Abstract
Exposure to stressful events during development has consistently been shown to produce long-lasting alterations in the hypothalamic-pituitary-adrenal (HPA) axis, which may increase vulnerability to disease, including PTSD and other mood and anxiety disorders. Recently reported genetic association studies indicate that these effects may be mediated, in part, by gene x environment (GxE) interactions involving polymorphisms within two key genes, CRHR1 and FKBP5. Data suggest that these genes regulate HPA axis function in conjunction with exposure to child maltreatment or abuse. In addition, a large and growing body of preclinical research suggests that increased activity of the amygdala-HPA axis induced by experimental manipulation of the amygdala mimics several of the physiological and behavioral symptoms of stress-related psychiatric illness in humans. Notably, interactions between the developing amygdala and HPA axis underlie critical periods for emotional learning which are modulated by developmental support and maternal care. These translational findings lead to an integrated hypothesis: high levels of early life trauma lead to disease through the developmental interaction of genetic variants with neural circuits that regulate emotion, together mediating risk and resilience in adults.
Keywords: Post-traumatic Stress Disorder, Depression, socioeconomic status, Trauma, Child Abuse, Childhood Maltreatment, amygdala
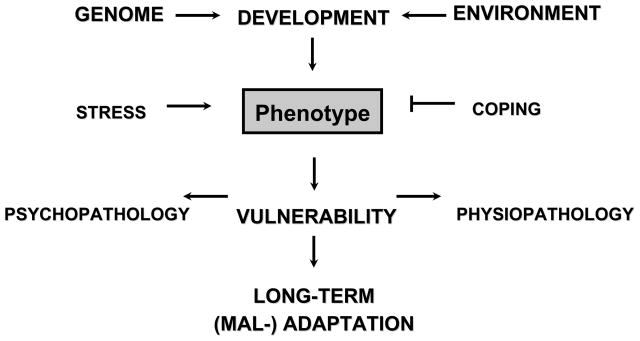
Epidemiological and clinical research studies have consistently identified exposure to trauma and neglect during early life as a major adverse influence on adult risk for mood and anxiety disorders (Chapman et al 2004; Dube et al 2001; Felitti et al 1998; Gladstone et al 2004; McCauley et al 1997). The parallel study of individuals who emerge from such adverse environments without significant mood or anxiety disorder (Rutter 2006) has resulted in the identification of psychosocial and biological variables associated with psychological resilience (Feder et al 2009). As with, environmental variables, predisposing genetic factors also influence vulnerability (Stein et al 2002; Sullivan et al 2000) and resilience (Rijsdijk et al 2003) in terms of aggregate risk for mood and anxiety disorders such as post-traumatic stress disorder (PTSD) and depression. However, the relationship between individual genetic variability and exposure to positive and negative life events as translated to risk for depression and PTSD remains unclear (Figure 1).
Figure 1. Schematic Diagram of Genetic and Environmental Effects on Development.
Gene x environment interactions affect critical periods of emotional neural system development, differentially mediating vulnerability and resilience.
To examine the relationship between genetic and environmental risk factors for depression and PTSD in more depth, our research group has recently focused on candidate genes related to the hypothalamic-pituitary-adrenal (HPA) axis, a collection of neural and endocrine structures that facilitate the adaptive response to stress. Briefly, parvocellular neurons of the paraventricular nucleus (PVN) of the hypothalamus project to the median eminence where they secrete corticotrophin-releasing hormone (CRH) into the primary plexus of blood vessels that comprise the hypothalamo-hypophyseal portal system (Swanson et al 1983). The secreted CRH is subsequently transported to the anterior pituitary gland where it activates CRH1 receptors on pituitary corticotrophs resulting in increased secretion of adrenocorticotrophic hormone (ACTH). ACTH released from the anterior pituitary into the systemic circulation stimulates the production and release of cortisol from the adrenal cortex. Feedback inhibition, mediated in part by activity of cortisol on mineralocorticoid and glucocorticoid receptors at the hippocampus, PVN, and pituitary (de Kloet et al 1991) reduces stress-induced activation of the HPA axis and limits excess secretion of glucocorticoids effectively dampening the stress response (Jacobson and Sapolsky 1991).
