Abstract
The distribution of type II and VI collagen was immunocytochemically investigated in bovine articular and nasal cartilage. Cartilage explants were used either fresh or cultured for up to 4 weeks with or without interleukin 1α (IL-1α). Sections of the explants were incubated with antibodies for both types of collagen. Microscopic analyses revealed that type II collagen was preferentially localized in the interchondron matrix whereas type VI collagen was primarily found in the direct vicinity of the chondrocytes. Treatment of the sections with hyaluronidase greatly enhanced the signal for both types of collagen. Also in sections of explants cultured with IL-1α a higher level of labeling of the collagens was found. This was apparent without any pre-treatment with hyaluronidase. Under the influence of IL-1α the area positive for type VI collagen that surrounded the chondrocytes broadened. Although the two collagens in both types of cartilage were distributed similarly, a remarkable difference was the higher degree of staining of type VI collagen in articular cartilage. Concomitantly we noted that digestion of this type of cartilage hardly occurred in the presence of IL-1α whereas nasal cartilage was almost completely degraded within 18 days of culture. Since type VI collagen is known to be relatively resistant to proteolysis we speculate that the higher level of type VI collagen in articular cartilage is important in protecting cartilage from digestion.
Keywords: Nasal cartilage, Articular cartilage, Collagen type II, Collagen type VI, Immunolocalization
Introduction
The extracellular matrix of hyaline cartilage contains a wide variety of non-collagenous proteins and several types of collagen. Of these collagens type II is the main component and constitutes about 80–95% of the total amount of collagen. In addition small amounts of type VI (1–2%), type IX (1%) and type XI (3%) are present (Chang et al. 1997; Poole et al. 1988; Smith et al. 1989). Type II collagen is essential for the structural integrity of the tissue, whereas the other collagens are likely to play a role in cellular attachment, fibril formation and/or the three dimensional organization of cartilage (Billinghurst et al. 1997). Of these collagens, type VI collagen is of considerable interest since it is assumed to function as an anchoring meshwork that connects cells to their surrounding environment (Everts et al. 1998; Kuo et al. 1997).
Type VI collagen is composed of three genetically different α chains that form triple helices with globular terminal domains. Repeats that have homology with the collagen-binding domain of von Willebrand factor are present in the globular domains and the triple helical part of the molecule contains at least 11 RGD sequences (Kuo et al. 1995; Lamande et al. 1998; Marcelino and McDevitt 1995). The multi-domain structure of type VI collagen not only makes it suitable for an interaction with components of the extracellular environment like collagen type II, decorin, fibromodulin, hyaluronan, and fibronectin, but also interacts with the cell surface by its integrin-binding properties (Loeser 1997, 2000). In line with its possible interaction with cells relatively high levels of type VI collagen have been observed in the pericellular environment of the chondrocyte (Chang and Poole 1996; Chang et al. 1997; Hagiwara et al. 1993; Hambach et al. 1998; Poole et al. 1992).
Although numerous studies have shown metabolism of collagen type II, hardly anything is known about synthesis and breakdown of type VI collagen in cartilage (Aigner et al. 1997; Billinghurst et al. 1997; Dodge and Poole 1989; Freemont et al. 1999; Kozaci et al. 1997; Nelson et al. 1998; Pullig et al. 1999; Stoop et al. 1999). It has been well established that under pathological conditions, such as osteoarthritis or rheumatoid arthritis the major part of the cartilage is broken down. Proteolytic enzymes, in particular matrix metalloproteinases and ADAMs (a disintegrin and metalloproteinase), mediate this degradation (Bohm et al. 1999; Flannery et al. 1999; McKie et al. 1997). Yet, several data indicate that type VI collagen is rather resistant to proteolytic attack (Kielty et al. 1993; Okada et al. 1992). In this respect it is of interest that in osteoarthritic cartilage higher levels of type VI collagen with a broader pericellular distribution have been noted (Arican et al. 1996; McDevitt et al. 1988; Poole et al. 1992; Ronziere et al. 1990). This increased amount has been suggested to be due to an elevated synthesis of type VI collagen (Hambach et al. 1998). An alternative possibility, however, is that type VI collagen was already present in these areas but masked by other matrix components. A (partial) breakdown of the latter constituents may result in accessibility of the antigenic sites of type VI collagen, thus making immunodetection possible (Dreiling et al. 2002a, b).
