Abstract
Malignant pleural mesothelioma (MPM) is a rare malignancy of the pleura with a very poor prognosis. Treatments evaluated for malignant mesothelioma, including chemotherapy, radiotherapy and surgery are of limited efficacy. However, the fact that the tumors of some patients with MPM regress spontaneously or respond to immunotherapy suggests that the immune system may respond to MPM under some circumstances. In this respect, animal studies have demonstrated immunoreactivity of MPM to different immunotherapies. In the case of MPM, several clinical studies have demonstrated a correlation between the presence of a lymphocyte infiltrate and a better prognosis and humoral response directed against specific antigens related to tumor. Thus, MPM immunotherapy is undoubtedly a highly promising but also very challenging approach to the treatment of this disease that has slipped through the defense lines of the immune system. This article reviews past and recent developments of the clinical strategies that concern immunotherapy of mesothelioma.
Key words: mesothelioma, immunotherapy, immune response, cancer virotherapy, chemo-immunotherapy
Introduction
Malignant mesothelioma are cancers affecting serous membranes (pleura, peritoneum, pericardium, vagina and ovary), more than 80% of which affect the pleura. In developed countries, pleural mesothelioma is a tumor associated, albeit not exclusively, with asbestos.1 In fact, asbestos exposure, primarily in the professional environment, is reported for 70% of cases.2 Although the incidence of mesothelioma is low in the general population, its incidence can be extremely high in certain industrial sites, especially those comprising shipbuilding harbors.3 According to various studies, the number of cases in Europe is estimated to reach a peak between 2010 and 2020. The latency period, defined as the time lapse between the first exposure to asbestos and the diagnosis of mesothelioma, is usually very long: between 30 and 40 years. The disease arises at a mean age of 60 years and is predominant in males (sex-ratio 4:1), thereby explaining its causal link to asbestos exposure. The most frequent manifestation of the disease is pleural extravasation.
Malignant Pleural Mesothelioma is a tumor with a very poor prognosis; mean survival from the time of diagnosis is less than 12 months and the 5-year survival rate is less than 5%. There are currently no recognized or standardized treatments for this disease.4 However, a large (456 eligible patients) multicentric, randomized phase III trial evaluated the association of Pemetrexed and Cisplatin and showed a survival gain of 2.8 months.5 In fact, there is no consensus today concerning the classical treatment strategies such as surgery (pleurectomy or pleuro-pneumonectomy), chemotherapy and radiotherapy.
Mesothelioma and the Immune Response
It is widely recognized that the immune system can play a fundamental role in the control of tumor growth within an organism, and especially within the context of surveillance during relapse. In the case of MPM, a study by Leigh and Webster6 demonstrated a correlation between the presence of a lymphocyte infiltration and a better prognosis. The findings of this study were supported a few years later by an immunohistochemical study showing a strong lymphocyte infiltration in a patient whose tumor showed spontaneous regression.7 Another tumor, noted later in the same patient, showed no lymphocyte infiltration and was probably at the origin of the patient’s death. In addition, a complementary serologic study showed a high titer humoral (antibody) response. The same type of humoral response was also reported by the same author in a larger number of patients.8 The latter study showed that a response directed against antigens specific to each tumor (a very specific autologous response) generally develops, whereas a response directed against “common” antigens shared by the different patients with malignant melanoma is rarely observed. Together these studies highlight the relevance and the difficulty in searching for antigens or tumor markers that could be specifically shared by patients suffering from mesothelioma.
More recently, the existence of a spontaneous humoral response that is relatively specific (shared with few other types of cancer) has been demonstrated, not only in the case of mesothelioma, but also in the case of ovarian cancer.9 Using the ELISA method, the authors reported a high level of anti-mesothelin antibodies, a glycoprotein expressed at the surface of several types of tumor cells, including mesothelioma, ovary and pancreas. Nearly 40% of patients suffering from mesothelioma and 42% of patients suffering from ovarian cancer present a high level of serum antibodies. Besides showing that the antibodies recognize mesothelin as being a highly immunogenic protein, this analysis also shows a strong potential for patients suffering from mesothelioma to mount an immune response to this relatively specific marker with antigenic properties.10
Immune Status of Patients Suffering from Mesothelioma
Reports from Robinson et al. have shown that solid tumors from patient suffering from malignant mesothelioma are potentially highly infiltrated by a population of immune cells. This tumor-immune cell association is also found within pleural liquids containing metastatic mesothelioma cells.11 However, the immunological status of patients suffering from mesothelioma is generally very “tolerogenic” towards the tumor cells. In fact, a study by Lew et al.,12 showed that although the overall lymphocyte count was not altered in patients suffering from mesothelioma, certain T lymphocyte populations (cytotoxic T lymphocytes [CTL]; helper T lymphocytes [Thelp] and “Natural Killers” cells [NK]), were significantly reduced. Very recently, Hegmans et al.13 completed a study on biopsies showing that despite a high degree of infiltration of certain tumors by macrophages and CD4+ and CD8+ T lymphocytes, antigen presenting cells (dendritic cells) were absent. However, such studies are infrequent and rarely discussed, probably because the results must be refined and correlated to the development stage of the tumor. In contrast, all of these studies point towards a significant increase in the population of “regulatory” T lymphocytes (Treg), in patient blood,14 as well as in pleural liquids, often to the detriment of effector cells.15 This shift in the immune response towards a tolerogenic state can be attributed to cytokines present in the tumor environment, whether they are in the tumor itself or in the pleural liquid. A study was carried out on the production of cytokines by mesothelioma tumor cells. This study, performed both in vivo (pleural effusion from patients) and in vitro (cell culture supernatants), demonstrated the production of angiogenin, VEGF, TGFβ as well as other immunosuppressive cytokines.16 TGFβ, a cytokine produced by numerous tumor cell types, including mesothelioma cells, is now recognized for its antigenic and immunosuppressive properties, as well as for its role in the generation and activation of Treg.17,18 The presence of numerous macrophages at the center of the tumor, an observation that has been frequently reported in the literature,19 is probably associated with the local production of anti-inflammatory cytokines with immunosuppressive functions. This production of immunoregulatory cytokines has also been described for the tumor cells themselves. Thus, the immune cell population associated with mesothelioma and its effects on the tumor environment through the production of soluble factors and cytokines can strongly favor a suppression of the immune response and thereby promote tumor development.