Elements of the HPA axis have previously been implicated in the physiological and pathological regulation of stress reactivity (Heinrichs and Koob 2004) and elevated cerebrospinal fluid (CSF) concentrations of CRH have repeatedly been reported in patients with depression (see e.g., Hartline et al 1996; Nemeroff et al 1984), as well as in combat veterans with PTSD (Baker et al 1999; Bremner et al 1997). In addition, several studied have found data indicating that the transcription (Raadsheer et al 1995) as well as expression (Raadsheer et al 1994) of CRH may be increased in postmortem tissue in patients with depression. Further, postmortem studies of individuals who have committed suicide have revealed elevated concentrations of CSF CRH (Arato et al 1989), decreased expression of CRH1 receptor mRNA within the frontal cortex (Merali et al 2004), increased CRH concentrations and decreased density of CRH receptors within the frontal cortex in comparison to controls (Merali et al 2006; Nemeroff et al 1988).
Developmental Trauma, CRH, and Depression
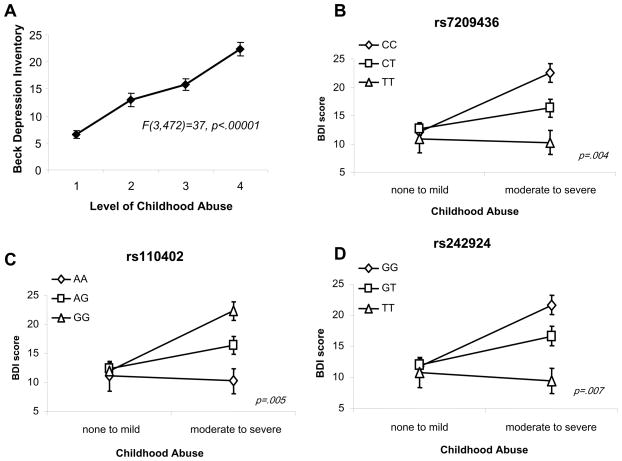
The experience of child abuse appears to pathologically alter the function of the HPA axis (Heim et al 2008b). Depressed patients who have a history of childhood adversity show elevated secretion of adrenocorticotrophic hormone (ACTH) and cortisol in response to a laboratory stress test (Heim et al 2000), as well as with neuroendocrine challenge tests including the dexamethasone-CRF test (Heim et al 2008a; Heim et al 2001). More recently reported data (McGowan et al 2009) identified epigenetic regulation of glucocorticoid receptors (GR) in post-mortem tissue from individuals with a history of child abuse. These data indicate that trauma exposure during childhood persistently alters the endogenous stress response, acting principally upon CRH and its downstream effectors, suggesting that a gene x environment (GxE) interaction at this locus may be important in mediating the effects of childhood trauma exposure on adult risk for depression. To examine whether the effects of child abuse on adult depressive symptoms (Figure 2A) are moderated by genetic polymorphisms within the CRH receptor (CRHR1) gene, our research group recently performed an association study examining GxE interaction between genetic polymorphisms at the CRHR1 locus and measures of child abuse on adult depressive symptomatology (Bradley et al 2008). This study was cross-sectional in design and participants were primarily low-income, African-American (>95%), men and women seeking care in the general medical care and obstetrics-gynecology clinics of an urban public hospital. The studied population reported high levels of exposure to childhood physical, sexual and emotional abuse. Fifteen single nucleotide polymorphisms (SNPs) spanning 57kb of the CRHR1 were examined (n = 422). We identified significant GxE interactions with multiple individual SNPS as well as with a common haplotype spanning intron 1 of the CRHR1 locus that modify adult risk of depression in the presence of childhood trauma exposure (Figure 2B,C,D). Specific CRHR1 polymorphisms appeared to moderate the effect of child abuse on the risk for adult depressive symptomatology but did not influence risk for adult post-traumatic stress symptomatology. These protective effects were supported with similar findings in a second independent sample (n=199). These data suggest that a GxE interaction is important for the expression of depressive symptoms in adults with CRHR1 risk or protective alleles who have a history of child abuse.