In the present study we investigated this by analyzing sections of cartilage that were either incubated with hyaluronidase or obtained from explants cultured with interleukin 1α (IL-1α), a cytokine known to induce digestion of cartilage (Xu et al. 1996). We compared two types of hyaline cartilage, nasal and articular, since it has been established that cartilage type-dependent differences exist in the IL-1α-induced degradation. In articular cartilage breakdown of type II collagen hardly occurs in the presence of IL-1α whereas this type of collagen is readily digested in nasal cartilage (Ellis et al. 1994; Kozaci et al. 1997; Xu et al. 1996).
Results
Type II collagen
Articular cartilage
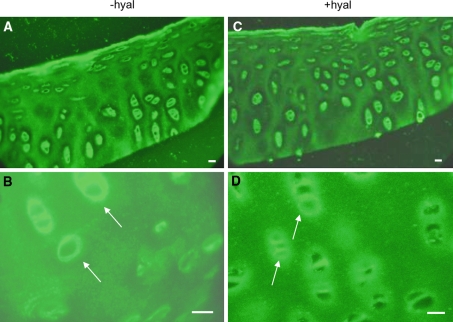
A low level of positively stained type II collagen was found in sections of non-cultured articular cartilage localized in the direct vicinity of the cells. In the interterritorial matrix between the chondrons it was present in a punctate pattern (Fig. 1a). Treatment of the sections with hyaluronidase resulted in a more intense staining of type II collagen around the chondron (Fig. 1b).
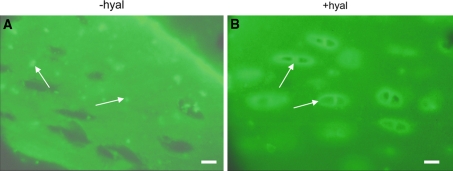
Fig. 1.
Localization of collagen type II in bovine articular cartilage. Cartilage sections were obtained form non-cultured explants and incubated with an antibody against type II collagen which was followed by a FITC-labeled second antibody. The sections were treated or not with hyaluronidase for 55 h. a without Hyaluronidase treatment (−hyal). Type II collagen label is present in a dot-like appearance (arrows). b with hyaluronidase treatment (+hyal). After hyaluronidase treatment the dot-like pattern is lost and the labeling is more uniformly distributed with a more intense staining around the chondron (arrows). In control sections incubated with buffer for 55 h the dot-like distribution was similar to that seen in untreated sections. Bars: 0.2 μm
In explants cultured in control medium a similar dot-like labeling was seen (Fig. 2a). Treatment with hyaluronidase resulted in a more intense staining around the chondrons (Fig. 2b). Culturing in the presence of IL-1α, however, resulted in uniform labeling and around the chondron (perichondral), a more intense staining was apparent. Treatment with hyaluronidase had no additional effect on the localization of type II collagen (Fig. 3a, b). The change in labeling pattern was already apparent after 1 week of culturing with IL-1α.
Fig. 2.
Localization of collagen type II in sections obtained from articular cartilage explants cultured for 1 week without IL-1α. Before labeling some sections were treated with hyaluronidase for 55 h. a without hyaluronidase treatment (−hyal). b with hyaluronidase. (+hyal) Treatment with hyaluronidase resulted in a more intense staining around the chondrons. Bars: 0.2 mm
Fig. 3.
Localization of collagen type II in sections from articular explants cultured for 1 week with IL-1α. Before labeling some sections were treated with hyaluronidase for 55 h. a without hyaluronidase treatment (−hyal) b with hyaluronidase treatment (+hyal). Culturing in the presence of IL-1α resulted in a change of the localization of type II collagen: the dot-like labeling became more uniform and around the chondron (perichondral) a more intense staining was apparent. Treatment with hyaluronidase had no additional effect on the localization of type II collagen. Bars: 0.2 mm
Nasal cartilage
In non-cultured nasal cartilage a low level of labeled type II collagen was found in the direct vicinity of the chondrocytes and in the interchondral area. Comparable with the articular cartilage explants, also in nasal cartilage the label had a dot-like appearance (not shown).
The over-all localization in sections of explants cultured in control medium was similar to that found in sections of non-cultured explants. Culturing with IL-1α changed the labeling pattern considerably. As with articular cartilage the dot-like labeling changed in a more uniform distribution already after a culture period of 1 week (Fig. 4a, b). Time intervals later than 2 weeks could not be studied since after 18 days of culturing in the presence of IL-1α the nasal cartilage explants were digested completely.
Fig. 4.
