Abstract
T-tubules are invaginations of the cardiomyocyte membrane into the cell interior which form a tortuous network. T-tubules provide proximity between the electrically excitable cell membrane and the sarcoplasmic reticulum, the main intracellular Ca2+ store. Tight coupling between the rapidly spreading action potential and Ca2+ release units in the SR membrane ensures synchronous Ca2+ release throughout the cardiomyocyte. This is a requirement for rapid and powerful contraction. In recent years, it has become clear that T-tubule structure and composition are altered in several pathological states which may importantly contribute to contractile defects in these conditions. In this review, we describe the “neighborhood” of proteins in the dyadic cleft which locally controls cardiomyocyte Ca2+ homeostasis and how alterations in T-tubule structure and composition may alter this neighborhood during heart failure, atrial fibrillation, and diabetic cardiomyopathy. Based on this evidence, we propose that T-tubules have the potential to serve as novel therapeutic targets.
1. Structure and Role of T-Tubules
1.1. Morphology
The plasma membrane of ventricular cardiomyocytes is comprised of both the surface sarcolemma and a branching network of T-tubules which project into the cell interior. These invaginations were so named since they were initially observed as transverse elements, which occur near the Z lines at regular intervals along the cell [1]. However, detailed imaging has shown that the T-tubule network is actually quite complex, and contains numerous longitudinal components which run from one Z line to the next [2, 3]. While T-tubule diameter varies between 20 and 450 nm throughout the cardiomyocyte, more than 50% of tubules have a diameter between 180 and 280 nm [3]. Their total volume has been estimated to be 0.8–3.6% of the cardiomyocytes volume [4, 5]. Estimates of the fraction of the total sarcolemma in the T-tubules (versus surface membrane) range from 21–64% [4], although in a recent review employing computer modeling, it was suggested that the true fraction is close to 50% [6]. The large variability in these estimates likely reflects the different methodologies used for calculation and differences between species, but might also be due to the considerable plasticity of the T-tubules. Indeed, T-tubules are absent in the neonatal heart [7, 8] and develop progressively after birth [9, 10]. Also, as we will discuss in this review, there is an important remodeling of the T-tubules during pathological conditions.
1.2. The Dyadic “Neighborhood”
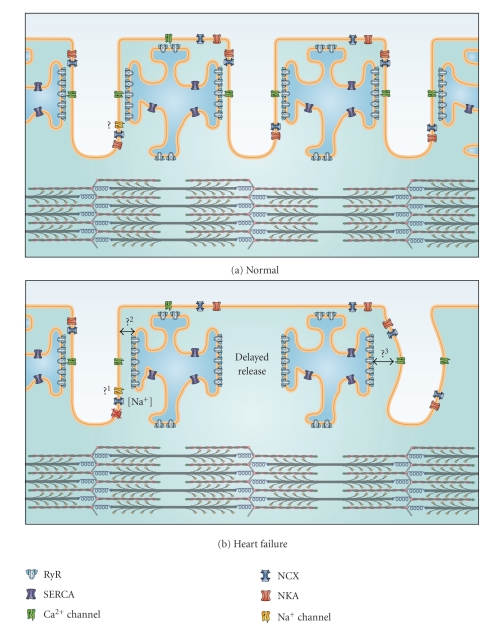
During the cardiac action potential, contraction is triggered in myocytes by a process known as excitation-contraction (EC) coupling [4]. During this process, electrical excitation of the cell membrane triggers a transient rise in intracellular Ca2+ concentration ([Ca2+]), which results in myocyte contraction as Ca2+ binds to the myofilaments. T-tubules play a pivotal role in EC coupling by allowing the action potential to propagate into the cell interior, and by providing proximity between the excitable cell membrane and the sarcoplasmic reticulum (SR), the main intracellular Ca2+ store. The SR membrane is apposed to the T-tubule membrane in highly specialized junctional microdomains (Figure 1). Here L-type Ca2+ channels face SR Ca2+ release channels, known as ryanodine receptors (RyRs), with a stoichiometry of 4–10 RyRs per L-type Ca2+ channel [11]. The two adjacent membranes are separated by a gap of 10–15 nm called the dyadic cleft. Clusters of RyRs and L-type Ca2+ channels and the dyadic cleft which separates them together constitute a functional unit called a couplon, or dyad [12] (Figure 1). The number of RyRs in a single dyad is still a matter of debate; analysis from electron micrographs reports numbers from 30 to 270, depending on species [13]. A recent study using a combination of confocal imaging and image processing suggested that the true number is in the upper range (120 to 260 RyRs) in rat [14]. However, a new electron microscopy tomography study in mouse demonstrated that there is a large variability in the size of the dyad [5]. The authors also showed that most dyads are significantly smaller than previously estimated; more than one-third of dyads are equal or smaller than the size necessary to hold ~15 RyRs, and the average dyad holds only 7.7 RyR tetramers.