Figure 2. CRHR1 Polymorphisms Strongly Interact with Level of Childhood Abuse in the Prediction of Adult Depression.
A) Level of childhood abuse by self report very strongly associates with current depressive symptoms measured with the Beck Depression Inventory. CRHR1 polymorphisms rs7209436 (B), rs110402 (C), and rs242924 (D) interact with level of reported child abuse. For all of these polymorphisms within the CRHR1 gene, at low levels of child abuse, there are similar levels of BDI-based depressive symptoms, but at moderate to severe levels of abuse, the CRHR1 SNPs separately associate with differential levels of depressive symptoms. (adapted from Bradley et al., 2008)
In addition to the above findings, there have been several additional reports of CRHR1 polymorphisms associated with depression and suicidality (Licinio et al., 2004; Liu et al., 2006; Wasserman et al., 2007; and Utge et al., 2009). Although these reports have not all used the same SNPs, several have found main effects for depressive symptomatology or antidepressant response. Licinio et al., (2004) examined the association of CRHR1 genotypes with the phenotype of antidepressant treatment response in 80 depressed Mexican-Americans in Los Angeles who completed a prospective randomized, placebo lead-in, double-blind treatment of fluoxetine or desipramine, with active treatment for 8 weeks. They found that a haplotype consisting of single-nucleotide polymorphisms rs1876828, rs242939 and rs242941 was associated with a greater antidepressant treatment response. These findings were generally supported by Liu and colleagues (2007), who also found a main effect of CRHR1 haplotype status with major depression frequency in a Chinese cohort (Liu et al., 2006). Wasserman and colleagues (2008–9) demonstrated in family trios with suicide attempter offspring (N=672) that CRHR1 polymorphisms (particularly rs4792887) were associated with suicide attempt history as a function of depression. Child abuse history was not reported in these studies. However, the extremely strong predictive value of history of child maltreatment for adult depression, suicide risk, and response to treatment suggests that stratification of these cohorts for child abuse may reveal interesting additional effects of CRHR1 on depressed phenotypes.
Fear Learning and CRH: Rodent Studies
Classical fear learning (Pavlovian fear conditioning) in animals has provided great insight into the mammalian fear response and the hypothesized role of the amygdala in patients with PTSD is complemented by two decades of work demonstrating the amygdala’s role in the learning and expression of fear in rodents (Davis, 1989; LeDoux, 2000; Maren, 2008). Within the brain, the amygdala appears to function as one component of a threat-responsive comparator system that acts to differentiate threatening from non-threatening environmental stimuli in real time on the basis of prototype matching to fear memories and initiate adaptive behavior to deal with any perceived threat (Bishop, 2008; Rodigues et al., 2009). Preclinical studies indicate that persistent hyperactivity of the HPA axis following developmental stress exposure is mediated, at least in part, by a hyperactive CRHR1 system (Ladd et al 2000; Lupien et al 2009; Plotsky et al 2005). In conjunction with data from functional brain imaging studies demonstrating increased activity of the amygdala in patients with depression (Drevets 2000) and PTSD (Hull 2002), these findings raise the question of whether developmental trauma exposure and fear-learning might adversely impact function of the HPA axis through effects at the level of the amygdala.