For this reason, in the future, the initiation of anti-tumor immunotherapy trials should also take into account the presence of considerable levels of immunosuppressive cytokines and Tregs that can be activated locally.20,21
Immunotherapy for Mesothelioma
Currently, immunotherapy includes the application of recombinant viral proteins, vaccines or antibody- and dendritic cell-based therapies. Immunotherapy is a conceptually attractive approach, because it is highly specific and can deal with disseminated disease with minimal impact on normal tissues. Ability to induce antigen-specific immune responses in patients with cancer is now well established in early-phase clinical trials using a variety of immunotherapeutic approaches. The therapeutic objective is to favor the recognition of tumor cells by cells of the immune system, to activate the cytotoxicity by targeting specific antigens to cancer cells and to generate immunological memory to ensure long-term remission. Anti-tumor immunotherapy can be performed in two ways: passive immunotherapy and active immunotherapy. Passive immunotherapy or adoptive immunotherapy, does not aim to activate the immune system in a systematic manner in situ, but relies on effectors isolated and activated in vitro before their re-injection. Active immunotherapy, or adaptative immunotherapy, also called “vaccination” although it is therapeutic, consists in presenting one or several antigens in an ideal context of stimulation so as to trigger an immune response.
Passive immunotherapy.
This term regroups a very large domain of immunologic cancer therapy. Several “agents” can be considered, such as cytokines, monoclonal antibodies, activated T lymphocytes or activated macrophages with cytotoxic properties (an antibody-dependent cellular toxicity—ADCC—response). The objective of these therapeutics is to provides immediate protection against antigen(s). This involves either non-specific (Lymphocytes Activated Killer cells [LAK] or macrophages), or highly specific (monoclonal antibodies, cytotoxic T cell clones [CTL] or Tumor Infiltrating Lymphocytes [TIL]) immune effectors.
Non specific immune activation. The first studies of immunotherapy for mesothelioma were initiated in the nineteen seventies with the “BCG” trial that favored a non-specific activation of the immune response.22 The nineteen eighties saw the development of a new type of vaccine based on the non-specific activation of the immune response by cytokines targeting NK cells or LAK, by the team of Rosenberg.23 This therapeutic strategy favored the implementation of new trials in several types of cancer, including mesothelioma. The principle aim of injecting strong doses of interleukin-2 (www.copewithcytokines.de/cope.cgi?8262), was to stimulate a cytotoxic immune response. Interleukin-2 is primarily produced by activated CD4+ T lymphocytes (helper T lymphocytes [Th1]). This interleukin is capable of activating and stimulating the proliferation of CTL and LAK in the absence of co-stimulation signals and antigen presentation. In the case of mesothelioma, this interleukin has also shown direct anti-proliferative effects on tumor cells in vitro.24 Several clinical trials have been performed that are based on IL-2 injection, generally via the intrapleural route (see Table 1), but sometimes via the intravenous or subcutaneous route. The median survival for this type of treatment varies from around 8 months for patients showing tumor progression, to 13.6 months for those presenting an objective response.
Table 1.
Cytokine investigations in clinical trials that involved an immunotherapy strategy for patients with malignant mesothelioma
| Treatments (Ul/m2/D) | Nb of pts | Year | Reference |
| IL-2—18.106 IV → 6.106 SC/D | 29 | 2001 | Mulatero CW, et al. Lung Cancer 31:67–72. |
| IL2—9.106 Intrapleural → 3.106 SC | 31 | 2001 | Castagneto B, et al. Lung Cancer 31:303–10. |
| IL2—21.106 Intrapleural | 22 | 1998 | Astoul Ph., et al. Cancer 83:2099–104. |
| IL2 | 6 | 1998 | Nano R, et al. Ooncol Rrep 5:489–92. |
| IL2 9.106 SC + epirubicin | 21 | 1998 | Bretti S. Tumori 84:558–61. |
| IL2 ↗ from 3.104 to 36.106 Intrapleural | 23 | 1995 | Goey SH, et al. Br J Cancer 72:1283–8. |
| IL2 —↗ from 3 to 24.106 Intrapleural | 15 | 1993 | Astoul P, et al. Chest 1993; 103:209–13. |
| IL12 ↗ Phase I—from 300 to 600 ng/kg I.P. | 3 | 2001 | Lenzi R, et al. Clin Cancer Res 8:3686–95. |
| GM-CSF + autol tumor cells | 22 | 2006 | Powell A, et al. Lung Cancer 52:189–97. |
| GM-CSF + doxorubicin + dexrazozane | 2001 | Kosty MP, et al. Lung Cancer 34:289–95. | |
| GM-CSF inside lesion (8 weeks) | 14 | 1998 | Davidson JA, et al. J Immunother 21:389–98. |
| IFNγ + macrophages—Intrapleural 8 weeks. | 19 | 2002 | Monnet I, et al. Chest 121:1921–7. |
| IFNγ (early stage mesothelioma — 45%) | 89 | 1994 | Boutin C, et al. Cancer 74:2460—7. |
| IFNβ-1 injection/D — 5 subsequent days | 14 | 1990 | Von Hoff DD, et al. J Interferon Res 10:531–4. |
| INFα + cisP + doxorubicin | 37 | 2001 | Parra HS, et al. Cancer 92:650–6. |
| INFα + carboplatin | 15 | 1999 | O’Reilly, et al. Cancer Invest 17:195–200. |
| INFα + CisP | 13 | 1998 | Purohit A, et al. Lung Cancer 22:119–25. |
| INFα + CisP | 23 | 1997 | Trandafir L, et al. Eur J Cancer 33:1900–2. |
| INFα + CisP | // | 1996 | Soulie P, et al. J Clin Oncol 14:878–85. |
| INFα + CiSP + mitomycin C | 20 | 1994 | Tansan S, et al. Oncology 51:348–51. |
| INFα + doxorubicin | 25 | 1993 | Upham JW, et al. Aust N Z J Med 23:683–7. |
| IFNγ + IL2 | 1990 | Boutin C, et al. Rev Pneumol Clin 1990; 46:211–5. |
Other types of cytokines, used with the objective of stimulating an immune response, have also been the subject of clinical trials for the treatment of mesothelioma. In the case of GM-CSF (www.copewithcytokines.de/cope.cgi?6747), a cytokine known to stimulate the activity of antigen presenting cells, a trial associating GM-CSF and autologous tumor cells was recently carried out by Powell and Colleagues (see Table 1). In this study, the median survival was 11.5 months, with a 50% survival at 1 year and a 27% survival at 2 years.
Several studies have also been performed with interferons (α, β, γ), whose beneficial effects are mediated by activating the immune response, with a considerable toxic effect against tumor cells.25,26 As such, in the trial conducted by Monnet et al. (see Table 1), based on the dual action of interferon gamma on both immune and tumor cells, the median survival was 29.2 months for 19 patients treated. It is also worth noting that in this study, 10 of 17 treated patients were able to subsequently receive a course of chemotherapy. Thus, the responses observed and the median survival observed during passive immunotherapy treatments are at least comparable in terms of therapeutic efficacy, to those currently reported for recent chemotherapy treatments.5
One can therefore note that certain treatments have provided pertinent information concerning the potential efficacy of this therapeutic approach. The trial of IFNγ treatment by Boutin et al. (see Table 1), performed at an early stage of the disease, reported an objective efficacy above 20%, including a complete histologically confirmed response for eight patients. However, this therapeutic approach has been abandoned because of the toxicity observed with interferon treatment. In fact, even though some treatments that activate the immune system using cytokines appear to be well-tolerated, especially those acting in the intrapleural cavity, others have either shown a high degree of toxicity (e.g., GM-CSF plus doxorubicin and dexamethasone) or have come across significant technical hurdles (e.g., intralesion GM-CSF for 8 weeks) that have resulted in the study being terminated.