Figure 1.
Schematic representation of the dyadic neighborhood in normal and failing cells. (a) Excitation-contraction coupling occurs at functional junctions between Ca2+ channels in the T-tubules and ryanodine receptors in the SR. Depending on their localization, other proteins in the dyadic neighborhood such as SR Ca2+ ATPase (SERCA), NCX, NKA, and Na+ channels can also regulate Ca2+ homeostasis. Question mark: The positioning of the Na+ channel at the dyad is still controversial. (b) During heart failure, T-tubule loss and/or disorganization occurs leading to the formation of orphaned ryanodine receptors, which do not have apposing Ca2+ channels. Ca2+ release in these regions is delayed leading to slower and weaker contractions. Other putative alterations in the dyadic neighborhood are indicated by the question marks: (1) it is unclear whether the Na+ channel is present in the dyad of failing cardiomyocytes. Some experimental evidence suggests that the distance between the SR and T-tubule is increased in heart failure (2), while T-tubule disorganization may lead to dyadic clefts with variable width (3).
When one or several L-type Ca2+ channels are open, Ca2+ release is triggered from RyRs in that couplon by an amplification system known as Ca2+-induced Ca2+ release (CICR) [15]. This can be observed as evoked Ca2+ sparks in unstimulated myocytes [16, 17], although Ca2+ sparks can also result from spontaneous RyR openings in the absence of L-type Ca2+ current [18]. When an action potential travels through the cardiomyocyte, thousands of individual Ca2+ sparks are triggered, and their spatiotemporal summation constitutes the Ca2+ transient [4]. The extent of CICR and magnitude of the Ca2+ transient are critically dependent on the SR Ca2+ content [19], which in turn is determined by the balance between SR refilling and release. However, efficient coupling between Ca2+ influx and SR Ca2+ release also requires the precise positioning of Ca2+ channels, RyRs, and other proteins within the dyadic “neighborhood”, as will be discussed below.
Following release, Ca2+ is recycled into the SR by the SR/ER Ca2+ ATPase (SERCA) and extruded from the cell by the Na+/Ca2+-exchanger (NCX) and the plasma membrane Ca2+ ATPase (PMCA). The PMCA is believed to be a quite minor contributor to overall Ca2+ removal due to its relatively slow kinetics [20]. In addition, it has not been demonstrated that the PMCA is present in the T-tubules, which is a requirement for an efficient Ca2+ extrusion pathway. On the other hand, NCX plays an important role in Ca2+ extrusion. It is localized both in the surface membrane and in the T-tubular membrane, but with a three times higher density in the T-tubules [21] (Figure 1). The contribution of NCX to total Ca2+ removal from the cytosol varies across species from ~30% in the rabbit to ~7% in the rat [22, 23]. The NCX is electrogenic since it exchanges 3 Na+ for 1 Ca2+, which means that its transport rate is dependent on both the membrane potential and transmembrane concentration gradients for Na+ and Ca2+. Since ionic concentrations may vary considerably in different submembrane spaces or ion pockets, NCX activity will very much depend on its localization as well as the localization of other nearby Na+ and Ca2+ handling proteins. For example, we have observed that the Na+/K+-ATPase α2 isoform is preferentially localized in the T-tubules where it regulates an Na+ pool which is shared by NCX [24]. Data from several other studies also support a close relationship between Na+/K+-ATPase and NCX function [25–28]. Since NCX and SERCA compete for Ca2+, this local control of Na+ homeostasis can importantly regulate SR Ca2+ load and the magnitude of the Ca2+ transient [29]. Thus, alterations in T-tubule structure or more subtle alterations in the dyadic neighborhood would be expected to have important functional consequences.