A persistent finding in patients with depression is the elevation in the numbers of CRH and arginine-vasopressin neurons in the PVN in comparison to control subjects (Purba et al 1995; Raadsheer et al 1994). Recently experiments conducted to examine CRH function in the amygdala may provide insight into these findings (Keen-Rhinehart et al 2009). Genetically modified lentiviral vectors were introduced into the Central Nucleus of the amygdala (CeA) resulting in overexpression of CRH and arginine-vasopressin within the CeA as well as the PVN. Overexpression of CRH in these structures was accompanied physiologically by decreased glucocorticoid negative feedback, and behaviorally by increased anxiety-like behavior (acoustic startle) and depressive-like behavior (forced swim). These data suggest that unrestrained CRH synthesis in the CeA may produce dysregulation of the HPA axis, which is associated with many of the behavioral, physiological, and reproductive consequences associated with stress-related disorders.
Developmental Trauma, FKBP5, and PTSD
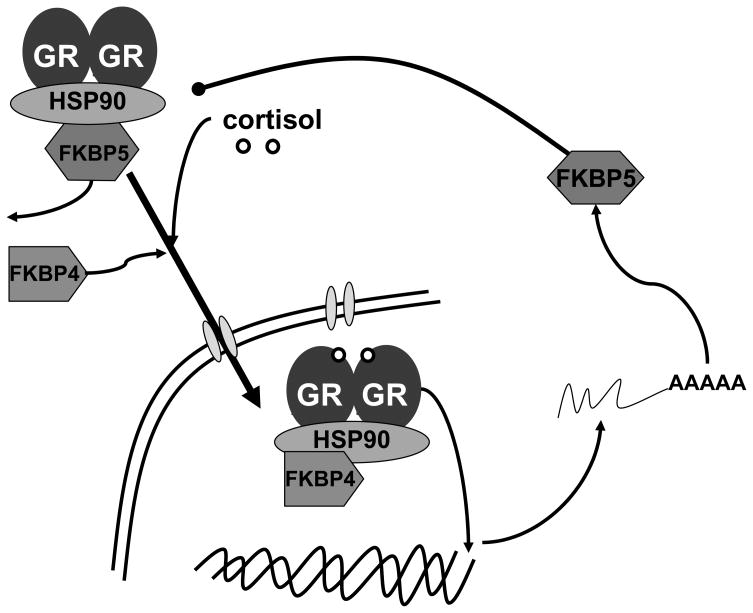
Increased HPA axis reactivity (Yehuda 2001; Yehuda et al 1991) and elevated GR sensitivity (Yehuda et al 2004a; Yehuda et al 2004b) are recurrent findings in patients with post-traumatic stress disorder (PTSD). FKBP5 (Figure 3) is a co-chaperone component of the GR heterocomplex (Schiene-Fischer and Yu, 2001; Binder, 2009) that plays a key role in the regulation of GR sensitivity and hence the expression of glucocorticoid-responsive genes by virtue of its participation in an ultra-short, intracellular negative feedback loop regulating GR activity (Vermeer et al 2003). Several lines of data suggest a role for FKBP5 in the pathophysiology of PTSD. Overexpression of FKBP5 reduces the hormone binding affinity (Denny et al 2000) and nuclear translocation of GR (Wochnik et al 2005). New world monkeys with naturally-occurring overexpression of FKBP5 experience increased GR resistance and hypercortisolemia (Denny et al 2000; Scammell et al 2001). In addition, clinical research has identified FKBP5 alleles associated with variation in GR resistance in depressed patients (Binder et al 2004) that are also associated with elevated peri-traumatic dissociation in medically-injured children (Koenen et al 2005), a psychological response to trauma which is predictive of PTSD risk in adults (Ozer et al 2003). Finally, level of FKBP5 expression in peripheral blood mononuclear cells at four months post-trauma exposure is predictive of PTSD diagnosis in trauma survivors (Segman et al 2005). Most recently, gene expression analysis from a study of subjects with PTSD following the World Trade Center Attacks found that FKBP5 showed reduced expression in PTSD, consistent with enhanced GR responsiveness (Yehuda et al., 2009).
Figure 3. Schematic of FKBP5 cellular function.
