Abstract
Vernonia amygdalina (VA) is an edible plant of the Asteraceae family used in many herbal formulations prescribed by herbalists for many diseases. We have previously reported that aqueous VA extracts inhibit the growth of estrogen receptor-positive human breast cancerous cells in vitro. Activity markers of the VA extracts have not been previously identified or characterized. Hence, the objective of this study was to identify activity markers of the VA extracts associated with cell growth inhibition. Extraction of VA with multiple solvents of various polarity indexes yielded three fractions (A1-2, B-3) that significantly inhibited cell growth (p <0.05) at 0.1 mg/ml concentration. At a higher concentration of 1 mg/ml, six fractions of hexane, chloroform, butanol, and ethyl acetate (A1-3, B2-4) inhibited DNA synthesis by 76, 98, 94, 98, 98, and 96% respectively. These fractions were UV-detected from 250–730 nm; and all showed three distinct peaks around 410, 431, and 664 nm. Furthermore, HPLC analysis of the fractions revealed similar retention times of 2.213, 2.167, and 2.151 min respectively. Bioactivity assays showed that HPLC retention of approximately 2 min is required for cell growth-inhibitory activity of VA fractions. Interestingly, all active fractions exhibited HPLC peaks at approximately 2 min. Therefore, the UV and HPLC peaks may be used as predictive tools to determine VA extracts activities.
Keywords: anti-cancer, vernonia amygdalina, activity prediction, standardization
INTRODUCTION
Vernonia amygdalina (VA), commonly known as bitter leaf, is a shrub that grows up to 3 meters high. It grows in African tropics and other parts of Africa, particularly South Africa, Zimbabwe and Nigeria (1–3). Vernonia amygdalina may be effective against amoebic dysentery (4); gastrointestinal disorders (5–9); microbial and parasitic activities (10–11); hepatotoxicities (12); and cancer (13–18). It is very unlikely that a single molecule is responsible for such varied activities; instead multiple molecules, working alone or in concert with others, are much more likely to be responsible for each biological activity. The biologically-active compounds of VA are saponins and alkaloids (19); terpenes, steroids, coumarines, flavonoids, phenolic acids, lignans, xanthones and anthraquinones (20); edotides (15); tannis (21) and; sesquiterpene lactone (13–14). These compounds isolated from VA extracts, using various solvents of different polarity indexes, have been attributed to specific biological activities. For example: the antiplasmodial (anti-malarial activity) of VA extracts may be related to the presence of flavonoids, saponins, alkaloids (19). Some studies have associated coumarines and flavonoids in most plants with antitumor activities in humans (22–24). Other cancer-fighting agents in VA extracts may include sesquiterpene lactones (SLs) (13–14) and Edotides (15).
The quantities, and qualities or activities of these compounds may vary with location, seasonal and propagation conditions, age at harvest, and storage conditions etc. Therefore, achieving consistency in botanical quality presents a daunting task for the botanical production industry. Several advanced techniques are available for qualitative analysis of complex mixtures. Typically, analysis of natural compounds has relied on a protocol involving: sample scale-up, extraction, solvent partitioning, column fractionation, profiling with an ultra violet (UV) detector and individual component spectroscopy using nuclear magnetic resonance (NMR). The combination of HPLC and thin layer chromatography (TLC) provides additional capabilities for mixture analysis for quality assurance and standardization of VA extracts. We have previously shown that low concentrations (µg/ml) of whole aqueous extracts of VA potently retards the growth of human estrogen receptor-positive (ER+) cell line (MCF-7) in vitro in a concentration-dependent manner by modulating the extracellular signal-regulated kinases 1 and 2 (ERK1/2) activities on MCF-7 cells (17), cytochrome P450 enzymes (16), membrane disruption (18). However, our efforts to purify or isolate the active component(s) of VA have not consistently and substantially improved the concentration required for 50 % inhibition of activity (IC50). Thus, suggesting the presence of multiple active components. The objectives of the present studies are to use solvent extraction, spectrophotometric and chromatographic techniques to fractionate and identify anti-cancer activity markers in VA extracts. The anti-cancer activity markers may be used as predictive tools for VA quality determination.
MATERIALS AND METHODS
Human breast cancerous cell line (MCF-7) was purchased from ATCC. RPMI 1640 Medium, Fetal Bovine Serum (FBS), and Phosphate Buffered Saline (PBS) were purchased from Gibco BRL (Grand Island, NY). [3H]-thymidine (1mCi/ml) was purchased from ICN Pharmaceuticals (Irving, CA). Methanol (AR grade) and other chemicals were obtained from Sigma Chemicals Company (St. Louis, MO. USA).
Sample Collection and Preparation of Aqueous Extracts
Pesticide-free fresh VA leaves were collected in Benin City, Nigeria. The leaves were rinsed with distilled water and spread out evenly on galvanized-wire screens with the edges bent upward 2 inches on all sides. The galvanized-wire screens were placed in a specially-constructed dryer and heated to 130–140 ° F for complete dryness within 4 h. Three hundred (300) g of dried leaves was soaked in 6 L of ddH20 (1:20 w/w) overnight at 40°C before squeezing by hand to a mixture. The mixture was then filtered through a clean white gauze to remove the particulate matter before filtration through a 0.45- µm filtration unit for sterilization. The resulting sample solution was lyophilized to dry powder (30 g) on a SpeedVac Concentrator (Savant SC210A), transferred into a 50 mL centrifugation tube and stored at −20° C for bioactivity assays, and HPLC, TLC, NMR, UV and IR spectroscopic analyses.