Although the NCX functions predominantly to extrude Ca2+ from the cell (forward mode), it can also function in reverse mode to facilitate Ca2+ entry. We have recently shown that NCX-mediated Ca2+ influx contributes to an early phase of Ca2+ entry which actually precedes that from Ca2+ channels [30]. Ca2+ influx via reverse-mode NCX can even trigger SR Ca2+ release, albeit with low efficiency [31]. We have calculated that such a role requires the presence of an Na+ channel in the dyad to locally elevate intracellular [Na+] [30] (Figure 1). However, the localization of both NCX and the Na+ channel in the dyad has been disputed [32]. Further study of the localization of these proteins is therefore required, both in normal cardiomyocytes and in disease states where the efficiency of NCX-mediated CICR may be altered.
1.3. T-tubule Density/Organization: Control of Ca2+ Release Synchrony
The vast majority of studies of T-tubular structure and function have been conducted on ventricular myocytes. Interestingly, other cardiac cell types are often stated to lack T-tubules. In reality, however, T-tubules have been observed in Purkinje cells [33] and their presence has been clearly documented in atrial myocytes from a number of species [34–42], albeit at a lower density than that observed in ventricular cells. To our knowledge, T-tubules have not yet been examined in human atrial cells, but are very likely to be present since large mammals such as sheep ([40, 41] see Figure 2(b), left panel) and dog [42] exhibit a surprisingly high T-tubule density.
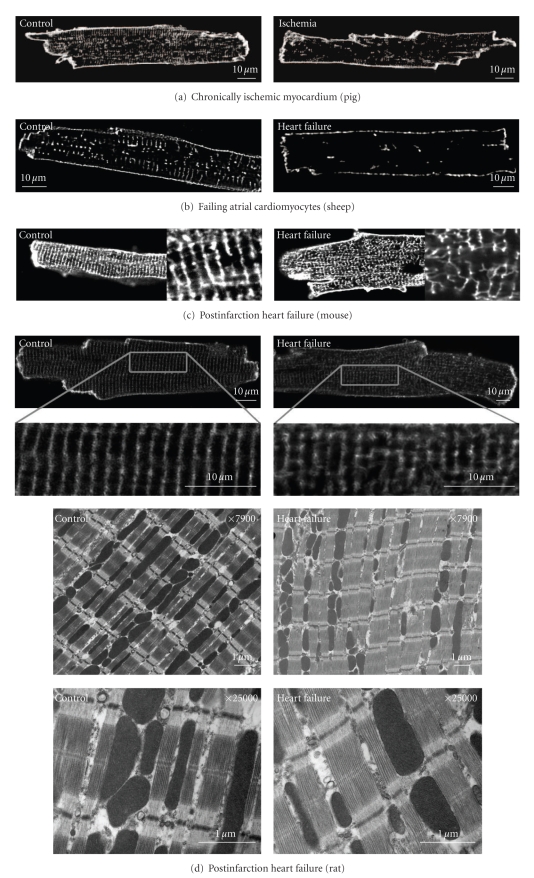
Figure 2.
Examples of T-tubule alterations in various pathological states. Left panels show images from controls; right panels show images from (a) chronically ischemic pig myocardium (from [43]), (b) failing atrial ovine cardiomyocytes (from [40]), and post-infarction heart failure models in (c) mouse (from [44]) and (d) rat (from [45]). Panel (d) shows representative electron micrographs from control and failing rat hearts showing T-tubule disruption. (e) Scanning ion conductance microscope images from the surface of human nonfailing (control) and failing cardiomyocytes showing loss of T-tubular openings (from [46]). All figures are reproduced with permission.