Localization of type II collagen in nasal cartilage cultured for 1 week with IL-1α. In a without hyaluronidase treatment (−hyal). b with hyaluronidase treatment (+hyal). Note the uniform labeling of type II collagen in nasal explants following culturing in the presence of IL-1α. Hyaluronidase proved to have no additional effect on type II collagen stainability. Bars: 0.2 mm
Comparable to the labeling found in articular cartilage, hyaluronidase pre-treatment resulted in a uniform distribution of type II collagen labeling. Treatment with hyaluronidase of sections of explants cultured with IL1α did not augment the staining intensity (Fig. 4b). Labeling of control sections pre-treated with buffer did not differ from untreated sections.
A striking difference between the two types of cartilage was the lack of any notable digestion of articular cartilage in the presence of IL-1α. Even after 4 weeks of culturing in the presence of the cytokine, the articular explants appeared to be intact and type II collagen could be detected easily. Under all conditions a more intense labeling was found in articular cartilage compared to nasal cartilage explants.
Type VI collagen
Articular cartilage
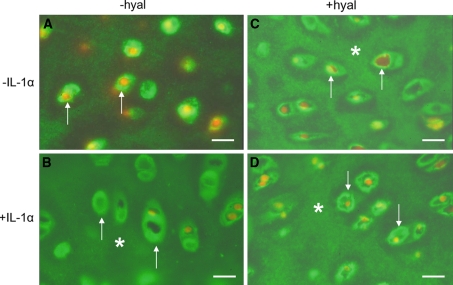
In articular cartilage sections obtained from non-cultured explants, type VI collagen was found as a distinct broad band surrounding the chondrocytes (Fig. 5a–d). A relatively weak labeling was present between the cells of a chondron and some labeling was found interchondrally.
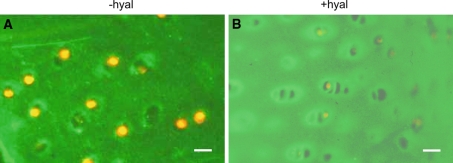
Fig. 5.
Localization of collagen type VI in non-cultured articular cartilage explants. Before labeling the sections were a, b without hyaluronidase treatment (−hyal) c, d with hyaluronidase treatment (+hyal). a, b A high labeling intensity is apparent in the direct vicinity of the chondrocytes (arrows) and some labeling is seen interchondrally. c, d Treatment with hyaluronidase resulted in a narrower but more intense staining area in between the chondrons (arrows). Bars: 0.2 mm
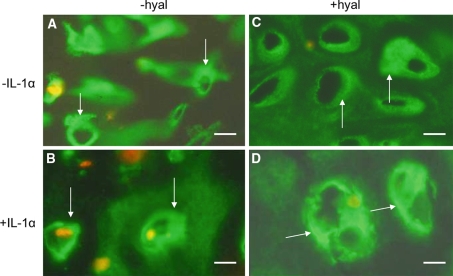
In sections of cultured control explants the site and amount of type VI collagen labeling was similar to non-cultured cartilage (Fig. 6a). A 1 week incubation with IL-1α had no effect on the distribution of type VI collagen (Fig. 6b) but after 4 weeks of culturing with IL-1α a higher level of label was found within and between the chondrons (Fig. 6d). The broad area of label surrounding the chondron changed into a thinner more intensely stained area. Also the interchondral labeling appeared to be more intense. Treatment of the sections of cultured control explants with hyaluronidase (Fig 6c, d) mimicked the results obtained following culturing with IL-1α (Fig. 6d). Also in the hyaluronidase treated sections the broad positive area surrounding the chondrons appeared to be as a more intense but narrower positively staining band.
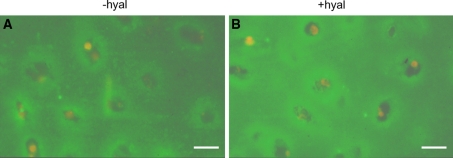
Fig. 6.
Localization of type VI collagen in articular cartilage cultured for 1 or 4 weeks a, b without hyaluronidase treatment (−hyal) c, d with hyaluronidase treatment (+hyal) a, c cultured without IL-1α (−IL-1α) b, d cultured with IL-1α (+IL-1α) Culturing in the presence of IL-1α resulted in a more intense staining. This was found both for the area in the direct vicinity of the chondrocytes (arrows) as well as for the interchondral areas (asterisks). Hyaluronidase treatment (c, d) also resulted in a more intense staining. In the direct vicinity of the chondron a narrow band of intense staining for type VI collagen is present. At some distance an unstained area is followed by an intense staining interchondral area. This staining pattern is found both after hyaluronidase treatment and after culture with IL-1α. Bars: 0.2 mm
Nasal cartilage
The distribution pattern of type VI collagen in sections of nasal cartilage (Fig. 7a–d) was comparable to the one found in articular cartilage. This was the case for sections of non-cultured explants as well as for explants cultured without IL-1α (Fig. 7a, c) or with the cytokine (Fig. 7b, d) and also for hyaluronidase-treated sections (Fig. 7c, d). However, the intensity of staining proved to differ considerably. In all sections of articular cartilage a more pronounced labeling of type VI collagen was apparent (compare Fig. 7 with Fig. 6).