Liquid-Liquid Extraction
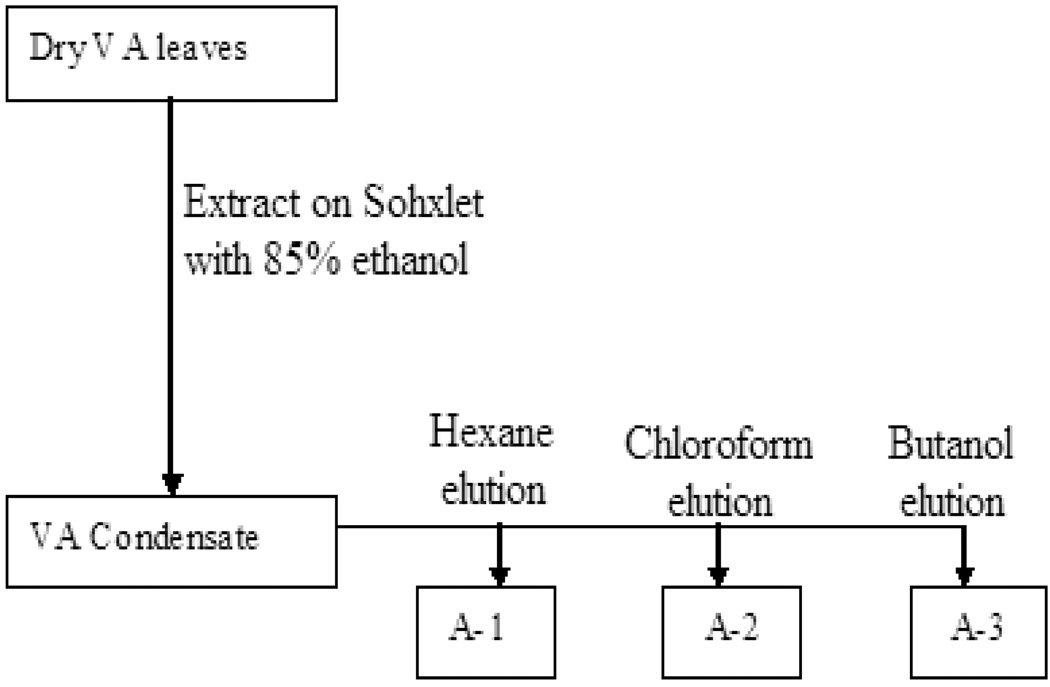
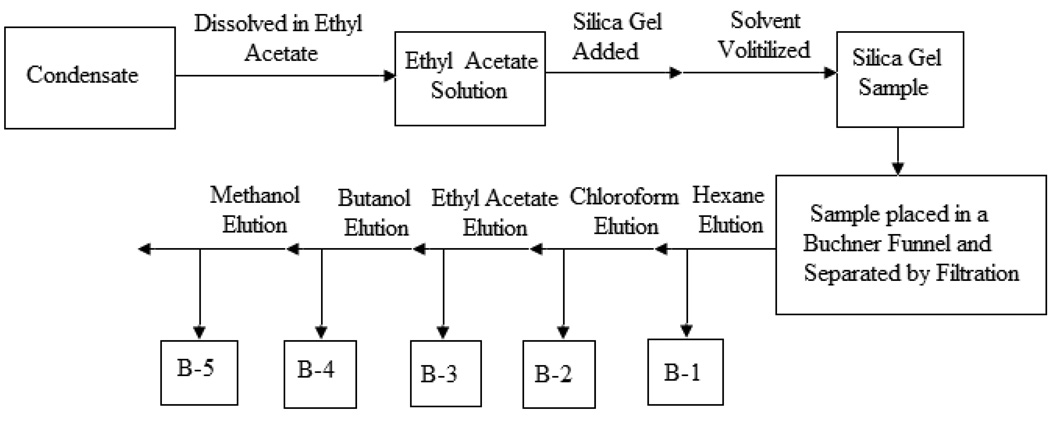
One hundred (100) g of dried VA leaves were pulverized and extracted on a soxhlet’s apparatus for 8–18 hours using 85% ethanol. The eluted condensate was divided into two fractions (A, B). Fraction A was further separated by liquid-liquid extraction technique using equal volumes of hexane, chloroform, and butanol. The separation yielded three fractions: A-1, A-2 and A-3 (Fig 1). Each of the eluted sample solutions was separately concentrated in a Rotavapor at 65° C and lyophilized to dry powder on a SpeedVac Concentrator (Savant SC210A). The dry powders were weighed and stored in 15 mL centrifuge tubes at −20° C for further chemical evaluation, and DNA synthesis determination in MCF-7 breast cancerous cells. Fraction B was separated by silica gel technique. In the silica gel method, the condensate of 85% ethanol-extracted VA was dissolved in ethyl acetate, and silica was added and mixed. The solvent (ethyl acetate) was then volatilized and the resultant silica gel sample was separated on a Buchner Funnel with filtration flushes of hexane, chloroform, ethyl acetate, butanol, and methanol. The resultant solvent elution of hexane, chloroform, ethyl acetate, butanol and methanol yielded fractions B-1, B-2, B-3, B-4 and B-5 respectively, as shown (Fig. 2). The Fractions (B1-5) solutions were then separately concentrated in a Rotavapor at 65° C and lyophilized to dry powder on a SpeedVac Concentrator (Savant SC210A).
Fig. 1.
Flow diagram showing the stages in the organic extraction and separation of fractions A-1, A-2 and A-3 using various solvents.
Fig. 2.
Flow Chart Diagram showing stages in the organic extraction and elution of fractions B-1, B-2, B-3, B-4 and B-5 from VA condensate using Silica gel. Bioactivity assays reveal that B2, and B3 are biologically-active.
Column Chromatography
This process was performed on silica gel 60 and Sephadex LH-20 (Sigma, 25–100 µm). The stationary phase consisted of silica gel, while the mobile phase was organic solvent poured on top of the loaded column. The organic solvents were forced out of the column by application of air-pressure, causing the components of sample mixture to distribute between the silica gel and the solvents, thus separating the components of the mixture so that as the solvents flowed out of the column, some components eluted with early collections and others eluted with late fractions.
High Pressure Liquid Chromatography (HPLC)
HPLC is a technique used to separate components of a mixture by using a variety of chemical interactions between the substance being analyzed and the chromatography column. The sample was injected through a column of the stationary phase by pumping a mobile phase (10% methanol) at high pressure through the column. The extracted VA sample was injected into the stream of mobile phase and retarded by chemical or physical interactions with stationary phase (90% deionized water). The time at which a specific analyte eluted (retention time) was considered an identifying characteristic of the eluent. Sample analyses for extracts were carried on an Agilent 1100 HPLC System (Agilent Technologies, Palo, Alto, CA).