Schematic diagram depicting the function of FKBP5 as a co-chaperone which regulates glucocorticoid receptor (GR) binding and translocation within the nucleus. When sufficient cortisol is present leading to GR dimerization and FKBP4 binding, FKBP5 is displaced allowing GR translocation and transcriptional activation. However, one of the gene targets of GR is the FKBP5 gene, which when increased in expression is thought to act as a negative intracellular feedback on the GR system within the cell. (figure courtesy of Elisabeth Binder, PhD)
To further evaluate the role of FKBP5 in the etiology of PTSD, our research group examined FKBP5 polymorphisms in association with level of adult PTSD symptomatology (Binder et al 2008). This cross-sectional study examined genetic and psychological risk factors for PTSD using a verbally-presented survey, combined with SNP genotyping, in a randomly-chosen sample of non-psychiatric clinic patients. Participants were primarily low-income, African-American (>95%), men and women seeking care in the general medical care and obstetrics-gynecology clinics of an urban public hospital. We found that this population had experienced significant levels of childhood abuse as well as non-child abuse trauma. 900 total participants were included in the overall analyses and 762 participants were included for all genotype studies. The primary outcome measure was severity of adult PTSD symptomatology, as measured with the modified PTSD Symptom Scale (mPSS). Independent factors included in the analyses were non-child abuse (primarily adult) trauma exposure and child abuse measured using the traumatic events inventory (TEI) and eight single nucleotide polymorphisms (SNPs) spanning the FKBP5 locus.
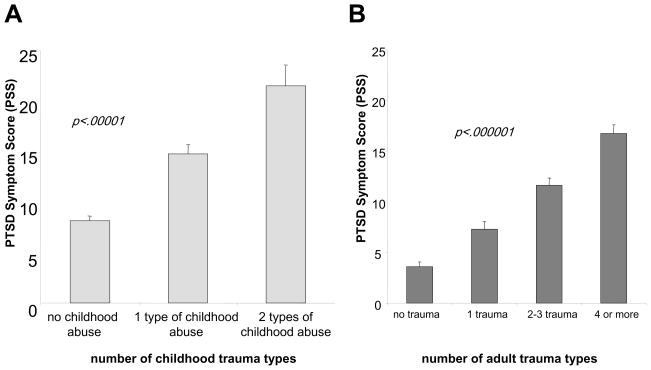
As expected based on prior research, we found that level of child abuse (Figure 4A) and non-child abuse trauma (Figure 4B) exposure separately predicted level of adult PTSD symptoms. Although FKBP5 SNPs did not directly predict PTSD outcome, or interact with level of non-child abuse trauma to predict PTSD, 4 SNPs in the FKBP5 locus significantly interacted with the severity of child abuse to predict level of adult PTSD (Figure 5). This gene x environment interaction remained significant when controlling for depression severity scores, age, gender, levels of non-child abuse trauma exposure and genetic ancestry. This genetic interaction was also paralleled by FKBP5 genotype- and PTSD-dependent effects on glucocorticoid receptor sensitivity as measured by the dexamethasone suppression test. Collectively, these data suggest a potential gene-childhood environment interaction for adult PTSD.
Figure 4. Child Abuse and Adult Trauma Predict PTSD symptoms.
Both level of childhood abuse (number of types of physical, sexual or emotional abuse) (A) and number of types of adult non-childhood trauma (B) each predict level PTSD symptoms in the adult. (adapted from Binder et al., 2008)
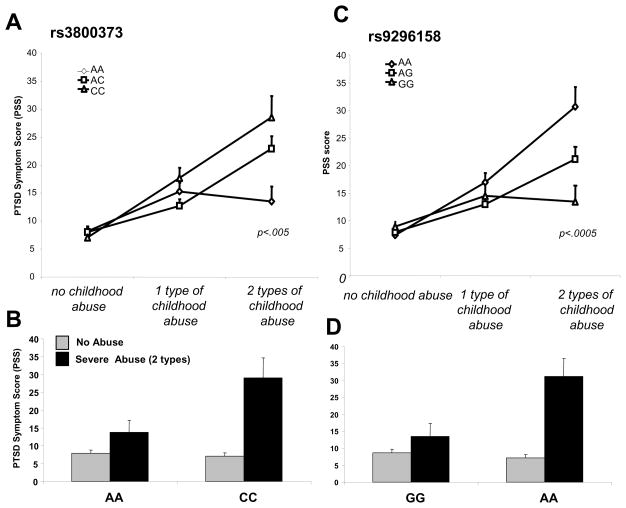
Figure 5. FKBP5 genotype interacts with level of child abuse to predict level of adult PTSD symptoms.