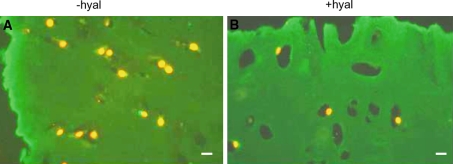
Fig. 7.
Localization of collagen type VI in sections from nasal cartilage explants cultured for 2 weeks with or without IL-1α. The sections were treated with or without hyaluronidase. a, b without hyaluronidase treatment (−hyal) c, d with hyaluronidase treatment (+hyal) a, c 2 week culture period without IL-1α (−IL-1α) b, d 2 week culture period with IL-1α (+IL-1α) Type VI collagen staining is present in a broad area around the chondrocytes (arrows). Treatment with hyaluronidase (c) resulted in a smaller but more intense staining area in between the chondrons (arrows). After a 2 week culture period with IL-1α (b, d), the distribution of type VI collagen is similar to that found in c following hyaluronidase treatment. Also here an intensively stained narrow area around the chondron (arrows) is next to a narrow unstained area. Interchondrally the staining becomes more intense after IL-1α incubation or hyaluronidase treatment. After 2 weeks culturing with IL-1α the explant is nearly completely digested. Bars: 0.2 μm
Zymography
Articular cartilage
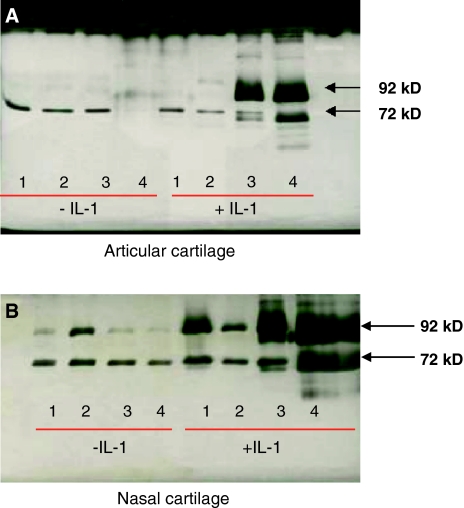
Hardly any gelatinolytic activity was detectable in media obtained from articular cartilage explants that were cultured for up to 4 weeks without IL-1α (Fig. 8a, lanes 1–4). Also in cultures with IL-1α during the first 2 weeks gelatinolytic activity was not increased (Fig. 8a, lanes 1, 2 with IL-1α). At the 3 and 4 week time points, however, activity of MMP-2 and -9 became apparent (Fig. 8a, lanes 3, 4 with IL-1α). In particular the level of pro-MMP-9 was increased.
Fig. 8.
Zymography of conditioned medium of: a Articular cartilage cultured for up to 4 weeks with or without IL-1α. b Nasal cartilage cultured for up to 4 weeks with or without IL-1α. But after 18 days of culture with IL-1α the nasal cartilage explant is nearly completely digested. The numbers 1–4 refer to the weeks of culturing. Hardly any gelatinolytic activity was detectable in media obtained from articular cartilage explants cultured without IL-1α or cultured for 1 or 2 weeks with IL-1α (panel A, lanes 1, 2, 3, 4 without IL-1α and lanes 1, 2 with IL-1α, respectively). Longer culture periods with IL-1α resulted in a detectable release of the pro- and active form of MMP-2 (72 and 68 kD, respectively) and MMP-9 activity in the media (panel A, lanes 3, 4 +IL-1α). The overloaded bands likely express MMP-9 in its pro- and mature form (92 and 88 kD, respectively). The same bands are present in the nasal cultures (panel B), but they are much more pronounced in the medium obtained from this type of cartilage after 3 and 4 weeks of culture with IL-1α
Nasal cartilage
In contrast to articular cartilage, zymographic analysis of conditioned media obtained from nasal cartilage cultured in the absence of IL-1α revealed that at all culture time intervals studied MMP-2 and -9 were detectable (Fig. 8b, lanes 1–4). Only the proform of these enzymes proved to be present.