Cell Proliferation Studies
The cells were seeded at 3 × 105 cells/ 100 mm plate and propagated to 60–65% confluence, the 100 mm plates were randomly selected for treatment with various concentrations (0.001 – 1 mg/ml) of the VA extracts with appropriate controls. The medium was aspirated from the cell monolayer and the cells were washed with PBS pH 7.4 to render the cells more sensitive to trypsin digestion. The resulting cell monolayer was treated with 1ml trypsin and incubated for at least 3 min at 37°C. The cells were viewed under the microscope to ensure complete cell detachment. The trypsin action was stopped by re-suspending the cells in RPMI 1640 medium before counting with a hemacytometer.
DNA Synthesis Assays
DNA synthesis was determined by the incorporation of [3H]-thymidine into cellular nucleic acids. The cells were grown to about 60% confluence in tissue culture plate (35 mm diameter wells) with RPMI 1640 medium supplemented with 1% antibiotic-antimycotic solution containing 10,000 U/ml penicillin, 10,000 µg/ml streptomycin and 25 µg/ml amphotericin B (fungizone) and 10% Fetal Bovine Serum. Stock solution (100 mg/ml) was prepared by dissolving 10 mg of fractions (A1-3, B1-5) of VA powder into 0.1ml of 85% ethanol, and the cells were treated with either 0.1 or 1mg/ml final concentrations of fractions A1-3, B1-5. Untreated cells served as negative controls while vehicle-treated cells served as positive controls. The cells were then incubated for 18 hours in a 37°C humidified 5% CO2 incubator before the addition of 1µCi/ml of [3H]thymidine per 35 mm well, followed by an additional 6 h. The incubation was stopped by medium aspiration, then followed by three sequential washes with cold PBS pH 7.4. Two (2 ml/ 35 mm) of ice cold 10% Trichloroacetic acid (TCA) was added and the cells were incubated for 10 min at 4°C. TCA solution was aspirated and the cells washed three times with ice-cold distilled water, then solubilized with 1ml of 0.5 M NaOH solution at 37°C for 30 min. Upon solubilization, 1ml per well aliquot samples was transferred to scintillation vials into which 5 ml of scintillation cocktail was added. DNA synthesis in treated and control cells was quantified by a Liquid Scintillation Analyzer (Try-Carb 2700TR).
Statistical Analysis
Results are expressed as the mean ± SD of values obtained from experiments conducted in triplicate. Data collected were analyzed using Analysis of Variance (ANOVA) with the General Linear Model (GLM) procedure of SAS version 9.1 (25) The testing hypothesis will be H1 = differences are expected between means. P value <0.5; if differences exist, the means were compared by students t-test.
RESULTS
Multiple-Solvent fractions of V. amygdalina extracts Inhibited DNA Synthesis
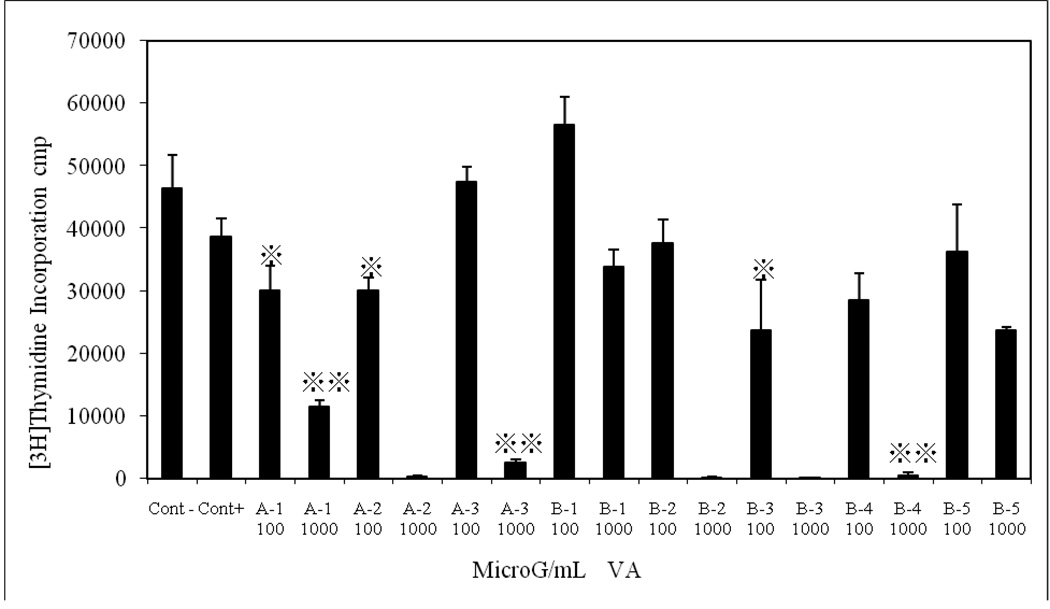
The exposure of MCF-7 cells to two concentrations (0.1 and 1 mg/ml) of multiple-solvent fractions (butanol, chloroform, ethyl acetate, hexane, and methanol) of VA inhibited DNA synthesis in both concentration and solvent-dependent fashion. Hexane, chloroform and ethyl acetate extracted fractions (A1, A2 and B3) at 0.1 mg/ml inhibited DNA synthesis by approximately 30 % (p < 0.05). One milligram/ml (1 mg/ml) of fractions of hexane, chloroform, butanol and ethyl acetate (A1-3, B2, B3, and B4) inhibited DNA synthesis by 76%, 98%, 94%, 98%, 98%, and 96%, (p < 0.001), respectively, as shown in Fig. 3. Next, we performed UV and HPLC analyses to better characterize the active component(s) of the VA fractions.
Fig. 3.
Multiple-Solvent fractions of V. amygdalina extracts Inhibited DNA Synthesis. Cells at the logarithmic growth phase were treated with either 100 or 1000 µg/ml for 18 h before the addition of 1µCi/ml [3H]thymidine for 6 h. Each data point represents the mean of three independent experiments done in duplicates (N=6). Exposure of cells to multiple-solvent fractions (butanol, chloroform, ethyl acetate, hexane, and methanol) of VA inhibited DNA synthesis in both concentration and solvent-dependent fashion. Hexane, chloroform and ethyl acetate extracted fractions (A1, A2 and B3) at 100 µg/ml inhibited DNA synthesis by approximately 30 %. One thousand (1000) microgram/ml of fractions of hexane, chloroform, butanol and ethyl acetate (A1-3, B2, B3, and B4) inhibited DNA synthesis by 76%, 98%, 94%, 98%, 98%, and 96%, (p < 0.001), respectively [3H]thymidine uptake was determined as described under the materials and methods section.