FKBP5 polymorphisms rs3800373 (A–B), and rs9296158 (C–D) interact with level of reported child abuse to predict level of adult PTSD symptoms. For these polymorphisms within the FKBP5 gene, at low levels of child abuse, there are similar levels of mPSS-based PTSD symptoms, but at moderate to severe levels of abuse, the FKBP5 SNPs separately associate with differential levels of PTSD symptoms. (adapted from Binder et al., 2008)
Integration of Data on Variations of CRHR1 and FKBP5 and Childhood Abuse in Predicting Adult Mood and Anxiety Disorders
As described above, data from several genetic association studies indicate that variation in CRHR1 and FKBP5 may influence risk for depressive and post-traumatic stress symptoms contingent on the experience of child abuse. One possible explanation for this finding is that a critical period exists for the normative development of an emotional regulatory system. The existence of such a system would not be unprecedented as other neurobiological systems such as the visual system have critical periods whereby normal development and functioning of the system is contingent on input from the environment (Wiesel and Hubel 1963).
In support of this hypothesis, preclinical data suggest that the quality of maternal care influences an emotional critical period in rodents (Moriceau and Sullivan 2005; Moriceau and Sullivan, 2006a). Rat pups have a sensitive period during which they have an increased capacity to learn preferences for novel odors, a decreased capacity to develop odor aversion, and a generalized hypo-responsiveness to environmental stress and corticosterone release. This phenomenon is thought to facilitate close maternal attachment during the period of early postnatal development when rat pups are maximally dependent on maternal care and proximity for survival (Sevelinges et al., 2007). During the sensitive period, odor-shock pairing produces either odor preference or odor aversion depending upon the presence or absence of the mother during conditioning. Maternal absence during odor-shock pairing results behaviorally in odor avoidance and physiologically in amygdala activation and corticosterone secretion. Conversely, maternal presence during odor-shock pairing results behaviorally in odor preference without amygdala activation or corticosterone secretion (Moriceau et al., 2006b). The effect of maternal presence on preference for the shock-paired odor and suppression of corticosterone secretion and amygdala activation following odor-shock pairing may be blocked by infusion of corticosterone into the amygdala of rat pups during odor-shock pairing suggesting that corticosterone (the rat equivalent of cortisol) plays a central role in facilitating and defining the temporal boundaries of such an emotional critical period as it relates to fear-learning (Moriceau and Sullivan 2006a).
In relation to the FKBP5 and CRHR1 GxE effects discussed above, it is important to note that these two studies were obtained with subjects that were primarily impoverished, highly traumatized populations (Bradley et al., 2008; Binder et al., 2008; Gillespie et al., in press). It has been previously reported that inner-city minority populations appear to be exposed to extreme amounts of trauma (Alim et al 2006; Breslau et al 1998). In particular, economically disadvantaged African Americans living within urban environments experience high levels of trauma (Breslau et al 1991; Breslau et al 1998; Fitzpatrick and Boldizar 1993; Selner-O’Hagan et al 1998; Shakoor and Chalmers 1991) and a very large amount of this exposure occurs during youth (Fitzpatrick and Boldizar 1993; Selner-O’Hagan et al 1998; Shakoor and Chalmers 1991).