In the presence of IL-1α (Fig. 8b, lanes 1–4 with IL-1α), not only the proform but also the active form of the enzymes could be detected. This was particularly apparent after 3 and 4 weeks of culture. At all time intervals more gelatinolytic activity was present in the media of nasal cartilage than in that of articular cartilage.
Discussion
In line with findings presented previously by others (Bruckner and van der Rest 1994; Cremer et al. 1998; Eyre 2002) hyaline cartilage contains both type II and VI collagen. We now show that this is the case for both types of hyaline cartilage studied, nasal and articular cartilage. Yet, differences in labeling intensity were noted among the two types. Articular cartilage stained much more intense with the two collagen antibodies than nasal cartilage did. Also following incubation with hyaluronidase or culturing with IL-1α, a more intense staining was apparent in articular cartilage compared to nasal cartilage. This finding strongly suggest that articular cartilage contains more of collagens type II and VI than nasal cartilage. The difference in composition of the two types of cartilage could perhaps be related to functional differences between the two cartilage types. The main function of nasal cartilage is to provide support whereas articular cartilage is essential in withstanding forces and wear in joints. It is not unlikely that such extreme functional demands of articular cartilage are reflected in higher levels of collagens that provide the necessary tensile strength to the tissue.
The highest level of type II collagen in cartilage was found interchondrally. Incubation of the sections with hyaluronidase resulted in enlargement of the positively stained areas. This finding indicates that under normal conditions a relatively large fraction of type II collagen is not accessible for the antibody. A comparable result was reached by culturing the cartilage explants with IL-1α. Under the influence of this cytokine proteoglycans are digested (Flannery et al. 1999; Xu et al. 1996) thus resulting in accessible antibody recognition sites. In this respect our data are in line with studies performed by others (Arican et al. 1996; Bathon et al. 1994) and demonstrate that large fractions of the collagen present in cartilage are masked by non-collagenous components.
A notable finding with respect to the localization of type VI collagen was its shift from a broad pericellular to a more intensely staining narrow band following culturing with IL-1α. Although this may suggest that under the influence of IL-1α a higher level of type VI collagen is produced, Bathon and coworkers (1994) demonstrated that this cytokine suppresses the expression of type VI collagen by chondrocytes. Therefore, a more plausible explanation is that due to IL-1α-induced loss of extracellular matrix constituents, a rearrangement of type VI collagen occurred. Data presented by Arican et al. (1996) support this view since they demonstrated a similar redistribution in osteoarthritic cartilage.
The highest levels of type II collagen were found at some distance from the chondrocytes whereas in the direct vicinity of the cells type VI collagen prevailed (Poole et al. 1988, 1992). The presence of the latter collagen in the direct vicinity of the cells has prompted the suggestion that a direct interaction exists between chondrocytes and type VI collagen (Marcelino and McDevitt 1995; Sherwin et al. 1999; Soder et al. 2002). Since it has been well established that collagens greatly affect the activity of cells, among which the expression and activity of enzymes like MMPs (Flannery et al. 1999; Kerkvliet et al. 2003; Ohta et al. 1998; Okada et al. 1992), a close association of the chondrocytes with this type of collagen may be important in the modulation of chondrocyte activity.
The presently described difference in the level of type VI collagen in close vicinity to chondrocytes of articular cartilage (high level) and nasal cartilage (low level) may provide an explanation for the difference in digestion of these two types of cartilage. In the present and in previous studies (Dodge and Poole 1989; Kozaci et al. 1997; Xu et al. 1996) it was shown that under the influence of IL-1α a complete digestion of nasal cartilage occurred within 2 weeks. Articular cartilage, however, proved highly resistant to IL-1α-induced digestion. Even after 4 weeks of culturing hardly any sign of degradation was noted. The difference in digestion was also expressed in the level of MMP-2 and -9 as assessed by zymography. A possible explanation for the observed resistance to digestion could be the relatively high level of type VI collagen in articular cartilage. Type VI collagen has been shown to resist digestion by many proteolytic enzymes (Kielty et al. 1993; Xu et al. 1996). A protective role of type VI collagen has been suggested previously by Horikawa et al. (2004). These authors found an accumulation of type VI collagen in the pericellular environment of chondrocytes after addition of TGF-β. Since this growth factor plays a beneficial role in preventing progression of osteoarthritis, they suggested that type VI collagen played a protective role. We now propose that the digestion of different types of hyaline cartilage (partially) depends on the amount of type VI collagen present; the higher the amount the more resistant to degradation.