The UV Spectra of Multiple-Solvent fractions of V. amygdalina Extracts
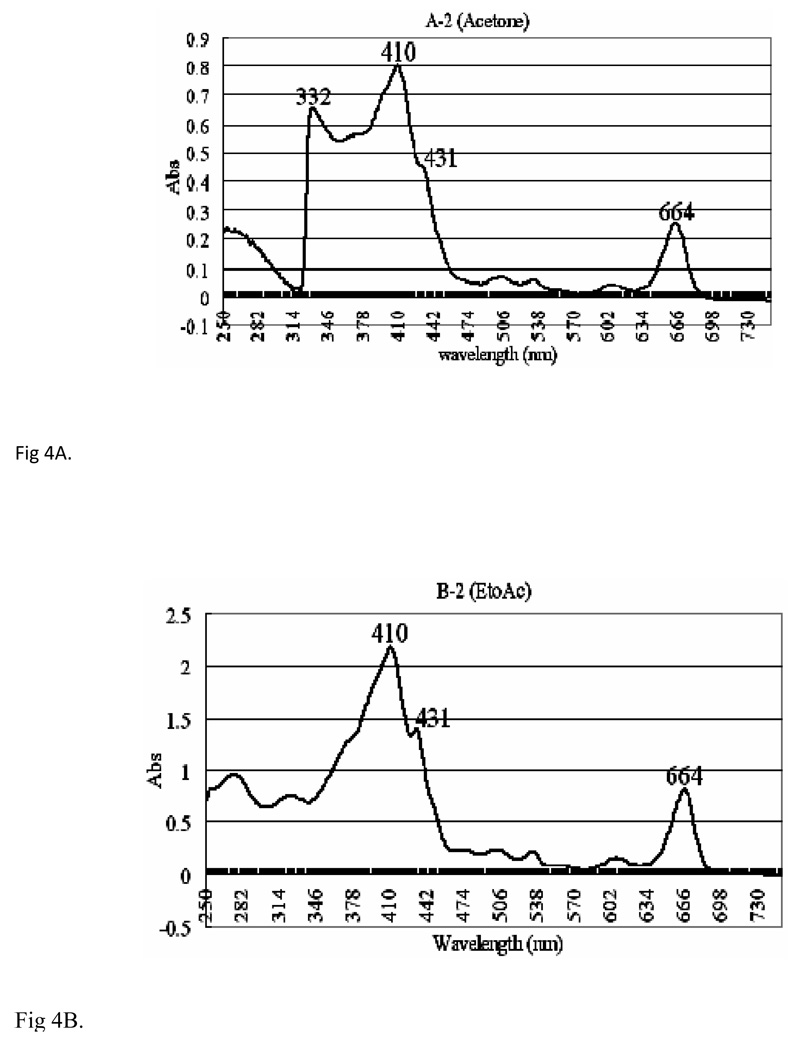
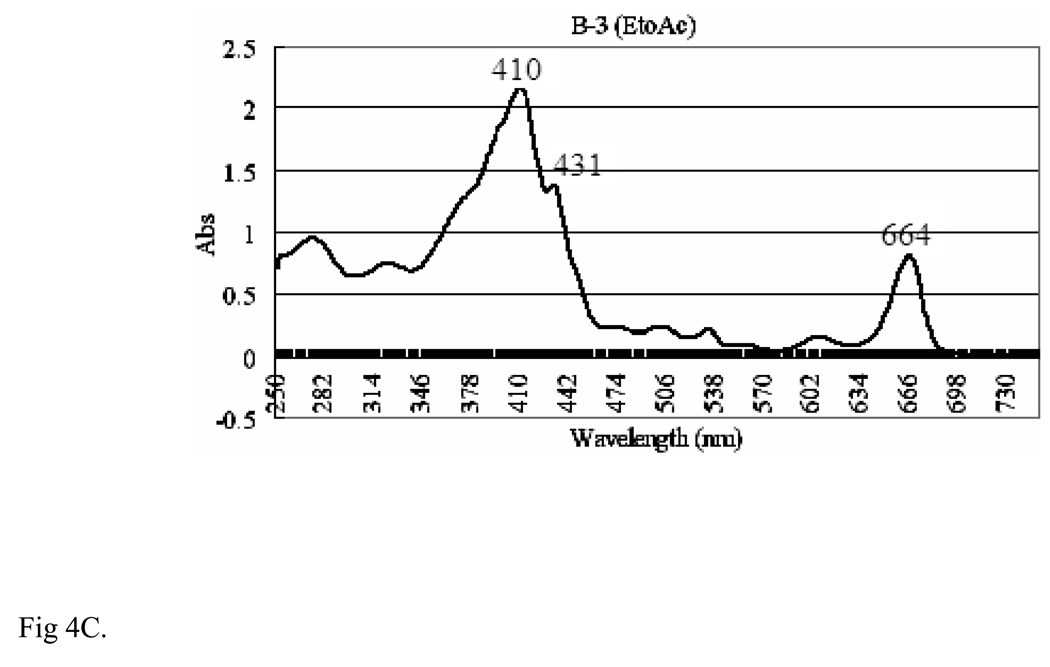
Fractions A2, B2 and B3 of VA extracts were UV-detected from 250–730 nm on a Varian UV-Visible spectrophotometer (CARY 300 BIO) using 1ml quartz cuvette. To examine the effect of solvents on the spectrophotometric peaks, various solvents were used, out of which ethyl acetate was found to be the most appropriate in the spectroscopic analysis. Therefore, ethyl acetate was used as a reference solvent for fractions B2 and B3, and acetone was used for fraction A2. The UV results (Figs 4A, 4B, and 4C) for all the fractions (A2, acetone; B2, EtOH, and B3, EtoAc) show three distinct high absorbance peaks around 410, 431 and 664 nm. Fraction A2 shows absorbencies of 0.8, 0.45 and 0.25 respectively (Fig. 4A), and 2.25, 1.40 and 0.80 respectively for fraction B2 (Fig. 4B). Fraction B3 shows peaks at absorbencies of 1.295 and 0.476 respectively (Fig. 4C). We observed that these peaks (410, 431, and 664 nm) were associated with activities.
Fig. 4.
