v-crk, BCR/ABL, E6).24 Considering MET specifically, it affects cell motility by regulating actin polymerization, depolymerization, and stress fiber formation. The signal pathway includes MET phosphorylation of the cytoskeletal proteins paxillin, p125FAK and Pyk2 (and potentially cdc42 and Rac), and the result is filopodia and lamellipodia formation and retraction.25 This pathway may explain why, for example, small cell lung cancer cells have increased motility after HGF stimulation.26
Among the many cytoskeletal proteins, paxillin may be especially important because it is activated by MET signaling and it interacts with the cytoskeleton. It stands at the crossroads of several molecular pathways, helping to integrate diverse signals promoting adhesion, growth, motility, actin reorganization and alterations of gene expression.8
While paxillin levels are often low or absent in small-cell cancers,24 high levels have been observed in a variety of non-small cell tumors. In one study, high expression of paxillin was seen in 51% of large cell carcinomas, 33% of adenocarcinomas, and 23% of squamous cell. The paxillin level also correlated with disease stage and was particularly high in metastatic samples. Many of these non-small cell lines had increased numbers of gene copies—for both paxillin and MET—suggesting an important mechanism underlying these increased paxillin levels.27
In addition to numeric expansion, mutations also increase paxillin’s effects. The overall rate of paxillin mutations in lung cancer is reported to be 9.4% and is particularly high in non-small cell cancers, with 18.4% of large cell carcinomas bearing a mutation (versus 0% of small cell carcinomas). One in particular, the A127T paxillin mutation, causes increased cell growth, focal adhesion formation, and colocalization with the anti-apoptosis protein Bcl-2 (likely to promote lung cancer cell survival). In vivo studies using mouse xenografts showed tumors with the mutation have increased growth, invasiveness, and nodularity, despite less stroma and less microvessel density (versus normal controls and wild type paxillin xenografts). Researchers also found that small interference targeting of the A127T mutation lead to decreased cell viability.27 Debate continues about the role of paxillin though, for a recent series of 159 patients with non-small cell lung cancer identified only two nonsynonymous polymorphisms, one mutation and six amplifications, without an obvious link to oncogenesis.28
Met as a Therapeutic Target in Cancer
The motivation behind so much study of MET is the hope that disrupting the pathway will inhibit cancer growth and metastasis. Various strategies have been considered (Table 2). One approach is to target MET RNA using small interference RNA (siRNA). This technique has been successful with non-small cell lung cancer cells (A549) in vitro, reducing MET protein expression by 50–60% and inhibiting viability by 57%.20 A paxillin-specific siRNA reduced cell viability by 40% in SK-LU-1 cancer cells transfected with paxillin.27 Alternatively microRNAs, which target messenger RNA, can potentially be used to regulate gene expression. In mouse models, Micro-RNA-1 (a molecule that targets MET, and which normally inhibits cell proliferation) was found to be downregulated in primary human lung cancer. Restoring this molecule, via ectopic expression of Micro-RNA-1, reduced A549 cancer cell proliferation in vivo, and reduced cell cancer cell migration and motility in vitro.29
Table 2.
MET targeted therapies
| Strategy | Molecules | Developmental stage |
| Silence MET RNA | Small interference RNA | In vitro success |
| Paxillin specific siRNA | In vitro success | |
| microRNA | In vivo success | |
| Inhibit HSP90 (chaperone protein) | Geldanamycin | Phase 2 clinical trials underway |
| Compete with HGF | Pro-HGF | In vivo success |
| NK2 | Failed in vivo study | |
| NK4 | In vivo success | |
| Bind HGF before it activates MET | Decoy MET receptor | In vivo success |
| Anti-HGF antibodies | Phase 2 clinical trials | |
| Broad spectrum (Non-specific) kinase inhibitors | K252a | In vivo success |
| RPI-1 | In vivo success | |
| Narrow spectrum (MET specific) kinase inhibitors | SU11274 | In vitro success |
| PHA665752 | In vitro success | |
| PF2341066 | In vivo success | |
| XL 880 | Phase 2 clinical trials | |
| XL184 | Phase 3 clinical trials | |
| ARQ197 | Phase 2 clinical trials | |
| SGX523 | Stopped in Phase 1 trials | |
| MGCD265 | Phase 2 clinical trials |
Another therapeutic approach is to inhibit Heat Shock Protein 90 (HSP90), a chaperone that is required for MET (and other kinases) to achieve conformational maturation and stability. Geldanamycins are the most developed of these compounds. While geldanamycin itself caused hepatotoxicity in dogs,30 the derivative 17-allylamino 17-demethoxy geldanamycin is less toxic.31 During in vitro trials with non-small cell lung cancer lines (H460, H358, H661 and H322) it was found to inhibit cell proliferation, MMP-9 secretion, and VEGF secretion.32 Phase 1 trials of 17-allylamino 17-demethoxy geldanamycin have been conducted,32,33 and phase 2 trials are being conducted for a variety of cancers.