Experimental procedures
Culture of cartilage explants
Cartilages from bovine nasal septa and metacarpophalangeal joints were cut into fragments of an average weight of 30 mg, washed with phosphate buffered saline (PBS) and cultured in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Paisley, UK), containing glutamine (2 mM), penicillin G (2,000 units/ml), streptomycin (0.1 mg/ml) and Hepes (10 mM) (all from Invitrogen). The explants were cultured for up to 4 weeks in 48 well tissue culture plates (Costar, High Wycombe, UK) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Each well contained two cartilage explants in 400 μl of medium.
Series of explants were incubated for various time intervals with recombinant IL-1α at a concentration of 50 ng/ml (Kozaci et al. 1997). Conditioned media were frozen and stored at −20°C for zymographic analysis. Cartilage explants were embedded in Tissuetek and frozen at −80°C until used for cryosectioning. Control cartilage explants were either not cultured and embedded immediately or cultured in DMEM only.
Immunolocalization of type II and type VI collagen
Cryosections of 8 μm were cut on a motor driven Bright cryotome at a cabinet temperature of −25°C. Sections were collected on Vectabond® coated slides (Vector Laboratories Inc., Burlingame, CA). Sections were either directly incubated for immunolocalization or first treated with hyaluronidase (from bovine testis, Sigma Chemical Co. St.Louis, MO), in a concentration of 5 mg/ml in 0.1 M sodium acetic acid and 0.15 M NaCl buffer (pH 5.2) for 55 h at 4°C. Control sections were incubated with buffer only. To prevent drying of the sections, a Teflon ring was placed around the sections. The ring was fixed with vacuum grease and covered by a coverslip. The sections were incubated in a humidified environment. In preliminary experiments sections were treated with hyaluronidase for different time intervals and analyzed for the presence of proteoglycans by Safranin O staining. A complete loss of staining was found after 55 h of hyaluronidase treatment, indicating that most if not all proteoglycans were lost by this enzyme incubation.
The cryosections were washed with PBS and fixed for 10 min with 4% formaldehyde in 0.15 M phosphate buffer. Free aldehyde groups were blocked with phosphate-buffered glycine (50 mM). After a 10 min wash in PBS containing 1% bovine serum albumin (BSA) the sections were incubated for 2 h with goat anti-human type II or type VI collagen antibody (Southern Biotechnology Associates Inc., Birmingham, AL) in a final concentration of 8 or 52 μg/ml in PBS, respectively. Control sections were incubated with similar concentrations of non-immune goat IgG (Southern Biotechnology Associates Inc.). The sections were washed with PBS/BSA for 10 min and incubated for 1 h with FITC-conjugated rabbit anti-goat IgG (Southern Biotechnology Associates Inc.) in a concentration of 7 μg/ml. Nuclei were counterstained with propidium iodide. Sections were washed with PBS/BSA and mounted in Vectashield (Vector laboratories Inc. Burlingame, CA) and fluorescent staining was viewed with a Leica-DMRA microscope. Micrographs were made using Kodak Ektachrome 400 ASA film. The negatives were scanned to digitalize the pictures.
Zymography
To assess the level of gelatinolytic activity in conditioned media of cultured explants, gelatin zymography was performed. Five micro litre of conditioned medium was 1:1 diluted with sample buffer (0.1 M Tris–HCL, 4% SDS, 20% glycerol, 0.005% bromophenol blue, 10 mM EDTA) and electrophoresed through a 10% polyacrylamide gel containing 2% gelatin, as described by (Heussen and Dowdle 1980). After electrophoresis, gels were washed in a 50 mM Tris–HCl buffer (pH 7.5) containing 2.5% Triton X-100 and 5 mM CaCl2 and subsequently incubated at 37°C, in Tris–HCL buffer (pH 7.5) supplemented with 5 mM CaCl2, 1% Triton X-100 and 0.02% NaN3.