The UV Spectra of Multiple-Solvent fractions of V. amygdalina Extracts [A] Ultraviolet (UV) spectrum of fraction A2 of VA extracts, showing distinct peaks at 410,431,664 and 332 nm at absorbencies of 0.8, 0.45, 0.25 and 0.65 respectively. The extracts were UV detected from 250 nm–730 nm on Varian UV-Visible spectrophotometer (CARY 300 BIO). Reference solvent used was acetone. [B] UV spectrum of fraction B2 of VA extracts, showing distinct peaks at 410, 431 and 664 nm at absorbencies of 2.25, 1.48 and 0.75 respectively. The extracts were UV detected from 250 nm–730 nm on Varian UV-Visible spectrophotometer (CARY 300 BIO). Reference solvent used was ethyl acetate. [C] UV spectrum of fraction B-3 of VA extract, showing distinct peaks at 664 nm, 410 nm and 431nm at absorbencies of 2.25, 1.48 and 0.75 respectively. The extracts were UV detected from 250 nm–730 nm on Varian UV-Visible spectrophotometer (CARY 300 BIO). Reference solvent used was ethyl acetate.
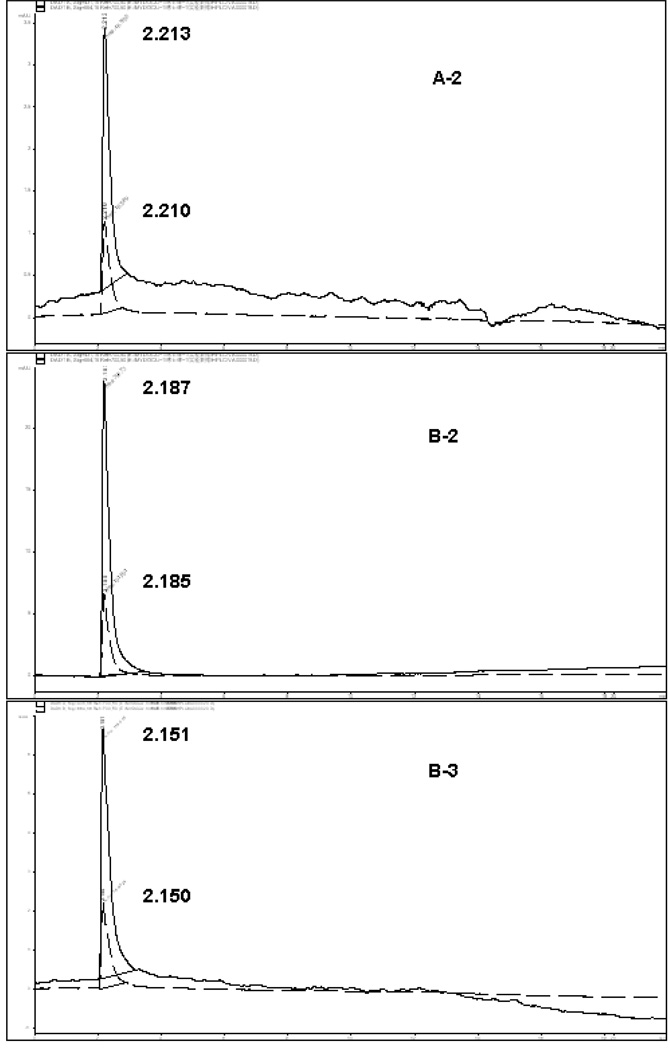
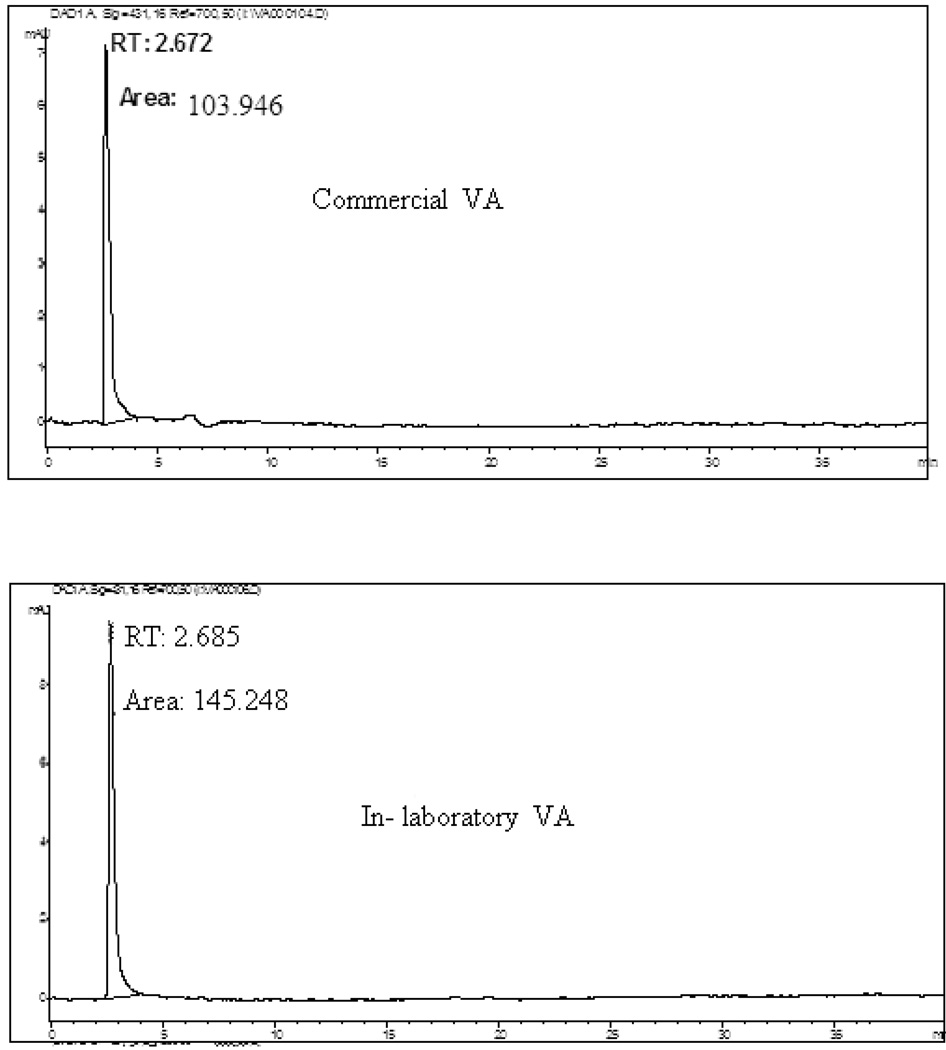
HPLC chromatograms of these three fractions (A-2, B2–B3) of VA extracts revealed retention times of 2.213, 2.187, and 2.151min respectively at 664 nm; and 2.210, 2.185, and 2.150 min respectively at 431 nm (Fig. 5). Sample fractions were ran at a flow rate of 0.6 ml/min with a solvent system of 90 % water and 10 % methanol for 40 min. To achieve this, a volume of 150 µl of filtered solutions of each sample was injected into the HPLC analyzer and each fraction was determined in triplicate. Peaks in the chromatograms were identified by comparing the retention times of individual fractions A2, B2- 3 with their corresponding activities as revealed by DNA synthesis assay (Fig.3). The chromatographic peaks with same retention time (RT) were considered common peaks in the extracted fractions. Two distinct peaks detected at 431 nm and 664 nm with close retention times were evident in the HPLC chromatograms of each of the three analyzed fractions (Table 1). The selected biologically-active fractions (A2, B2-3) shared common peaks at 431 nm and 664 nm with a retention time of approximately 2 min. Thus, suggesting that compounds with these peaks are required for VA anticancer activities (DNA synthesis inhibition). If this assertion is correct, the presence and sizes of these peaks may be used for quality assurance purposes: fingerprints for authentication and anti-cancer activity of VA extracts. Based on this fingerprint analysis, the qualitative results of the peak groups were very similar in the three fractions. However, they differed markedly on quantitative basis, with fractions A2, B2 and B3 showing a mean peak heights of 2.35, 15.95, and 4.475 mAU respectively (Table 1). The concentrations of all the peak fractions were highest in B-2, suggesting the presence of highest active compounds in the fractions. Taken together, the UV and HPLC data suggest that two peaks (431 and 664 nm) are required for VA anticancer activities. Therefore, we hypothesized that compound(s) contained in these two fractions that correspond to these peaks (431 and 664 nm) are required for the anti-cancer (DNA synthesis inhibition) of VA extracts. To test this hypothesis, dried VA leaves were obtained from a commercial source in Jackson, MS, for activity comparison with our in-laboratory VA. Equal amounts (1gram) of each sample was extracted with ethanol (85 %) and processed as described under the materials and methods section. Both extracts expressed peaks at approximately 2.6 min, suggesting the presence of similar chemical qualities (Fig. 6). However, they greatly differed on