Several modified versions of HGF have been found to compete with natural HGF. For instance Pro-HGF, the precursor of HGF, binds MET without activating it. Researchers found that in mice harboring A549 lung carcinoma cells, wild-type HGF increased tumor weight and metastasis, while mice receiving an uncleavable version of Pro-HGF had reduced tumor weight and no metastases.34 NK2 and NK4, which are variants of the HGF alpha chain containing just the N-terminal hairpin domain and either two Kringle domains (NK2) or four Kringle domains (NK4), also compete with HGF. NK2, a naturally occurring molecule, can act as an antagonist or partial agonist of MET, depending on the target cell and the culture conditions.8 NK2 has limited therapeutic potential, for although it reduced melanoma growth in mice (which were genetically modified to overexpress NK2), it also increased metastasis nine-fold relative to wild type mice.35 However NK4 has shown more promising experimental results. NK4 is produced by proteolytic digestion of HGF, and acts as an antagonist—binding MET without activating it.36 NK4 also inhibits angiogenesis, possibly due to its resemblance to angiostatin,37 or by inhibiting extracellular fibronectin assembly in endothelial cells.36 In mouse models using Lewis Lung Carcinoma, researchers used plasmid lipofection (adeno-associated virus vector) to administer NK4, and found reduced average metastatic burden compared with the untreated group.38
Binding HGF before it can activate MET is another strategy. With this in mind, decoy MET receptors have been developed that bind both HGF and endogenous MET. The decoy receptors inhibited MET activation in vitro, and caused impaired tumor growth and no metastases in mice harboring various human tumor cells (including A549 lung carcinomas).39 There are also antibodies to HGF40 that have been developed, and show synergy with docetaxel and temozolomide in U-87 glioblastoma mouse models.41 Currently anti-HGF (AMG 102) is being tested on lung cancer and several other cancers in phase II clinical trials.
A variety of direct MET inhibitors can also interfere with signaling. RPI-1 was previously known to inhibit Ret tyrosine kinase oncoproteins, but recently was found to inhibit the MET receptor in vitro. Additionally, in vivo studies of H460 non-small cell lung cancer cells in mice indicated orally administered RPI-1 significantly reduced lung metastases and angiogenesis.42 Another molecule, K252a, is a broad spectrum kinase inhibitor, derived from a group of natural alkaloids. It inhibits kinase activity by competing with ATP for binding at the catalytic domain. While active against MET, particularly MET proteins with the activating mutation M1268T, it is not particularly selective for MET; it is also active against serine/threonine kinases, it inhibits members of the Trk family, and is a partial inhibitor of the PDGF receptor tyrosine kinase.43 Consequently enthusiasm for broad spectrum inhibitors is tempered by concerns that they will have more adverse effects than narrow spectrum inhibitors.8
The narrow spectrum kinase inhibitor SU11274 is active against MET, but not active against other tyrosine kinase oncoproteins such as BCR-ABL, TEL-JAK2, TELPDGFβR and TEL-ABL. Indeed it is 50x more selective for MET than for other tyrosine kinases. SU11274 acts by competing with ATP for the catalytic site on MET. It also inhibits autophosphorylation at TPR-MET domains TYR361/365/366 (autophosphorylation sites), Tyr480 (Grb2 binding site), and Tyr 496 (important in cell morphogenesis). The downstream effect is inhibition of molecules in the PI3K pathway: AKT, FKHR, GSK3β. In vitro Studies conducted with TPR-MET transformed BaF3 cells and lung cancer cells overexpressing MET (H69 and H345 cell lines) showed that SU11274 causes a dose-dependent reduction of cell growth, induction of G1 cell cycle arrest, promotion of apoptosis with increased caspase 3 activity and Annexin V staining and reduced migration.25
Two other narrow spectrum kinase inhibitors warrant mention. PHA665752 selectively inhibits MET in tumor cells (BaF3 cells with TPR-MET mutation and H441 non-small cell lung cancer cells), thus causing cell cycle arrest, decreasing motility and migration, and inducing apoptosis. Activity of this drug was enhanced by simultaneous use of rapamycin, suggesting a possible combination therapy.44 In further studies of mouse xenografts, PHA665752 reduced tumorigenicity by 99% in small cell lung cancer and by 75% in non-small cell lung cancer (NCI-H69 and NCI H441 mouse models), probably via significant inhibition of angiogenesis.45 Another compound, PF2341066, is an orally available selective competitor for MET that inhibits tumor cell growth both in vitro and in vivo (mouse xenografts).46 Recently, PF2341066 has been shown also to inhibit the ALK (anaplastic lymphoma kinase) pathway,46 which suggests it could be effective against non-small cell lung cancers harboring ALK gene alterations.47 Several other kinase inhibitors also show promise, and have reached clinical trials: XL 880,48 XL184,49 ARQ197, SGX523,50 (stopped in phase I due to renal toxicity),6 and MGCD265.