Recently, McEwen and colleagues reported that higher cortisol levels were found in children of low socioeconomic status compared to children of high socioeconomic status. Most interestingly, among this group, the child’s cortisol levels correlated with the extent of maternal depressive symptomatology (Lupien et al 2000). These combined data suggest that children of low socioeconomic status residing in urban areas with high trauma exposure are at increased risk for dysregulation of cortisol levels beginning at an early age (Shonkoff et al., 2009).
The data described above suggest that a critical period exists during which brain exposure to corticosterone affects fear learning that is modulated by the quality of maternal care. Together, this convergent series of data suggest the following hypothesis (Figure 6): During a sufficiently supported development, there develops an amygdala-dependent emotional circuit that is able to appropriately differentiate threatening from non-threatening environmental stimuli. In contrast, when child abuse is combined with these biological risk factors, amygdala development may be altered through interactions of elevated stress / cortisol and genetic risk / resilience factors such as described with variation in FKBP5 or CRHR1. This developmental interaction may lead to an amygdala-dependent emotional circuit is altered and always primed for stress responsiveness. In the case of child maltreatment with combined genetic risk, this emotion circuit is unable to differentiate threat appropriately. Thus, in the presence of an adult trauma these individuals may be at a higher risk for PTSD or other trauma-related psychopathology, such as depression.
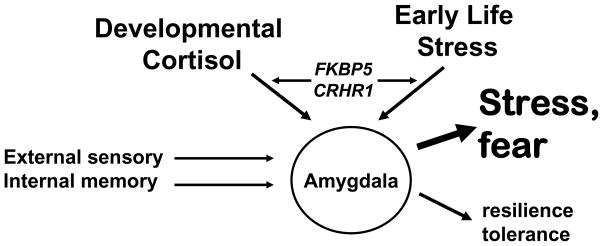
Figure 6. Early Life Stress – HPA axis Gene Interaction.
This diagram represents the function of the adult amygdala. It continually compares neural inputs containing external sensory information with emotion-related internal memory to rapidly activate systems leading to tolerance to aversion and resilience vs. the fight or flight, fear and stress reaction. The data reviewed here suggests that with sufficiently supportive development, a dynamic amygdala-dependent emotional circuit is created allowing proper interpretation of threat responses. In contrast, child abuse combined with biological risk factors (e.g. increased stress-dependent cortisol interacting with FKBP5 or CRHR1 polymorphisms) may lead to an adult amygdala-dependent emotional circuit that is always ‘primed’ for stress responsiveness. It is hypothesized that this latter hyper-active stress response may, in the presence of adult trauma, lead to a higher risk for trauma-related psychopathology.
In summary, heritability accounts for 30–40% of the variance contributing to risk for PTSD and for other mood and anxiety disorders. It is also well known that childhood exposure to abuse and other early life adverse events increases risk for the later development of these disorders. Recent research from a number of areas suggests that childhood experiences in combination with genetic factors appear to contribute to alterations in biologically based stress response systems. Taken together, these data suggest that a greater understanding of risk, resiliency, and stress-related illness will rely on further progress in dissecting the interactions between genes and the environment during the developmental critical periods of neural circuits that underlie emotion.
Acknowledgments
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also received from National Institute of Mental Health (MH082256) and NARSAD (CFG), the American Foundation for Suicide Prevention (RGB) and the Burroughs Wellcome Fund (KJR).