quantitative basis, as represented by higher peak area (145.248 mAU) in our In-laboratory VA, and lower peak area (103.946 mAU) in the Commercial VA (Fig.6). We therefore predicted that our In-laboratory VA would be more active compared to the Commercial VA. To test this prediction, both VA samples were processed and activities were determined as described under the materials and methods section. These results show that in-laboratory VA inhibited cell growth by 51% (p < 0.01) compared to 31% (p < 0.05) by commercial VA at 0.1 mg/ml. This confirms the notion that positive relationships exist between the 410 and 431 nm peaks and anticancer activities of VA fractions.
Fig. 5.
HPLC chromatograms of VA fractions Fractions (A-2, B2–B3) of VA extracts revealed retention times of 2.213, 2.187, and 2.151min respectively at 664 nm; and 2.210, 2.185, and 2.150 min respectively at 431 nm. Sample fractions were ran at a flow rate of 0.6 ml/min with a solvent system of 90 % water and 10 % methanol for 40 min.
Table1.
Retention time (RT) and heights of two characteristic peaks in HPLC finger-prints of four fraction extracts of a batch of VA. All fraction peaks expressed at a relatively similar retention time, while higher peak height was expressed in fractions 4 and B-3. Fingerprint experiment was conducted in duplicates and mean and standard deviations for peak height and retention time computed.
|
V.amygdalina fraction No. |
Peak 1 RT (min) |
Peak 2 RT (min) |
Mean RT (min) |
S.D. RT (min) |
Peak 1 height (mAU) |
Peak 2 height (mAU) |
Mean height (mAU) |
S.D height (mAU) |
|---|---|---|---|---|---|---|---|---|
| 4 | 2.206 | 2.208 | 2.207 | 0.001 | 5.50 | 14.0 | 9.75 | 6.010 |
| A-2 | 2.210 | 2.213 | 2.2115 | 0.002 | 1.2 | 3.5 | 2.35 | 1.626 |
| B-2 | 2.185 | 2.187 | 2.186 | 0.001 | 6.9 | 25.0 | 15.95 | 12.799 |
| B-3 | 2.150 | 2.152 | 2.151 | 0.001 | 2.25 | 6.7 | 4.475 | 3.147 |
Fig. 6.
HPLC profiles of solutions of in-laboratory and commercial VA extracts obtained by direct extraction with 85 % ethanol. Hypersil ODS C18 column (4.6 mm × 250 mm, 5µm, Hypersil) at room temperature. Mobile phase: 10 % methanol for 40 min in 90 % water, flow rate 0.6 ml/min. Detection was set at 431 nm and 664 nm.
DISCUSSION
Conventional medicine (CM) is very popular in most developed countries. It is equally important to note that traditional medicine (TM) serves as a major source of healthcare for billions of people in the developing countries. Antagonistic relationships exist between CM and TM practitioners. The contention is that conventional drugs are standardized and chemically-defined; the quantities and structures of the active ingredients are known. Therefore, therapeutic dosages may be determined. In contrast, traditional medicines are often native or non-purified botanical extracts with limited knowledge of their chemical compositions. There is a strong, growing evidence to support the medicinal benefits of plant products and extracts. This evidence is predicated on first, the observation and/or reports that inverse relationships exist between consumption of vegetables and risks of developing many types of cancer (26–33). Thus, suggesting the presence of cancer-fighting phytochemicals in plants. Secondly, many highly effective anti-cancer drugs (Paclitaxel or Taxol, Vincrastine, Vinblastine, Camptothecin, Etoposides) representing four classes of anti-cancer drugs (taxanes, camptothecins, vinca, and epipodophyllotoxins) trace their origins directly or indirectly to plants (34). Therefore, there is a consensus amongst scientists that plant products/extracts do possess active compounds of medicinal benefits.