Genetic therapy and activation of the immune response. The introduction of viral vectors into tumor cells, favoring the production of immune-activating cytokines at the site of tumor development, has also been performed in MPM.27 Nevertheless, in contrast to other types of cancer, few clinical studies based on genetic therapy have been carried out for the treatment of mesothelioma.28 Even so, several pre-clinical studies in a murine model based on the adenoviral-mediated introduction of a gene coding for either interleukin 12,29 or CD40 ligand,30 close to or in the surroundings of the tumor, have demonstrated true therapeutic efficacy. Also, a study was initiated to treat mesothelioma via the injection of a gene coding for GM-CSF coupled to an autologous tumor lysate.31 More recently, in a pre-clinical investigation, Willmon et al. Tested IFNbeta as a therapeutic transgene expressed by vesicular stomatitis virus as a novel treatment for mesothelioma.32 VSV-IFNbeta showed significant efficacy against AB12 murine mesothelioma in the context of both local and locoregional viral deliveries. Immune monitoring suggested the participation of CD8+ T cells.
Besides in situ cytokine activation, immunomodulatory genetic therapy has also been orientated towards the expression of costimulatory molecules (B7.1 and/or B7.2),33 providing signals that promote the recognition of tumor cells by the immune system. Moreover, in support of the encouraging preclinical34 and clinical35 studies where transduction of an adenovirus coding for the HSV-tk gene was performed, this strategy promoted the initiation of efficient and specific immune recognition of tumor cells. In the clinical study, an antibody response to the transgene was observed for three patients and a mononuclear cell proliferation was observed for 12 of the 21 patients treated. Thus, in the case of mesothelioma, immunotherapy via gene transfer appears to be an attractive strategy. In fact, in this disease, the possibility of local vector administration via the pleural cavity is an advantage. The clinical trials of Albelda’s team showed the efficacy of infecting tumor cells with VSV via this route of administration, but without the therapeutic efficacy observed in animal models.36 However, an optimization of the viral vector production methods should rapidly lead to more encouraging clinical results.
These studies using passive immunotherapy, which have been ongoing for several years in the case of mesothelioma, have shown a potential for the immune response in patients suffering from mesothelioma, albeit without providing real evidence of a therapeutic benefit. On the whole, these results are comparable to those observed for other therapeutic strategies used in this disease. The information that can be retained from these different treatments using passive immunotherapy is that the immune system can develop a response, albeit weak in terms of anti-tumor efficacy. For this reason it is now necessary to move towards stronger, and above all, more specific forms of immunotherapy strategies. This is the objective of the targeted cell or antibody therapies based on the ex vivo or in vivo activation of the immune system. The objectives of these novel therapeutic strategies are to act against specific targets by calling upon specific and more efficient effector populations.
Active immunotherapy.
Tumor antigens. The identification of tumor-associated antigens has enabled the development of clinical trials focusing on vaccine therapy, especially in the treatment of melanoma and prostate cancers. Because of the distinctive nature of mesothelioma, no specific antigen has yet been identified. However, several studies have recently shown that certain non-specific antigens, such as “Large T antigen (Tag)”, can be expressed by the tumor cells following the integration of viral SV40 DNA, through vaccinations against polio in the sixties.37,38 Nonetheless, this notion of presence of the Large T of SV40 is still debatable.39,40 Similarly, the expression of the p53 protein in mesothelioma41 is not unanimously accepted and is still debated, unfortunately more in mouse models than in human.42,43
A study by Robinson’s team demonstrated the presence of antibodies directed against Topoisomerase IIb in the serum of 13 of 14 patients analyzed.44 Also, albeit this is not specific to mesothelioma, tumor cells have also been shown to express MAGE-1, MAGE-3 or GAGE antigens 1–6, which are associated with tumors and expressed by germinal cells. This was observed after treatment with DNA methyl transferase (DNMT) inhibitors.45 Nevertheless, the results obtained by the authors, together with our own results from a larger group of cell lines (approximately 15 human epithelioid mesothelioma cell lines) suggest a high degree of heterogeneity in the nature and expression intensity of these tumor-associated antigens. However, we recently investigated on the in vitro effect of a sequential treatment of mesothelioma cells with DNMT and histone de-acetylase inhibitors, and demonstrated the expression of cancer testis antigens (CTA) such as the New-York esophageal cancer (NY-ESO-1) and the melanoma-associated antigen (MAGE-A1). Moreover, we demonstrated immunological response in the presence of the treated cancer cells (manuscript submitted). These CTAs are of growing interest as immunotherapeutic targets because of their in vivo immunogenicity, their expression by several types of tumors, and their absence in normal tissues. We demonstrated for instance, that the CTA NY-ESO-1 is preferentially induced in human mesothelioma cells by sequential treatment with 5-AZACdR and valproate or vorinostat (SAHA) and that mesothelioma cells expressing NY-ESO-1 display sensitivity to NY-ESO-1-specific cytotoxic T lymphocytes.