References
- Alim TN, Charney DS, Mellman TA. An overview of posttraumatic stress disorder in African Americans. J Clin Psychol. 2006;62:801–813. doi: 10.1002/jclp.20280. [DOI] [PubMed] [Google Scholar]
- Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biological psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. The American journal of psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature genetics. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009 Jun 25; doi: 10.1016/j.psyneuen.2009.05.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci. 2008;1129:141–52. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of general psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. The American journal of psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of general psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of general psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of affective disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Ann N Y Acad Sci. 1989;563:165–83. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Oitzl M, Sutanto W. Implication of brain corticosteroid receptor diversity for the adaptation syndrome concept. Methods Achiev Exp Pathol. 1991;14:104–132. [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KM, Boldizar JP. The prevalence and consequences of exposure to violence among African-American youth. J Am Acad Child Adolesc Psychiatry. 1993;32:424–430. doi: 10.1097/00004583-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Malhi GS, Wilhelm K, Austin MP. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. The American journal of psychiatry. 2004;161:1417–1425. doi: 10.1176/appi.ajp.161.8.1417. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma Exposure and Stress-Related Disorders in Inner City Primary Care Patients. General Hospital Psychiatry. 2009 doi: 10.1016/j.genhosppsych.2009.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Annals of the New York Academy of Sciences. 1996;780:96–105. doi: 10.1111/j.1749-6632.1996.tb15114.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biological psychiatry. 2008a;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American journal of psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008b;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hull AM. Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Molecular psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in brain research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, Lake S, Tantisira KG, Weiss ST, Wong ML. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004 Dec;9(12):1075–82. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006 Sep 1;404(3):358–62. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007 Mar 6;414(2):155–8. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008 Oct;28(8):1661–6. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biological psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental psychobiology. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature neuroscience. 2006a;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006b Jun 21;26(25):6737–48. doi: 10.1523/JNEUROSCI.0499-06.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Archives of general psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science (New York, NY. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Purba JS, Raadsheer FC, Hofman MA, Ravid R, Polman CH, Kamphorst W, et al. Increased number of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of patients with multiple sclerosis. Neuroendocrinology. 1995;62:62–70. doi: 10.1159/000126989. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. The American journal of psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Snieder H, Ormel J, Sham P, Goldberg DP, Spector TD. Genetic and environmental influences on psychological distress in the population: General Health Questionnaire analyses in UK twins. Psychological medicine. 2003;33:793–801. doi: 10.1017/s0033291703007451. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Ledoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rutter M. Implications of resilience concepts for scientific understanding. Annals of the New York Academy of Sciences. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and comparative endocrinology. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Schiene-Fischer C, Yu C. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001;495:1–6. doi: 10.1016/s0014-5793(01)02326-2. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Molecular psychiatry. 2005;10:500–513. 425. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- Selner-O’Hagan MB, Kindlon DJ, Buka SL, Raudenbush SW, Earls FJ. Assessing exposure to violence in urban youth. Journal of Child Psychology and Psychiatry. 1998;39:215–224. [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007 Nov 15;62(10):1070–9. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Shakoor BH, Chalmers D. Co-victimization of African-American children who witness violence: effects on cognitive, emotional, and behavioral development. J Natl Med Assoc. 1991;83:233–238. [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. The American journal of psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. The American journal of psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Utge S, Soronen P, Partonen T, Loukola A, Kronholm E, Pirkola S, Nyman E, Porkka-Heiskanen T, Paunio T. A population-based association study of candidate genes for depression and sleep disturbance. Am J Med Genet B Neuropsychiatr Genet. 2009 Jun 22; doi: 10.1002/ajmg.b.31002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. The Journal of clinical endocrinology and metabolism. 2003;88:277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008 Feb;7(1):14–9. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Wasserman J, Rozanov V, Sokolowski M. Depression in suicidal males: genetic risk variants in the CRHR1 gene. Genes Brain Behav. 2009 Feb;8(1):72–9. doi: 10.1111/j.1601-183X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. The Journal of clinical psychiatry. 2001;62:41–46. [PubMed] [Google Scholar]
- Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biological psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Halligan SL, Meaney MJ, Bierer LM. The ACTH response to dexamethasone in PTSD. The American journal of psychiatry. 2004a;161:1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological psychiatry. 2004b;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Müller-Myhsok B, Holsboer F, Buxbaum JD. Gene Expression Patterns Associated with Posttraumatic Stress Disorder Following Exposure to the World Trade Center Attacks. Biol Psychiatry. 2009 Apr 24; doi: 10.1016/j.biopsych.2009.02.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]