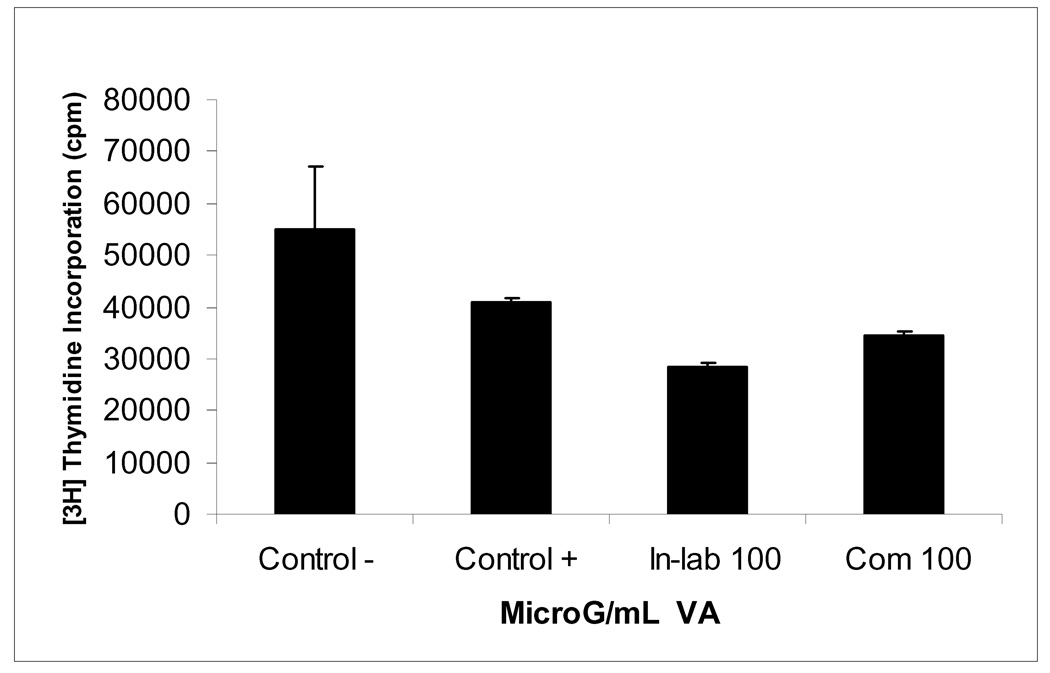
Despite this general acceptance, an interphase to bring AM and TM into harmony is needed. The following may promote such harmony between TM by AM practitioners: native extracts must be fractionated to identify the active components, and elucidation of structures and mechanisms of actions. However, in cases where fractionation compromises the activities or health benefits of the native extracts, then the extracts should be standardized (active components). Here, we have provided the initial steps toward the formulation and standardization of a promising medicinal botanical, VA extracts. The literature shows that VA has been used to treat a myriad of biological disorders and diseases including cancer (4–18). The present studies corroborate the anti-cancer activities of VA. Aqueous and organic extraction and chemical profiling of VA by UV and HPLC spectroscopic techniques have effectively aided in the separation, evaluation and provided insights on the chemical compositions of VA. Selected biologically-active fractions (A2, B2-3) shared common peaks at 431 nm and 664 nm with a retention time of approximately 2 min. Thus, suggesting that the compound(s) present in these fractions and represented in these peaks are required for VA anti-cancer activities. Therefore, the presence and sizes of these peaks may be used for quality assurance purposes. We hypothesized that positive relationships exist between heights of these peaks (visa vice quantities of the compounds contained) and DNA synthesis inhibitory activities of the fractions. The hypothesis was validated (Fig. 7).Taken together, these results show reliability in the utilization of spectroscopic and chromatographic techniques to monitor or predict the quality of medicinal botanical products. Efforts to complete the isolation and elucidation of the active compounds are underway in our laboratories.
Fig. 7.
Inhibition of DNA synthesis in MCF-7 cells by extracts of In-laboratory and Commercial VA. Cells at the logarithmic growth phase were treated with either 100 µg/ml for 18 h before the addition of 1µCi/ml [3H]thymidine for 6 h. Each data point represents the mean of three independent experiments done in duplicates (N=6). Exposure of cells to 100 µg/ml 85 % ethanolic extractions of in-laboratory and commercial VA inhibited DNA synthesis 51 % and 31 % respectively. DNA synthesis was determined as described under materials and methods section.
ACKNOWLEDGEMENTS
Our appreciation also goes to Dr. Joseph L. Bryant (University of Maryland School of Medicine, Baltimore, MD.) for his technical assistance.