Over the past few years, new and potentially antigenic markers have been found to be expressed by mesothelioma tumor cells and in physiologic liquids (blood, pleural and peritoneal fluids). For example, mesothelin, a surface glycoprotein that is strongly expressed in pancreatic and ovarian cancers as well as in mesothelioma, also has a soluble part that can be found in biological fluids. This soluble form of mesothelin, which is a 42–45 kDa protein was quantified by Robinson et al. in the serum of patients exposed to asbestos and in patients with mesothelioma.46 These authors showed that soluble mesothelin could potentially be a diagnostic tumor marker as well as a marker to monitor patient’s outcome.47 These results were recently confirmed by Hassan’s48 and Scherpereel’s49–51 groups. This blood marker was proposed for the screening of MPM in a population of subjects exposed to asbestos. In fact, in their first report,46 Robinson et al. reported that 7/40 asbestos-exposed subjects without a diagnosis of mesothelioma had a significantly elevated level of soluble mesothelin. Among these subjects, 3 of 7 patients were diagnosed for a mesothelioma during the 5 subsequent years, suggesting a value of mesothelin in MPM screening. However, a larger and more recent epidemiologic study from the same Australian group concluded that serum mesothelin did not permit the screening of MPM in a population of subjects clearly exposed to asbestos.52 Recently, a work on a large cohort of patients showed, however, that pleural fluid mesothelin provides additional diagnostic value relative to cytological examination.53 They additionally demonstrated that soluble mesothelin measurements are reproducible and not affected by pleural inflammatory processes. Thus even though mesothelin is not the specific marker of mesothelioma that everyone has been waiting for, it is nevertheless a marker of choice and quite simply an important antigenic target for immunotherapy.54
With this in mind, Hassan et al. have developed a chimeric protein—SS1P—made up of the Fv fragment of the anti-mesothelin antibody, coupled to a truncated form of the exotoxin of Pseudomonas.55 The cytotoxic efficacy directed specifically against tumor cells observed in vitro and in vivo in a murine model, was sufficient for the initiation of a phase I clinical trial.56 The SS1P chimeric protein was administered I.V. in patients suffering from mesothelioma and known to express mesothelin. Thirty four patients, of whom 21 were suffering from mesothelioma, have been included to date. The authors reported a partial response for 4 patients and disease stability for 19 others.57 The toxic limiting dose in this study gave a reversible pleuritis for a few patients. The preclinical studies in murine models also suggest a synergy in efficacy between Gemcitabine (14 day survival), SS1P (11 day survival) and a combination of Gem plus SS1P (60 day survival). A humanized monoclonal antibody against mesothelin, MORAb-009 (Morphotek Inc.,),58 has also been developed and used in a phase I clinical study, combined with Gemcitabine chemotherapy in six patients.59 Other antigens of relative specificity, such as osteopontin,60,61 and MUC-1 (CA15-3), are markers as well as potential antigenic targets expressed by the large majority of mesothelioma. These markers/targets are, however, generally shared by numerous types of tumor and are therefore less specific than mesothelin.62
Cell immunotherapy. An increasing number of scientific and clinical studies have used immune cells to battle against tumor progression.63 This is particularly true in the context of cell immunotherapy for the treatment of melanomas where several antigens have been reported as being associated, although not yet for mesothelioma. In fact, if one queries the website that indexes clinical trials that are either underway or have been completed (http://clinicaltrials-dev.ifpma.org/), one can note that more than 40 trials are or have been performed using activated T lymphocytes and more than 30 with dendritic cells for the treatment of melanoma (out of 500 indexed trials). In contrast, one can note that in the case of mesothelioma, no clinical trial has tested activated lymphocyte injection and only one single phase I study was done using injection of dendritic cells loaded with autologous tumor lysates (out of 170 indexed trials). However, we have shown in vitro, using human cells in autologous conditions, the possibility of generating cytotoxic T cell responses directed specifically against mesothelioma tumors by stimulating naive autologous peripheral blood lymphocytes.64,65 In our experiments, immature dendritic cells derived from circulating monocytes and having phagocyted apoptotic mesothelioma cells were matured then put in contact with autologous T lymphocytes. This active ex-vivo immunization is based on the efficient presentation of one or several tumor antigens and the subsequent stimulation of an immune response. This immune response was analyzed by the activation of autologous CTL against cancer cells originating from the tumor but also against mesothelioma cells expressing the same HLA. The efficacy of the immune response activation in vitro was also found to be efficient in vivo, in a murine model.66 Hegmans et al. have shown that the development of an intraperitoneal mesothelioma tumor in mice, obtained by injecting syngeneic tumor cells, could be halted by injecting dendritic cells loaded with tumor lysates. The predominant factor shown to limit this therapeutic efficacy in the experimental setting is the time between the development of the tumor and the vaccine injection. It is thought that the relatively weak observed effect was due to a stage of tumor development that was too advanced, implying a tumor volume and stroma-reaction that limited the access of immune cells to the cancer cells and/or the development of regulatory T cells (Treg). On the other hand, an immunization or preventative vaccination protected all animals subsequently receiving an injection of tumor cells. Together, these experimental, preclinical results prompted the authors to initiate a phase I trial in patients suffering from epithelioid mesothelioma, having undergone chemotherapy (http://clinicaltrials-dev.ifpma.org/; key words: dendritic—mesothelioma). Ten patients who responded to pemetrexed-cisplatin chemotherapy were selected. This study demonstrated the safety and feasibility of tumor lysate-pulsed dendritic cells as therapeutic adjuvants in mesothelioma patients and identified distinct immune and antitumor responses in these patients.67
Using a very similar therapeutic regimen, Robinson’s group has also initiated a phase I trial based on the injection of recombinant GM-CSF (for the in situ activation of dendritic cells) and autologous tumor lysates.68 The treatment was not associated with significant side effects (>grade II). Of the 21 patients, 16 completed the treatment and 5 had to drop out of the study because of tumor progression. The current results of these studies point more towards an immunological response rather than to an objective response to treatment. Nevertheless, stabilization of tumor progression was observed in 46% of patients during the vaccination phase. The median patient survival in this small cohort was 11.5 months when considering all patients included, and 14.5 months if only considering the 16 patients that had completed the treatment schedule. This median survival is only slightly better, if at all different, from that reported after chemotherapy.5 Thus, overall these results are only moderately successful and are insufficient for such strategies to become standardized as a first line treatment.69,70 However, in a recent series reported by Rosenberg and Dudley,71 based on clinical trials in melanoma using increasing lympho-depletion before infusion of autologous infiltrating lymphocytes (TIL), significant (49 to 72%) objective responses were seen. As such, this strategy could become part of a multimodal therapy as an adjuvant phase of treatment and could be easily adapted to MPM treatment.72
Multimodal and Immunotherapy Strategies
To date, there is no validated immunotherapy for efficient treatment of mesothelioma. However, clinical trials investigating new trends in the treatment of stage I and II malignant mesothelioma (http://www.mesotheliomaweb.org/clinical.htm) have shown promising prospects for both immunotherapy and systemic chemo-immunotherapy.73,74 In addition, recent progress in early detection techniques also provides hope that patients can be treated efficiently, at an earlier stage, and with the possibility of monitoring. The majority concern a therapeutic approach using a surgery-radiotherapy combination associated with neoadjuvant chemotherapy.75 Only one promising phase I immunotherapy study, using autologous dendritic cell vaccination, subsequent to Cisplatin-ALIMTA chemotherapy has been proposed to date. However, one cannot exclude the possibility of future immunotherapy trails (cell therapy) associated with first line treatment using a relevant chemotherapy.76
Chemotherapy and Immunotherapy
Conventional cancer treatments against MPM mediate their effects via the direct elimination of tumor cells. Nonetheless, recent evidence indicates that radiotherapy and/or some chemotherapeutic agents can also induce specific immune responses that contribute to therapeutic outcomes.77 It is now known that certain chemotherapies have no effect on the subsequent development of an immune response.78 Thus, given the now well-established importance of the immune system in controlling and shaping developing tumors, defining the immunological consequences of activating various tumor suppressor pathways may provide important insights into the development of more effective cancer therapies.79,80 Understanding the molecular interactions governing tumor suppression and immunity may thus lead for development of novel integrated approaches based on the simultaneous harnessing of latent tumor suppressor pathways and the activation of anti-cancer immune responses. The main hypothesis is the clearance of dead cells, which is essential in the maintenance of tissue homeostasis. Phagocytes that recognize ligands differently expressed by dead and living cells are able to provide the “danger signals” that induced DC maturation.81 Mature DCs present neo expressed antigen to cytotoxic T lymphocytes and induce a specific immune response.82 Two major tumor-intrinsic changes that determine the immune response to tumors have been identified: the translocation of calreticulin83 to the plasma membrane and the release of high-mobility group box 1 protein.84 Several phagocytic signals, which belong to the so-called “danger signals,”85 and are provided by apoptotic cells, mobilize complement proteins, which in turn can promote immune responses.86,87 The notion that tumor cells may be “stressed” before death, making them recognizable by immune cells, is of interest for immunotherapy. Indeed, “eat-me,” “danger” and “killing” signals released by stressed tumor cells under the pressure of cytotoxic compounds may serve as links between the chemotherapy-elicited response of tumor cells and subsequent immune responses.