Following 18 h incubation, gels were stained with 0.1% Coomassie Brilliant Blue and cleared with 7% acetic acid with 4% methanol to visualize unstained proteolytic bands.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aigner T, Gluckert K, von der Mark K. Activation of fibrillar collagen synthesis and phenotypic modulation of chondrocytes in early human osteoarthritic cartilage lesions. Osteoarthr Cartil. 1997;5:183–189. doi: 10.1016/S1063-4584(97)80013-1. [DOI] [PubMed] [Google Scholar]
- Arican M, Carter SD, Bennett D, Ross G, Ayad S. Increased metabolism of collagen VI in canine osteoarthritis. J Comp Pathol. 1996;114:249–256. doi: 10.1016/S0021-9975(96)80046-6. [DOI] [PubMed] [Google Scholar]
- Bathon JM, Hwang JJ, Shin LH, Precht PA, Towns MC, Horton WE. Type VI collagen-specific messenger RNA is expressed constitutively by cultured human synovial fibroblasts and is suppressed by interleukin-1. Arthritis Rheum. 1994;37:1350–1356. doi: 10.1002/art.1780370913. [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm BB, Aigner T, Gehrsitz A, Blobel CP, Kalden JR, Burkhardt H. Up-regulation of MDC15 (metargidin) messenger RNA in human osteoarthritic cartilage. Arthritis Rheum. 1999;42:1946–1950. doi: 10.1002/1529-0131(199909)42:9<1946::AID-ANR21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bruckner P, van der Rest M. Structure and function of cartilage collagens. Microsc Res Tech. 1994;28:378–384. doi: 10.1002/jemt.1070280504. [DOI] [PubMed] [Google Scholar]
- Chang J, Poole CA. Sequestration of type VI collagen in the pericellular microenvironment of adult chrondrocytes cultured in agarose. Osteoarthr Cartil. 1996;4:275–285. doi: 10.1016/S1063-4584(05)80105-0. [DOI] [PubMed] [Google Scholar]
- Chang J, Nakajima H, Poole CA. Structural colocalisation of type VI collagen and fibronectin in agarose cultured chondrocytes and isolated chondrons extracted from adult canine tibial cartilage. J Anat. 1997;190(Pt 4):523–532. doi: 10.1046/j.1469-7580.1997.19040523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer MA, Rosloniec EF, Kang AH. The cartilage collagens: a review of their structure, organization, and role in the pathogenesis of experimental arthritis in animals and in human rheumatic disease. J Mol Med. 1998;76:275–288. doi: 10.1007/s001090050217. [DOI] [PubMed] [Google Scholar]
- Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989;83:647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiling FJ, Henson MM, Henson OW. Immunolabeling type II collagen in the basilar membrane, a pre-embedding approach. Hear Res. 2002;166:181–191. doi: 10.1016/S0378-5955(02)00313-1. [DOI] [PubMed] [Google Scholar]
- Dreiling FJ, Henson MM, Henson OW. The presence and arrangement of type II collagen in the basilar membrane. Hear Res. 2002;166:166–180. doi: 10.1016/S0378-5955(02)00314-3. [DOI] [PubMed] [Google Scholar]
- Ellis AJ, Curry VA, Powell EK, Cawston TE. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994;201:94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- Everts V, Niehof A, Jansen D, Beertsen W. Type VI collagen is associated with microfibrils and oxytalan fibers in the extracellular matrix of periodontium, mesenterium and periosteum. J Periodontal Res. 1998;33:118–125. doi: 10.1111/j.1600-0765.1998.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery CR, Little CB, Caterson B, Hughes CE. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–237. doi: 10.1016/S0945-053X(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Byers RJ, Taiwo YO, Hoyland JA. In situ zymographic localisation of type II collagen degrading activity in osteoarthritic human articular cartilage. Ann Rheum Dis. 1999;58:357–365. doi: 10.1136/ard.58.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Schroter-Kermani C, Merker HJ. Localization of collagen type VI in articular cartilage of young and adult mice. Cell Tissue Res. 1993;272:155–160. doi: 10.1007/BF00323581. [DOI] [PubMed] [Google Scholar]
- Hambach L, Neureiter D, Zeiler G, Kirchner T, Aigner T. Severe disturbance of the distribution and expression of type VI collagen chains in osteoarthritic articular cartilage. Arthritis Rheum. 1998;41:986–996. doi: 10.1002/1529-0131(199806)41:6<986::AID-ART5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Horikawa O, Nakajima H, Kikuchi T, Ichimura S, Yamada H, Fujikawa K, Toyama Y. Distribution of type VI collagen in chondrocyte microenvironment: study of chondrons isolated from human normal and degenerative articular cartilage and cultured chondrocytes. J Orthop Sci. 2004;9:29–36. doi: 10.1007/s00776-003-0737-4. [DOI] [PubMed] [Google Scholar]