Combination therapies are also being considered, for MET inhibition can restore chemotherapy susceptibility in some chemotherapy resistant tumors. In particular tyrosine kinase inhibitors, such as Gefitinib and erlotinib, are used to treat non-small cell lung cancers that have an activating mutation in the EGFR gene. Most of these tumors respond initially but ultimately become resistant to therapy with tyrosine kinase inhibitors. Researchers have recently attributed some of this resistance to focal amplification of MET. Exposing these cells to the MET inhibitor PHA665752 restored sensitivity to gefitinib—inhibiting growth, reducing EGFR phosphorylation, and inducing apoptosis. Likewise, RNA interference directed against two regions of MET restored gefitinib sensitivity, giving further evidence that MET amplification causes gefitinib resistance, which can be overcome with MET inhibition.51
Another study reported MET amplification in 9 of 43 patients with acquired resistance to gefitinib or erlotinib. In contrast, only 2 of 62 untreated patients showed similar MET amplification, suggesting that MET amplification is triggered (or selected) by EGFR kinase inhibitors such as gefitinib. These researchers also reported that in vitro studies (using H820 lung adenocarcinoma cells which harbor an EGFR mutation and MET amplification) demonstrated the MET inhibitor XL880 was more effective at inhibiting lung adenocarcinoma cell viability than EGFR inhibitors (namely erlotinib and CL-387,785). Perhaps compounds like XL880 will become an important treatment for patients with EGFR mutant lung adenocarcinomas, where MET amplification occurs and treatment resistance appears.52
Recent reports have identified Tolfenamic acid, a non-steroidal anti-inflammatory drug, as another anti-cancer molecule that can decrease MET expression. It acts by downregulating or degrading several Sp-dependent genes and proteins, especially Sp1 and Sp3, which mediate MET expression. In nude mice harboring A549 and CRL5803 human lung cancer cells, Tolfenamic acid inhibited cell survival and increased apoptosis in a dose dependent manner, ultimately causing mice to have smaller tumors.53
Conclusion
Many future directions exist for research on MET. Room exists to improve the specificity of tyrosine kinase inhibitors. Many combination therapies have yet to be studied. Genetic profiling might identify subsets of patients likely to benefit from MET kinase inhibitors. Various MET mutations in tumors may respond differently to kinase inhibitors, with some having resistance and others having increased susceptibility. Targeting proteins downstream from MET could also interfere with MET induced oncogenesis. Another frontier involves experimenting with C. elegans worms that have been genetically modified to express MET mutations. Compared with mouse studies, this may prove to be a cheaper and faster way to screen for MET inhibitors.54 Resolving the crystal structure of MET could also assist the rational design of specific MET kinase inhibitors.
Knowledge about MET signaling in cancer has grown tremendously since the oncogene was discovered in the early 1980s. Still there is much work to be done to clarify the molecular mechanisms causing oncogenesis, and to harness this knowledge for the production of effective cancer therapies.