Infection or local inflammation could also provide the appropriate signals to immature DC that further facilitate their differentiation and maturation. Thus, signals such as pathogen-associated molecular patterns (PAMPs) that are recognized by pattern recognition receptors such as the toll-like receptors (TLR)88 must be induced in the tumor cells by various treatments. That’s why chemotherapies and immunotherapies can form partnership in cancer treatment and particularly in mesothelioma because of the local strategy that could support the pleural (or peritoneal) cavity.
Cancer Virotherapy and Immunotherapy
During the past decade, there has been an increasing interest in cancer virotherapy, namely the use of replicating viruses for cancer treatment.89 Numerous live-attenuated viruses, such as adenovirus (AdV), vesicular stomatitis virus (VSV), herpes simplex virus (HSV), Newcastle disease virus (NDV), Vaccinia viruses (VV) and measles virus (MV) are now considered as potential cancer therapeutics.90,91 In these strategies, a virus is rendered conditionally replicative for tumor cells. Indeed, the use of replicative viruses as anti-tumor therapeutics depends on the observation that certain viruses replicate preferentially in tumor cells; in contrast, normal non-transformed cells remain barely sensitive, or insensitive, to infection by these “oncolytic viruses.”
Among these virus, MV has already demonstrated promising oncolytic properties.92,93 For example, we recently investigated both the oncolytic activity and immuno-adjuvant properties of Schwarz measles vaccine on a panel of mesothelioma cells derived from pleural effusions of mesothelioma patients.94 We used a cloned Schwarz MV produced from an infectious cDNA that was previously described.95,96 We have analyzed infection susceptibility and cytolytic activity in both tumoral and non-transformed mesothelial cells and we observed that mesothelioma tumor cells are more susceptible than non-transformed mesothelial cells to MV infection. The increased susceptibility of mesothelioma cells to MV infection was assessed by the analysis of cell surface expression of the MV vaccine receptor (CD46). We demonstrated that phagocytosis of apoptotic MV-infected mesothelioma cells induced spontaneous DC maturation and activation, as evidenced by an increased expression of MHC and co-stimulatory molecules, the production of pro-inflammatory cytokines with Th1 polarizing capacities, and a significant amplification of MSLN-specific CD8 T-cells.
Thus, cancer virotherapy might be an interesting opportunity for an efficient improvement in mesothelioma cancer treatment, when combined with chemotherapy.97 Indeed, cisplatin was demonstrated to potentiate, in malignat pleural mesothelioma, the oncolytical properties of an attenuated Herpes simplex virus type 1.98 However, the major issue remains the search for high tumor specificity in order to achieve an efficient targeting of tumor cells while leaving the normal healthy cells unharmed. The second challenge is to find a viral agent that is cytotoxic; this is a central point that will ensure the pertinence of using viruses in anti-tumor therapeutic protocols.
Conclusions and Perspectives
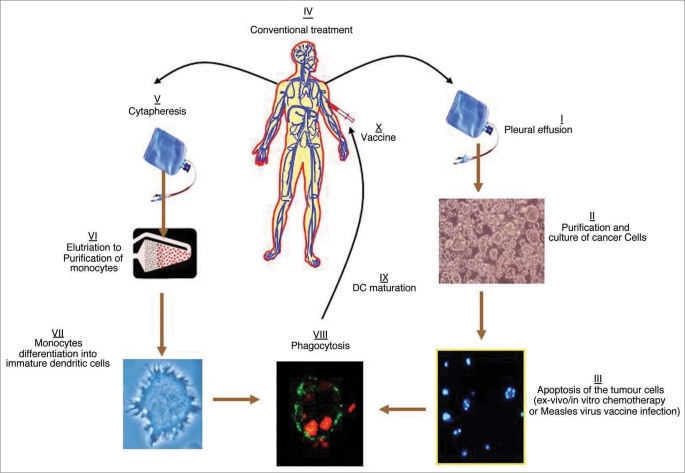
Targeted therapies associated to standard chemotherapy and multimodal treatment are now investigated in order to offer new opportunities for an efficient strategy to treat MPM. Interestingly, radiotherapy and certain chemotherapies drugs can induce death in a large number of cells that could thereafter release tumor antigens. Subsequently, these antigens can be taken up by antigen presenting cells, such as dendritic cells (Fig. 1). These tumor antigen-loaded cells can then activate an immune response that can be further sensitized by a therapeutic vaccination.99 There is already some experiences with this type of vaccination strategy for mesothelioma.67 Nevertheless, the selection of patients is also of utmost importance for anti-tumor efficacy. This is because the adjuvant therapeutic vaccination based on the injection of immune cells is more efficient when performed at an early stage, as previously demonstrated for melanoma.100 As a complement to this type of strategy and in order to increase efficacy, recent data from previous clinical trials suggest the utility of targeting and inactivating the regulatory T cell population.101 For this reason, if one takes these different results into account i.e., the observations concerning the immune populations in patients suffering from mesothelioma and the ease of access to the local tumor site within the pleural (or peritoneal) cavity, this pathology should be the subject of several cellular immunotherapy trials in the near future.
Figure 1.
Proposal clinical application of immune therapy to treat MPM with dendritic cells loaded with autologous apoptotic tumor cells. The cancer cells can be treated in vivo and in vitro with either chemotherapeutic/immunogenic drugs or infected with the Measles virus vaccine. Part I to III could be proposed before the conventional treatment of the Patient, and part V to X after the treatment, when clinical efficacy is noticed.