- Kerkvliet EH, Jansen IC, Schoenmaker T, Beertsen W, Everts V. Collagen type I, III and V differently modulate synthesis and activation of matrix metalloproteinases by cultured rabbit periosteal fibroblasts. Matrix Biol. 2003;22:217–227. doi: 10.1016/S0945-053X(03)00035-0. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Lees M, Shuttleworth CA, Woolley D. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem Biophys Res Commun. 1993;191:1230–1236. doi: 10.1006/bbrc.1993.1349. [DOI] [PubMed] [Google Scholar]
- Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- Kuo HJ, Keene DR, Glanville RW. The macromolecular structure of type-VI collagen. Formation and stability of filaments. Eur J Biochem. 1995;232:364–372. doi: 10.1111/j.1432-1033.1995.tb20820.x. [DOI] [PubMed] [Google Scholar]
- Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272:26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- Lamande SR, Sigalas E, Pan TC, Chu ML, Dziadek M, Timpl R, Bateman JF. The role of the alpha3(VI) chain in collagen VI assembly. Expression of an alpha3(VI) chain lacking N-terminal modules N10–N7 restores collagen VI assembly, secretion, and matrix deposition in an alpha3(VI)-deficient cell line. J Biol Chem. 1998;273:7423–7430. doi: 10.1074/jbc.273.13.7423. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Growth factor regulation of chondrocyte integrins. Differential effects of insulin-like growth factor 1 and transforming growth factor beta on alpha 1 beta 1 integrin expression and chondrocyte adhesion to type VI collagen. Arthritis Rheum. 1997;40:270–276. doi: 10.1002/art.1780400211. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37:109–116. [PubMed] [Google Scholar]
- Marcelino J, McDevitt CA. Attachment of articular cartilage chondrocytes to the tissue form of type VI collagen. Biochim Biophys Acta. 1995;1249:180–188. doi: 10.1016/0167-4838(95)00026-q. [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Pahl JA, Ayad S, Miller RR, Uratsuji M, Andrish JT. Experimental osteoarthritic articular cartilage is enriched in guanidine soluble type VI collagen. Biochem Biophys Res Commun. 1988;157:250–255. doi: 10.1016/S0006-291X(88)80040-8. [DOI] [PubMed] [Google Scholar]
- McKie N, Edwards T, Dallas DJ, Houghton A, Stringer B, Graham R, Russell G, Croucher PI. Expression of members of a novel membrane linked metalloproteinase family (ADAM) in human articular chondrocytes. Biochem Biophys Res Commun. 1997;230:335–339. doi: 10.1006/bbrc.1996.5957. [DOI] [PubMed] [Google Scholar]
- Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC, Dieppe P, Robin PA. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Imai K, Yamashita K, Matsumoto T, Azumano I, Okada Y. Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Lab Invest. 1998;78:79–87. [PubMed] [Google Scholar]
- Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66:680–690. [PubMed] [Google Scholar]
- Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(Pt 4):635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Gilbert RT. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organisation in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103(Pt 4):1101–1110. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- Pullig O, Kladny B, Weseloh G, Swoboda B. Metabolic activation of chondrocytes in human osteoarthritis. Expression of type II collagen. Z Orthop Ihre Grenzgeb. 1999;137:67–75. doi: 10.1055/s-2008-1037039. [DOI] [PubMed] [Google Scholar]
- Ronziere MC, Ricard-Blum S, Tiollier J, Hartmann DJ, Garrone R, Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990;1038:222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- Sherwin AF, Carter DH, Poole CA, Hoyland JA, Ayad S. The distribution of type VI collagen in the developing tissues of the bovine femoral head. Histochem J. 1999;31:623–632. doi: 10.1023/A:1003811310619. [DOI] [PubMed] [Google Scholar]
- Smith GN, Jr, Hasty KA, Brandt KD. Type XI collagen is associated with the chondrocyte surface in suspension culture. Matrix. 1989;9:186–192. doi: 10.1016/s0934-8832(89)80049-6. [DOI] [PubMed] [Google Scholar]
- Soder S, Hambach L, Lissner R, Kirchner T, Aigner T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthr Cartil. 2002;10:464–470. doi: 10.1053/joca.2002.0512. [DOI] [PubMed] [Google Scholar]
- Stoop R, van der Kraan PM, Buma P, Hollander AP, Poole AR, van den Berg WB. Denaturation of type II collagen in articular cartilage in experimental murine arthritis. Evidence for collagen degradation in both reversible and irreversible cartilage damage. J Pathol. 1999;188:329–337. doi: 10.1002/(SICI)1096-9896(199907)188:3<329::AID-PATH371>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Xu C, Oyajobi BO, Frazer A, Kozaci LD, Russell RG, Hollander AP. Effects of growth factors and interleukin-1 alpha on proteoglycan and type II collagen turnover in bovine nasal and articular chondrocyte pellet cultures. Endocrinology. 1996;137:3557–3565. doi: 10.1210/en.137.8.3557. [DOI] [PubMed] [Google Scholar]