Acknowledgements
Supported in part by NIH/National Cancer Institute (5P01HL058064-140009; 5R01CA100750-06; 5R01CA125541-03), V-Foundation (Guy Geleerd Memorial Foundation), Kate McMullen Foundation, Respiratory Health Association of Chicago, and Mesothelioma Applied Research Foundation (Jeffrey P. Hayes Memorial Grant).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10973
References
- 1.Fell JW. A treatise on cancer, and its treatments. London: John Churchill, New Burlington Street; 1857. [Google Scholar]
- 2.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 3.Dean M, Park M, LeBeau MM, Robins TS, Diaz MO, Rowley JD, et al. The human met oncogene is related to the tyrosine kinase oncogenes. Nature. 1985;315:385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto Narsimhan CRP, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim ES, Salgia R. MET pathway as a therapeutic target. J Thoracic Oncol. 2009;4:444–447. doi: 10.1097/JTO.0b013e31819d6f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 8.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Can Meta Rev. 2003;22:209–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 9.Ma PC, Tretiakova MS, Mackinnon AC, Ramnath N, Johnson C, Dietrich S, et al. Expression and mutational analysis of MET in human solid cancers. Genes, Chrom Cancer. 2008;47:1025–1037. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong S, Segal S, Anver M, Resau JH, VandeWoude GF. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Nati Acad Sci. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth FR. 2002;13:41–59. doi: 10.1016/s1359-6101(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 12.Sattler M, Ma PC, Salgia R. Therapeutic targeting of the receptor tyrosine kinase met. Cancer Treat Res. 2004;119:121–138. doi: 10.1007/1-4020-7847-1_7. [DOI] [PubMed] [Google Scholar]
- 13.Maulik G, Madhiwala P, Brooks S, Ma PC, Kijima T, Tibaldi EV, et al. Activated c-Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J Cell Mol Med. 2002;6:539–553. doi: 10.1111/j.1582-4934.2002.tb00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma PC, Kijima T, Maulik G, Fox EA, Sattler M, Griffin JD, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–6281. [PubMed] [Google Scholar]
- 15.Ponzetto C, Bardelli A, Zhen Z, Maina F, della Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 16.Besser D, Bardelli A, Didichenko S, Thelen M, Comoglio PM, Ponzetto C, et al. Regulation of the urokinase-type plasminogen activator gene by the oncogene Tpr-Met involves GRB2. Oncogene. 1997;14:705–711. doi: 10.1038/sj.onc.1200879. [DOI] [PubMed] [Google Scholar]
- 17.Wojta J, Nakamura T, Fabry A, Hufnagl P, Beckmann R, McGrath K, et al. Hepatocyte growth factor stimulates expression of plasminogen activator inhibitor type 1 and tissue factor in HepG2 cells. Blood. 1994;84:151–157. [PubMed] [Google Scholar]
- 18.Dunsmore SE, Rubin JS, Kovacs SO, Chedid M, Parks WC, Welgus HG. Mechanisms of hepatocyte growth factor stimulation of keratinocyte metalloproteinase production. J Biol Chem. 1996;271:24576–24582. doi: 10.1074/jbc.271.40.24576. [DOI] [PubMed] [Google Scholar]
- 19.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivero M, Rizzo M, Madeddu R, Casadio C, Pennacchietti S, Nicotra MR, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer. 1996;74:1862–1868. doi: 10.1038/bjc.1996.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in Non-small cell lung cancer. Cancer Res. 2005;65:147914–147988. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 22.Kanteti R, Yala S, Furguson MK, Salgia R. MET HGF, EGFR and PXN gene copy number in lung cancer using DNA extracts from FFPE archival samples, and prognostic significance. J Environ Pathol Toxicol Oncol. 2009;28:89–98. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnaswamy S, Kanteti R, Duke-Cohan JS, Loganathan S, Liu W, Ma PC, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res. 2009;15:5714–5723. doi: 10.1158/1078-0432.CCR-09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgia R, Li JL, Ewaniuk D, Wang YB, Sattler M, Chen WC, et al. Expression of the focal adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene. 1999;18:67–77. doi: 10.1038/sj.onc.1202273. [DOI] [PubMed] [Google Scholar]
- 25.Sattler M, Pride YB, Ma PC, Gramlich JL, Chu SC, Quinnan LA, et al. A novel small molecule Met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63:5462–5469. [PubMed] [Google Scholar]
- 26.Maulik G, Kijima T, Ma PC, Ghosh SK, Lin G, Shapiro GI, et al. Modulation of the c-met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8:620–627. [PubMed] [Google Scholar]
- 27.Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, et al. Paxillin is a target for somatic mutations in lung cancer: Implications for cell growth and invasion. Cancer Res. 2008;68:132–142. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallier K, Houllier AM, Cazes A, Laurent-Puig P, Blons H. No somatic genetic change in the paxillin gene in nonsmall-cell lung cancer. Mol Carcinog. 2009;48:581–585. doi: 10.1002/mc.20538. [DOI] [PubMed] [Google Scholar]