In addition, the intrinsic features of pleural mesothelioma, such as its accessibility and its localized nature, associated with a relative lack of distant metastasis, make it also a suitable candidate for virotherapy. MPM tumors spread early and aggressively in the serosal cavity, but rarely metastasize at distant sites through lymphatic or systemic circulation.102 Moreover, the pleural cavity is a confined compartment that could allow an efficient interaction between cancer cells and a viral therapeutic agent, such as the measles vaccine. Thus, mesothelioma constitutes an ideal target for the local administration of attenuated virus contained in the vaccine. This intra-pleural administration pathway could also be a solution to limit virus inactivation by circulating Measles virus-neutralizing antibodies present as a result of previous vaccination. In this field of application, the use of other oncolytic viruses, such as the HSV NV1066 strain and the Adenovirus for recombinant IFNβ, has already demonstrated the feasibility of a virotherapy approach for this cancer.103,104 Under these conditions, an adjuvant immunotherapy performed when a patient has recovered a satisfactory general condition, could activate an immune surveillance mechanism, thereby avoiding or delaying the frequently observed relapse. The low incidence of side effects reported during numerous trials of immunotherapy involving the injection of immune cells for other cancers lends credence to such multimodal approaches.
Acknowledgements
The authors thank Professor Gerard Dabouis and Dr. Laurent Cellerin for their clinical collaboration and their advice concerning the clinical studies and this work. The research performed by M. G.’s team was supported by INSERM, the Ligue contre le Cancer (Ligue interregionale du Grand Ouest) and the Region Pays de Loire. We extend special thanks to Joanna Ashton-Chess for English editing of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11361
References
- 1.Jaurand MC, Renier A, Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part Fibre Toxicol. 2009;12:6–16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippmann M. Asbestos exposure indices. Environ Res. 1988;46:86–106. doi: 10.1016/s0013-9351(88)80061-6. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M, Imbernon E, Rolland P, Gilg Soit Ilg A, Saves M, et al. The French national mesothelioma surveillance program. Occup Environ Med. 2006;63:390–395. doi: 10.1136/oem.2005.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, et al. Guidelines of the European respiratory society and the European society of thoracic surgeons for management of malignant pleural mesothelioma. Eur Respir J. 2009;35:479–495. doi: 10.1183/09031936.00063109. [DOI] [PubMed] [Google Scholar]
- 5.Vogelzang NJ, Rustoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of Pemetrexed in combination with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 6.Leigh RA, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J. 1982;61:1007–1009. [PubMed] [Google Scholar]
- 7.Robinson BWS, Robinson C, Lake RA. Localised spontaneous regression in mesothelioma-possible immunological mechanism. Lung Cancer. 2001;32:197–201. doi: 10.1016/s0169-5002(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 8.Robinson C, Robinson BWS, Lake RA. Sera from patients with mesothelioma can contain autoantibodies. Lung Cancer. 1998;20:175–184. doi: 10.1016/s0169-5002(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Hassan R, Zhang J, Wang Q-C, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 10.Creaney J, Yeoman D, Demelker Y, Segal A, Musk AW, Skates SJ, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol. 2008;3:851–878. doi: 10.1097/JTO.0b013e318180477b. [DOI] [PubMed] [Google Scholar]
- 11.DeLong P, Carroll RG, Henry AC, Tanaka T, Ahmad S, Leibowitz MS, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4:342–346. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 12.Lew F, Tsang P, Holland JF, Warner N, Selikoff IJ, Bekesi JG. High frequency of immune disfunctions in asbestos workers and in patients with malignant mesothelioma. J Clin Immunol. 1986;6:225–233. doi: 10.1007/BF00918702. [DOI] [PubMed] [Google Scholar]
- 13.Hegmans JP, Hemmes A, Hammad H, Boon L, Hoogsteden HC, Lambrecht BN. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Resp J. 2006;27:1080–1095. doi: 10.1183/09031936.06.00135305. [DOI] [PubMed] [Google Scholar]
- 14.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. FoxP3 expressing CD4+CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Atanackovic D, Block A, de Weerth A, Faltz C, Hossfeld D, Hegewisch-Becker S. Characterization of effusion-infiltrating T cells: benign versus malignant effusions. Clin Cancer Res. 2004;10:2600–2610. doi: 10.1158/1078-0432.ccr-03-0239. [DOI] [PubMed] [Google Scholar]
- 16.Kumar-Singh S, Weyler J, Martin MJ, Vermeulen PB, Van Marck E. Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and -2, and TGFbeta expression. J Pathol. 1999;189:72–78. doi: 10.1002/(SICI)1096-9896(199909)189:1<72::AID-PATH401>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGFbeta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 18.Rao PE, Petrone AL, Ponath PD. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGFβ. J Immunol. 2005;174:1446–1455. doi: 10.4049/jimmunol.174.3.1446. [DOI] [PubMed] [Google Scholar]
- 19.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 20.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster I, Cochrane JW, Burkhardt KR. Immunotherapy with BCG vaccine in 30 cases of mesothelioma. South Afr med J. 1982;61:277–278. [PubMed] [Google Scholar]
- 23.Rosenberg SA, Lotze MT. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- 24.Porta C, Danova M, Orengo AM, Ferrini S, Moroni M, Gaggero A, et al. Interleukin-2 induces cell cycle perturbations leading to cell growth inhibition and death in malignant mesothelioma cells in vitro. J Cell Physiol. 2000;185:126–134. doi: 10.1002/1097-4652(200010)185:1<126::AID-JCP12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Zucchella M, Pacchiarini L, Meloni F, Ballabio P, Saporiti A, Brocchieri A, et al. Effect of interferon-alpha, interferon-gamma and tumor necrosis factor on the procoagulant activity of human cancer cells. Haematologica. 1993;78:282–286. [PubMed] [Google Scholar]
- 26.Christmas TI, Manning LS, Garlepp MJ, Musk AW, Robinson BWS. Effect of interferon-alpha-2a on malignant mesothelioma. J Interferon Res. 1993;13:9–12. doi: 10.1089/jir.1993.13.9. [DOI] [PubMed] [Google Scholar]
- 27.Albelda SM. Gene therapy for lung cancer and mesothelioma. Chest. 1997;111:144–149. doi: 10.1378/chest.111.6_supplement.144s. [DOI] [PubMed] [Google Scholar]
- 28.VanDerMost RG, Robinson BWS, Nelson DJ. Gene therapy for malignant mesothelioma: beyond the infant years. Cancer Gene Therapy. 2006;13:897–904. doi: 10.1038/sj.cgt.7700935. [DOI] [PubMed] [Google Scholar]