The extent and organization of the T-tubule network is an important determinant of the spatial homogeneity of SR Ca2+ release throughout the cardiomyocyte. A dense, well-organized T-tubule network, such as that observed in mouse and rat ventricle myocytes, (Figures 2(c) and 2(d), left panels) allows for very synchronized CICR in these cells (Figure 3(a)) [43, 44, 47]. Heinzel et al. [48] demonstrated that a somewhat lower T-tubule density in pig ventricular myocytes was associated with less synchronous Ca2+ release (Figure 3(a)). In cat atrial cells, which have a very low T-tubule density, there is a wave-like propagation of the Ca2+ transient from the sarcolemma to the cell interior [49] (Figure 3(a)). Thus, in all of these cell types, Ca2+ release is initially triggered following influx of Ca2+ at the surface sarcolemma and at locations where T-tubules (and Ca2+ channels) are present, followed by propagation of released Ca2+ into regions where T-tubules are absent. Since there is a uniform distribution of RyRs across myocytes [47, 48, 50], this diffusing Ca2+ may then trigger SR Ca2+ release. Meethal et al. [51] observed spontaneous Ca2+ sparks at sites of irregular gaps occurring between adjacent T-tubules in dog cardiomyocytes. This shows the presence of functional RyRs and suggests that a propagating Ca2+ wave could trigger CICR at these locations. In atrial myocytes, the extent of CICR propagation into the interior of the cell has been shown to depend on both SR content and β-adrenergic tone [50]. Thus, control of Ca2+ release synchrony does not solely rely on T-tubular structure, but also on cardiomyocyte status. These considerations may have particular significance in pathological conditions, as will be described in the following chapters. This discussion will initially and most thoroughly address T-tubule alterations in heart failure, where there are now considerable data available, followed by a briefer discussion of more preliminary data from atrial fibrillation and diabetes.
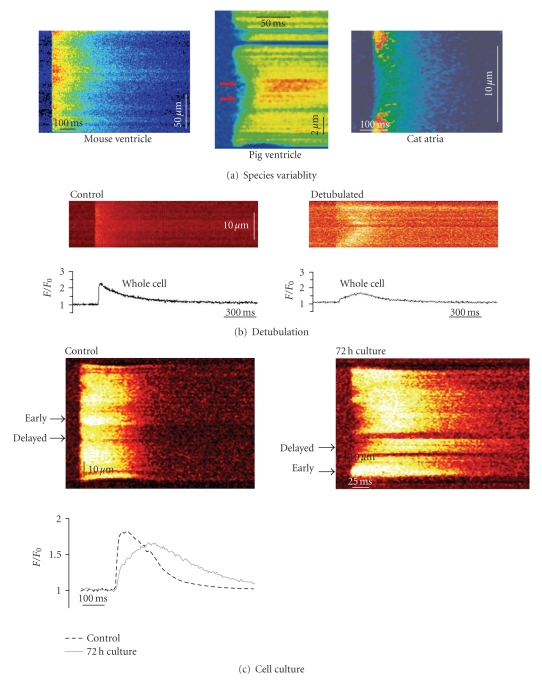
Figure 3.
T-tubule density affects Ca2+ release synchrony. (a) Variable amount of T-tubules in different cell types affects the homogeneity of the Ca2+ transient, as illustrated in confocal line scans of myocytes from mouse ventricle ((a), unpublished data), pig ventricle ((b), from [43]), and cat atria ((c), from [49]). (b) Experimental loss of T-tubules using the detubulation technique causes delayed Ca2+ release in the centre of the cardiomyocyte (from [52]). (c) T-tubules are lost when cardiomyocytes are kept in culture resulting in dyssynchronous slowing of the Ca2+ transient (from [53]). All figures are reproduced with permission.
2. Heart Failure
Heart failure is a progressive and chronic disease, characterized by an impaired ability of the heart to pump blood. This condition is on the rise in the western world, and diagnosis carries an alarmingly high mortality rate of more than 60% after 5 years [54]. Depressed contractility in heart failure is widely believed to result, at least in part, from reduced magnitude of the cardiomyocyte Ca2+ transient [29]. A number of studies have indicated that decreased SR Ca2+ release in this condition results from lowered SR Ca2+ content [55, 56], due to reduced SERCA function and/or greater Ca2+ leak from the SR [29]. In addition, the ability of the Ca2+ current to trigger CICR, the so-called “gain of Ca2+ release”, is reduced in failing cells [43, 57–60]. Slowing of SR Ca2+ release also occurs in heart failure, which slows contraction and additionally reduces the power of the heartbeat [46, 61–69].
2.1. T-tubule Loss/Disorganization
A growing body of evidence indicates that impaired Ca2+ homeostasis in failing myocytes may involve alterations in T-tubular structure. Kamp and colleagues [70, 71] observed loss of T-tubules in ventricular cardiomyocytes in a dog model of tachycardia-induced heart failure. This finding has since been confirmed in other animal models of heart failure including post-infarction rabbit [72] and rat [46], and pigs with chronic ischemia [43] (Figure 2). Similar observations have also been reported in failing human ventricular myocytes [46, 73]. A recent investigation of atrial T-tubules in heart failure also showed dramatic T-tubule loss in sheep following atrial pacing [40] (Figure 2).