- 29.Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, et al. Downregulation of Micro-Rna-1 (miR-1) in lung cancer: suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem. 2008;283:33394–33405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 31.Nowakowski GS, McCollum AK, Ames MM, Mandrekar SJ, Reid JM, Adjei AA, et al. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin Cancer Res. 2006;12:6087–6093. doi: 10.1158/1078-0432.CCR-06-1015. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen DM, Desai S, Chen A, Weiser TS, Schrump DS. Modulation of metastasis phenotypes of non-small cell lung cancer cells by 17-allylamino 17-demethoxy geldanamycin. Ann Thorac Surg. 2000;70:1853–1860. doi: 10.1016/s0003-4975(00)01810-5. [DOI] [PubMed] [Google Scholar]
- 33.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 34.Mazzone M, Basilico C, Cavassa S, Pennacchietti S, Risio M, Naldini L, et al. An uncleavable form of pro-scatter factor suppresses tumor growth and dissemination in mice. J Clin Invest. 2004;114:1418–1432. doi: 10.1172/JCI22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuka T, Jakabczak J, Vieira W, Bottaro DP, Breckenridge D, Larochelle WJ, et al. Disassociation of met-mediated biological responses in vivo: the natural hepatocyte growth factor/scatter factor splice variant NK2 antagonizes growth but facilitates metastasis. Mol Cell Biol. 2000;20:2055–2065. doi: 10.1128/mcb.20.6.2055-2065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai K, Nakamura T, Matsumoto K, Nakamura T. Angioinhibitory action of NK4 involves impaired extracellular assembly of fibronectin mediated by perlecan-NK4 association. J Biol Chem. 2009;284:22491–22499. doi: 10.1074/jbc.M109.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du W, Hattori Y, Yamada T, Matsumoto K, Nakamura T, Sagawa M, et al. NK4, an antagonist of hepatocyte growth factor (HGF), inhibits growth of multiple myeloma cells: molecular targeting of angiogenic growth factor. Blood. 2007;109:3042–3049. doi: 10.1182/blood-2006-02-003103. [DOI] [PubMed] [Google Scholar]
- 38.Buhles A, Collins SA, van Pijkeren JP, Rajendran S, Miles M, O’Sullivan GC, et al. Anti-metastatic effects of viral and non-viral mediated Nk4 delivery to tumours. Genet Vaccines Ther. 2009;7 doi: 10.1186/1479-0556-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 40.Kakkar T, Ma M, Zhuang Y, Patton A, Hu Z, Mounho B. Pharmacokinetics and safety of a fully human hepatocyte growth factor antibody, AMG 102, in cynomolgus monkeys. Pharm Res. 2007;24:1910–1918. doi: 10.1007/s11095-007-9316-2. [DOI] [PubMed] [Google Scholar]
- 41.Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 42.Cassinelli G, Lanzi C, Petrangolini G, Tortoreto M, Pratesi G, Cuccuru G, et al. Inhibition of c-Met and prevention of spontaneous metastatic spreading by the 2-indolinone RPI-1. Mol Cancer Ther. 2006;5:2388–2397. doi: 10.1158/1535-7163.MCT-06-0245. [DOI] [PubMed] [Google Scholar]
- 43.Morotti A, Mila S, Accornero P, Tagliabue E, Ponzetto C. K252a inhibits the oncogenic properties of Met, the HGF receptor. Oncogene. 2002;21:4885–4893. doi: 10.1038/sj.onc.1205622. [DOI] [PubMed] [Google Scholar]
- 44.Ma PC, Schaefer E, Christensen JG, Salgia R. A selective small molecule c-MET inhibitor, PHA665752, cooperates with rapamycin. Clin Cancer Res. 2005;11:2312–2319. doi: 10.1158/1078-0432.CCR-04-1708. [DOI] [PubMed] [Google Scholar]
- 45.Puri N, Khramtsov A, Salman A, Nallasura V, Hetzel JT, Jagadeeswaran R, et al. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67:3529–3534. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 46.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 47.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 48.Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69:8009. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 49.Sugawara M, Geffner DL, Martinez D, Hershman JM. Novel treatment of medullary thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2009;16:367–372. doi: 10.1097/MED.0b013e3283304f0c. [DOI] [PubMed] [Google Scholar]
- 50.Buchanan SG, Hendle J, Lee PS, Smith CR, Bounaud PY, Jessen KA, et al. SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol Cancer Ther. 2009;8:1–10. doi: 10.1158/1535-7163.MCT-09-0477. [DOI] [PubMed] [Google Scholar]
- 51.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to Gefitinib resistance in lung cancer by activating ERBB3 Signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 52.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colon J, Basha MR, Madero-Visbal R, Konduri S, Baker CH, Herrera LJ, et al. Tolfenamic acid decreases c-Met expression through Sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice. Invest New Drugs. 2009. In press. [DOI] [PubMed]
- 54.Siddiqui S, Loganathan S, Krishnaswamy S, Faoro L, Jagadeeswaran R, Salgia R. C. elegans as a model organism for in vivo screening in cancer: effects of human c-Met in lung cancer affect C. elegans vulva phenotypes. Cancer Biol Ther. 2008;7:856–863. doi: 10.4161/cbt.7.6.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]