- 29.Caminschi I, Venetsanakos E, Leong CC, Garlepp MJ, Scott B, Robinson BW. Interleukin-12 induces an effective antitumor response in malignant mesothelioma. Am J Respir Cell Mol Biol. 1998;19:738–746. doi: 10.1165/ajrcmb.19.5.3257m. [DOI] [PubMed] [Google Scholar]
- 30.Friedlander PL, Delaune CL, Abadie JM, Toups M, LaCour J, Marrero L, et al. Efficacy of CD40 ligand gene therapy in malignant mesothelioma. Am J Resp Cell Mol Biol. 2003;29:320–330. doi: 10.1165/rcmb.2002-0226OC. [DOI] [PubMed] [Google Scholar]
- 31.Powell A, Creaney J, Broomfield S, Van Bruggen I, Robinson BWS. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer. 2006;52:189–197. doi: 10.1016/j.lungcan.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J. Expression of IFNbeta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Nelson D, Loh S, van Bruggen I, Palmer LJ, Leong C, et al. The immune anti-tumor effects of GM-CSF and B7-1 gene transfection are enhanced by surgical debulking of tumor. Cancer Gene Ther. 2001;8:580–588. doi: 10.1038/sj.cgt.7700347. [DOI] [PubMed] [Google Scholar]
- 34.Smythe WR, Kaiser LR, Hwang HC, Amin KM, Pilewski JM, Eck SJ, et al. Successful-mediated gene transfer in an in vivo model of human malignant mesothelioma. Ann Thorac Surg. 1994;57:1395–1401. doi: 10.1016/0003-4975(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 35.Sterman DH, Recio A, Vachani A, Sun J, Cheung L, DeLong P, et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 36.Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V, et al. Evaluation of an Attenuated Vesicular Stomatitis Virus (VSV) Vector expressing Interferon-beta for Use in Malignant Pleural Mesothelioma: Heterogeneity in Interferon-Responsiveness Defines Potential Efficacy. Hum Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo P, Carbone M, Fisher SG, Matker C, Swinnen LJ, Powers A, et al. Simian virus 40 is present in most United States human mesotheliomas, but it is rarely present in non-Hodgkin’s lymphoma. Chest. 1999;116:470–473. doi: 10.1378/chest.116.suppl_3.470s. [DOI] [PubMed] [Google Scholar]
- 38.Carbone M, Pass HI. Evolving aspects of mesothelioma carcinogenesis: SV40 and genetic predisposition. J Thorac Oncol. 2006;1:169–171. [PubMed] [Google Scholar]
- 39.Pershouse MA, Heivly S, Girtsman T. The role of SV40 in malignant mesothelioma and other human malignancies. Inhal Toxicol. 2006;18:995–1000. doi: 10.1080/08958370600835377. [DOI] [PubMed] [Google Scholar]
- 40.Henzi T, Blum WV, Pfefferli M, Kawecki TJ, Salicio V, Schwaller B. SV40-induced expression of calretinin protects mesothelial cells from asbestos cytotoxicity and may be a key factor contributing to mesothelioma pathogenesis. Am J Pathol. 2009;174:2324–2336. doi: 10.2353/ajpath.2009.080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cote RJ, Jhanvar SC, Novick S, Pellicer A. Genetic alterations of the p53 gene are a feature of malignant mesothelioma. Cancer Res. 1991;51:5410–5416. [PubMed] [Google Scholar]
- 42.Ichihara G, Castranova V, Tanioka A, Miyazawa K. Re: Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:381–384. doi: 10.2131/jts.33.381. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson K, Stone V, Seaton A, Tran L, Aitken R, Poland C. Re: Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:385–388. doi: 10.2131/jts.33.385. [DOI] [PubMed] [Google Scholar]
- 44.Robinson C, Callow M, Stevenson S, Scott B, Robinson BW, Lake RA. Serologic responses in patients with malignant mesothelioma: evidence for both public and priate specificities. Am J Respir Cell Mol Biol. 2006;22:550–556. doi: 10.1165/ajrcmb.22.5.3930. [DOI] [PubMed] [Google Scholar]
- 45.Sigalotti L, Coral S, Altomonte M, Natali L, Gaudino G, Cacciotti P, et al. Cancer testis antigens expression in mesothelioma: role of DNA methylation and bioimmunotherapeutic implications. Br J Cancer. 2002;86:979–982. doi: 10.1038/sj.bjc.6600174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 47.Creaney J, Yeoman D, Naumoff LK, Hof M, Segal A, Musk AW, et al. Soluble mesothelin in effusions: a useful tool for the diagnosis of malignant mesothelioma. Thorax. 2008;62:566–569. doi: 10.1136/thx.2006.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 49.Scherpereel A, Grigoriu BD, Conti M, Gey T, Gregoire M, Copin M-C, et al. Soluble Mesothelin-related Protein in the Diagnosis of Malignant Pleural Mesothelioma. Am J Respir Crit Care Med. 2006;173:1155–1160. doi: 10.1164/rccm.200511-1789OC. [DOI] [PubMed] [Google Scholar]
- 50.Grigoriu BD, Scherpereel A. Diagnostic value of soluble mesothelin in malignant mesothelioma. Thorax. 2008;63:85–88. [PubMed] [Google Scholar]
- 51.Grigoriu B, Chahine B, Zerimech F, Gregoire M, Balduyck M, Copin MC. Serum mesothelin has a higher diagnostic utility than hyaluronic acid in malignant mesothelioma. Clin Biochem. 2009;42:1046–1050. doi: 10.1016/j.clinbiochem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Park E-K, Sandrini A, Yates DH, Creaney J, Robinson BW, Thomas PS, et al. Soluble Mesothelin-related Protein in an Asbestos-exposed Population. Am J Respir Crit Care Med. 2008;178:832–837. doi: 10.1164/rccm.200802-258OC. [DOI] [PubMed] [Google Scholar]
- 53.Davies HE, Sadler RS, Bielsa S, Maskell NA, Rahman NM, Davies RJO, et al. Clinical Impact and Reliability of Pleural Fluid Mesothelin in Undiagnosed Pleural Effusions. Am J Respir Crit Care Med. 2009;180:437–444. doi: 10.1164/rccm.200811-1729OC. [DOI] [PubMed] [Google Scholar]
- 54.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 55.Hassan R, Williams-Gould J, Steinberg SM, Liewehr DJ, Yokokawa J, Tsang KY, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12:4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 56.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5147. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 58.Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20–27. [PMC free article] [PubMed] [Google Scholar]
- 59.Hassan R, Schweizer C, Lu KF, Schuler B, Remaley AT, Weil SC, et al. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: Implications for cancer therapy. Lung Cancer. 2009. In press. [DOI] [PMC free article] [PubMed]
- 60.Pass HI, Dan Lott BS, Lonardo F, Harbut M, Liu Z, Tang N, et al. Asbestos exposure, pleural mesothelioma and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 61.Ivanov SV, Ivanova AV, Goparaju CM, Chen Y, Beck A, Pass HI. Tumorigenic properties of alternative osteopontin isoforms in mesothelioma. Biochem Biophys Res Commun. 2009;382:514–518. doi: 10.1016/j.bbrc.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Grigoriu BD, Scherpereel A, Devos P, Chahine B, Letourneux M, Lebailly P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res. 2007;13:2928–2935. doi: 10.1158/1078-0432.CCR-06-2144. [DOI] [PubMed] [Google Scholar]