This research was supported in part by the Research Centers in Minority Institutions (RCMI)/NIH grant # G122RR13459-07S1; National Center for Minority Health Disparities (NCMHD)/NIH grant # P20MD000534-01
REFERENCES
- 1.Oleszek W, Igile G, Burda S, Jurzysta M. Nutritional assessment of vernonia amygdalina leaves in growing mice. J. Agric. Food Chem. 1995;43:2162–2166. [Google Scholar]
- 2.Farombi EO. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. African journal of biotechnology. 2003;2(12):662–671. [Google Scholar]
- 3.Afolayan AJ, Grierson DS, Eraso P. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacology. 2006;106:117–120. doi: 10.1016/j.jep.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Moundipa FP, Kamini G, Melanie F, Bilong FC, Bruchhaus I. In vitro amoebicidal activity of some medicinal plants of the Bamun region (Cameroon) African J Trad CAM. 2006;2(2):113–121. [Google Scholar]
- 5.Akah PA, Ekekwe RK. Ethnopharmacology of some of the asteraceae family used in the Nigerian traditional medicine. Fitoterapia. 1995;66:352–355. [Google Scholar]
- 6.Akah PA, Okafor CL. Phytother Res. 1992;6:171. [Google Scholar]
- 7.Huffman MA, Page JE, Sukhdeo MVK, Gotoh S, Kalunde MS, Towers GHN. Leaf swallowing by chimpanzees: a behavioral adaptation for the control of strong nematode infections. International J of Primatology. 1996;72:475–503. [Google Scholar]
- 8.Hamill FA, Apio S, Murbiru NK, Masango M, Bukenya-Ziraba R, Maganyi OW, Soejarto DD. Traditional herbal drug of southern Uganda. J Ethnopharmacology. 2000;70:281–300. doi: 10.1016/s0378-8741(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 9.Olajide OA, Makinde JM, Awe SO. Cathartic effect of the leaf extract of Vernonia amygdalina. Fitoterapia. 1999;70:161–165. [Google Scholar]
- 10.Akinpelu DA. Antimicrobial activity of Vernonia amygdalina leaves. Fitoterapia. 1999;70:432–234. [Google Scholar]
- 11.Hladik C, Krief S, Haxaire C. Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. Journal of Ethnopharmacology. 2005;101:1–5. doi: 10.1016/j.jep.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Iwalokun BA, Efedede BU, Alabi-sofunde JA, Oduala T, Magbagbeola OA, Akinwande AI. Hepatoprotective and antioxidant activities of vernonia amygdalina on acetaminophen-induced hepatic damage on mice. 2006;9(4):524–530. doi: 10.1089/jmf.2006.9.524. [DOI] [PubMed] [Google Scholar]
- 13.Kupchan SM, Hemingway RJ, Karim A, Werner D. Tumor inhibitors.XLVII. Vernodaline and Vernomygdine,two new cytotoxic sesquiterpene lactones from Vernonia amygdalina. Del. Journal of Organic chemistry. 1969;34:3908–3911. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 14.Jasaka M, Ohigashi H, Takagaki T, Nozaki H, Tada T, Hiroto M, Hirie R, Huffman MA, Nishida T, Kaji KK. Bitter steroid glucosides, Vernoniosides A1, A2, A3 and related B1 from possible medicinal plant-Vernonia amygdalina used by wild chimpanzees. Tetrahedron. 1992;48(4):625–632. [Google Scholar]
- 15.Izevbigie EB. Discovery of water soluble anticancer agents (Edotides) from vegetables found in Benin City, Nigeria. Exp.Biol.Med. 2003;228:293–298. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- 16.Howard CB, Stevens J, Izevbigie EB, Walker A, McDaniel O. Time and dose-dependent modulation of phase 1 and phase 2 gene expression in response to treatment of MCF-7 cells with a natural anti-cancer agent. Cell. Mol. Biol. 2003;49(7):1057–1065. [PubMed] [Google Scholar]
- 17.Izevbigie EB, Bryant JL, Walker A. A novel natural inhibitor of extracellular signal-regulated kinases and human breast cancer cell growth. Exp.Bio.Med. 2004;229:163–169. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- 18.Opata M, Izevbigie EB. Aqueous V. amygdalina Extracts Alter MCF-7 Cell Membrane Permeability and Efflux. Int. J. Environ. Res. Public Health. 2006;3(2):174–179. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayed MD, Zaki AY, El-Marzabai MM, Doss SL. Phytochemistry. 1982;21:944. [Google Scholar]
- 20.Cimanga RK, Tona L, Mesia K, Musuamba CT, De Bruyne T, Apers S, Hernans N, Miert VS, Pieters L, Totte J, Vlietink AJ. In Vitro Antiplasmodial activity of extracts and fractions of seven medicinal plants used in the Democratic Republic of Congo. Journal of Ethnophamacology. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973. p. 278. [Google Scholar]
- 22.Monroe EW, Mansukh CW, Govindranjan M, Philip A, Harold T, Thomas JH, Warner J, MacGivney R. Plant antimutagenic agents, flavonoids. Journal of natural products. 1988;51:1084–1091. doi: 10.1021/np50060a006. [DOI] [PubMed] [Google Scholar]
- 23.Lin YL, Juan IM, Chen YL, Liang YC, Lin JK. Composition of polyphenols in fresh tea leaves and associations with their oxygen-radical absorbing capacity with antiproliferative actions in fibroblast cells. J. Agric. Food.Chem. 1996;44:1387–1394. [Google Scholar]
- 24.Wanzel U, Kunz S, Brendel MD, Daniel H. Diatery flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000;60:3823–3831. [PubMed] [Google Scholar]
- 25.SAS Institute. SAS® Institute User’s Reference Guide: Statistics. edition. Cary, North Carolina: SAS Institute Inc.; 1990. [Google Scholar]
- 26.Singletary K. Natural products and cancer chemoprevention. J. Nutr. 2000;130:465–466. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- 27.Jahansson S, Gullbo J, Lindholm P, Ek B, Thunberg E, Samuelsson G, Larsson R, Bohlin L, Claeson P. Small, novel proteins from the mistletoe phoradendron tomentosum exhibit highly selective cytotoxicity to human breast cancer cells. Cell Mol. Life Sci. 2003;60:165–175. doi: 10.1007/s000180300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karkabounas S, Assimakopoulos D, Malamas M, Skaltsounis AL, Leonce S, Zelovitis J, Stefanou D, Evangelou A. Antiproliferative and anticarcinogenic effects of an aqueous preparation of Abies alba and Viscum album se abies on a L-1210 malignant cell line and tumor-bearing Wistar rats. Anticancer Res. 2000;20(6B):4391–4395. [PubMed] [Google Scholar]
- 29.Florack DE, Stickema WJ. Thionins: properties, possible biological roles and mechanisms of action. Plant Mol. Biol. 1994;26:25–37. doi: 10.1007/BF00039517. [DOI] [PubMed] [Google Scholar]
- 30.Froy O, Gurevitz M. Membrane potential modulators: a thread of scarlet from plants to animals. FASEB J. 1998;12:1793–1796. doi: 10.1096/fasebj.12.15.1793. [DOI] [PubMed] [Google Scholar]
- 31.Rajabalian S. Methanolic extract of Teucrium polium L. potentiates the cytotoxic and apoptotic effects of anticancer drugs of vincristine, vinblastine and doxorubicin against a panel of cancerous cell lines. Exp Oncol. 2008;30(2):133–138. [PubMed] [Google Scholar]
- 32.Hou W, Chen L, Yang G, Zhou H, Jiang Q, Zhong Z, Hu J, Wang X, Yuan Y, Tang M, Wen J, Wei Y. Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytother Res. 2008;22(8):1125–1132. doi: 10.1002/ptr.2472. [DOI] [PubMed] [Google Scholar]
- 33.Mazzio EA, Soliman KF. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother Res. 2008 doi: 10.1002/ptr.2636. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53(2):121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]