- 63.D’Elios MM, Del Prete G, Amedei A. New frontiers in cell-based immunotherapy of cancer. Expert Opin Ther Pat. 2009;19:623–641. doi: 10.1517/13543770902817820. [DOI] [PubMed] [Google Scholar]
- 64.Gregoire M, Ligeza-Poisson C, Juge-Morineau N, Spisek R. Anti-cancer therapy using dendritic cells and apoptotic tumour cells: pre-clinical data in human mesothelioma and acute myeloid leukemia. Vaccine. 2003;21:791–794. doi: 10.1016/s0264-410x(02)00600-x. [DOI] [PubMed] [Google Scholar]
- 65.Ebstein F, Sapede C, Royer P-J, Marcq M, Ligeza-Poisson C, Barbieux I, et al. Cytotoxic T cell responses against mesothelioma by apoptotic cell-pulsed dendritic cells. Am J Respir Crit Care Med. 2004;169:1–9. doi: 10.1164/rccm.200312-1683OC. [DOI] [PubMed] [Google Scholar]
- 66.Hegmans JP, Hemmes A, Aerts JG, Hoogsteden HC, Lambrecht B. Immunotherapy of murine malignant mesothelioma using tumor lysates-pulsed dendritic cells. Am J Respir Crit Care Med. 2005;171:1168–1177. doi: 10.1164/rccm.200501-057OC. [DOI] [PubMed] [Google Scholar]
- 67.Hegmans JP, Veltman J, Lambers M, de Vries IJ, Figdor C, Hendriks R, et al. Consolidative dendritic-cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010. In press. [DOI] [PubMed]
- 68.Powell A, Creaney J, Broomfield S, Van Bruggen I, Robinson B. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer. 2006;52:189–197. doi: 10.1016/j.lungcan.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy? J Immunother. 2008;31:793–805. doi: 10.1097/CJI.0b013e31818403bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dauer M, Schnurr M, Eigler A. Dendritic cell-based cancer vaccination: quo vadis? Expert Rev Vaccines. 2008;7:1041–1053. doi: 10.1586/14760584.7.7.1041. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Cur Op Immun. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang T, Herlyn D. Combination of active specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer Immunol Immunother. 2009;58:475–492. doi: 10.1007/s00262-008-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kukreja J, Jaklitsch MT, Wiener DC, Sugarbaker DJ, Burgers S, Baas P. Malignant pleural mesothelioma: overview of the North American and European experience. Thorac Surg Clin. 2004;14:435–445. doi: 10.1016/j.thorsurg.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 74. ( http://www.eortc.be/protoc/details.asp?protocol=08031). Phase II feasibility trial of induction chemotherapy followed by extrapleural pneumonectomy and postoperative radiotherapy in patients with malignant pleural mesothelioma.
- 75.Stahel RA, Weder W. Improving the outcome in malignant pleural mesothelioma: nonaggressive or aggressive approach? Curr Opin Oncol. 2009;21:124–130. doi: 10.1097/CCO.0b013e328324bc30. [DOI] [PubMed] [Google Scholar]
- 76.McCoy MJ, Nowak AK, Lake RA. Chemoimmunotherapy: an emerging strategy for the treatment of malignant mesothelioma. Tissue Antigens. 2009;74:1–10. doi: 10.1111/j.1399-0039.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 77.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 78.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 79.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang T, Herlyn D. Combination of active specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer Immunol Immunother. 2009;58:475–492. doi: 10.1007/s00262-008-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 82.Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L. Tumor stress, cell death and the ensuing immune response. Cell Death Differ. 2008;15:21–28. doi: 10.1038/sj.cdd.4402266. [DOI] [PubMed] [Google Scholar]
- 83.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 84.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 85.Matzinger P. Tolerance, danger and the extended family. Ann Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 86.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 88.Atkinson TJ. Toll-like receptors, transduction-effector pathways and disease diversity: evidence of an immunobiological paradigm explaining all human illness? Int Rev Immunol. 2008;27:255–281. doi: 10.1080/08830180801959072. [DOI] [PubMed] [Google Scholar]
- 89.Boisgerault N, Tangy F, Gregoire M. New perspectives in cancer virotherapy: bringing the immune system into play. Immunotherapy. 2010. In press. [DOI] [PubMed]
- 90.Liu T, Kirn D. Systemic Efficacy with Oncolytic Virus Therapeutics: Clinical Proof-of-Concept and Future Directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 91.Parato K, Senger D, Forsyth P, Bell J. Recent progress in the battle between oncolytic viruses and tumors. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 92.Fielding A. Measles as a potential oncolytic virus. Rev Med Virol. 2005;15:135–142. doi: 10.1002/rmv.455. [DOI] [PubMed] [Google Scholar]
- 93.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 95.Combredet C, Labrousse V, Mollet L, Lorin C, Delebecque F, Hurtel B, et al. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol. 2003;77:11546–11554. doi: 10.1128/JVI.77.21.11546-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parks C, Lerch R, Walpita P, Sidhu M, Udem S. Enhanced measles virus cDNA rescue and gene expression after heat shock. J Virol. 1999;73:3560–3566. doi: 10.1128/jvi.73.5.3560-3566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Curiel DT, Zhu ZB. Combining chemotherapy with virotherapy: a novel Treatment Strategy for Malignant Pleural Mesothelioma. Cancer Biol Ther. 2006;5:236–237. doi: 10.4161/cbt.5.2.2564. [DOI] [PubMed] [Google Scholar]
- 98.Adusumilli PS, Chan M-K, Chun YS, Hezel M, Chou T-C, Rush VW, et al. Cisplatin-Induced GADD34 Upregulation Potentiates Oncolytic Viral Therapy in the Treatment of Malignant Pleural Mesothelioma. Cancer Biol Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lake RA, Robinson BWS. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–3405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 100.Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B, et al. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532–538. doi: 10.1007/s00262-002-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101:14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robinson B, Musk A, Lake R. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 103.Sterman D, Recio A, Carroll R, Gillespie CT, Haas A, Vachani A, et al. A Phase I Clinical Trial of Single-Dose Intrapleural IFNβ Gene Transfer for Malignant Pleural Mesothelioma and Metastatic Pleural Effusions: High Rate of Antitumor Immune Responses. Clin Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 104.Adusumilli P, Stiles B, Chan M, Mullerad M, Eisenberg DP, Ben-Porat L. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J Gen Med. 2006;8:603–615. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]