Others have not observed decreased T-tubule density in failing myocytes, but rather a structural disorganization. We reported that heart failure progression in mice and rats following myocardial infarction was associated with loss of the uniform, transverse T-tubule pattern, with a greater proportion of tubules present in the longitudinal direction ([44, 45], Figure 2). We additionally observed the appearance of irregular gaps between adjacent T-tubules in failing cells. Similar T-tubular disorganization has been observed in myocytes from failing spontaneously hypertensive rats [47] and in failing human left ventricle [74]. Dilated T-tubules have also been reported in human heart failure [53, 73, 75]. Thus, there is now compelling evidence supporting altered T-tubular structure in heart failure, although it is unclear under what conditions this may be manifested as T-tubule loss or as reorganization.
2.2. Slowed, Dyssynchronous Ca2+ Release
The consequences of T-tubule alterations for EC-coupling were initially investigated by experimentally promoting T-tubule loss by either cell culture [53, 76] or detubulation [52, 77]. In both cases, T-tubule loss was associated with desynchronization of Ca2+ release across the cell. When cells were detubulated, wave-like propagation of the Ca2+ transient from the sarcolemma to the cell interior was observed [52, 77, 78] (Figure 3(b)), which resembled the pattern of Ca2+ release reported in other cells with very low T-tubule density [8, 49, 79, 80] (Figure 3(a)). With less dramatic T-tubule reduction during cell culture, a fragmented Ca2+ release pattern was observed [53] (Figure 3(c)). This indicated that SR Ca2+ release was initially triggered at sites where T-tubules were present, followed by propagation into regions devoid of T-tubules [53]. This situation is similar then to that observed in normal myocytes which have a moderate T-tubular density, such as pig ventricular myocytes ([43], Figure 3(a)), and sheep atrial myocytes [40]. Spatially summating Ca2+ release in cells which had lost T-tubules showed that the overall Ca2+ transient became slowed and reduced in magnitude [52, 53] (Figures 3(b) and 3(c)). Therefore, such experiments served as proof of principle that large reductions in T-tubule density could reproduce reported alterations in the failing Ca2+ transient.
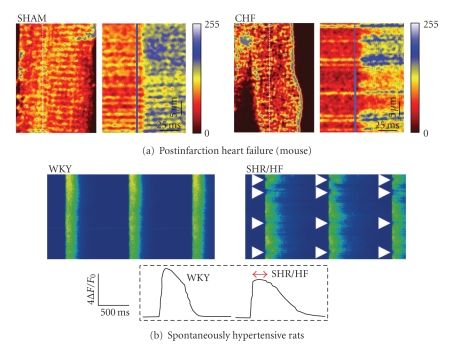
Although dyssynchronous Ca2+ release was first observed in failing myocytes by Litwin et al. [81], it was not until later studies by Louch et al. [44] and Song et al. [47] that such alterations were directly linked to alterations in T-tubular structure. In both studies, small regions of delayed Ca2+ release were observed to occur at irregular gaps between adjacent T-tubules following T-tubule disorganization (Figure 4). We demonstrated this phenomenon by simultaneously visualizing the T-tubule network and intracellular [Ca2+] (Figure 4(a)). Song et al. [47] employed an alternative approach, and demonstrated that when intracellular Ca2+ was highly buffered, regions of delayed Ca2+ release did not occur, as Ca2+ diffusion into the gaps between T-tubules was prevented. They observed, however, that the ryanodine receptor distribution remained intact in failing myocytes, suggesting that T-tubule disorganization resulted in some ryanodine receptors becoming “orphaned”, without opposing Ca2+ channels. More recent work has similarly linked T-tubule loss in failing ventricular [43, 46] and atrial myocytes [40] to reduced Ca2+ release synchrony. Importantly, as in studies with experimental loss of T-tubules [52, 53, 76, 78], reduced Ca2+ release synchrony in failing cells has been shown by a number of investigators to promote slowing and broadening of the overall Ca2+ transient [43, 44, 46, 47], a hallmark of the failing condition. Results from studies with experimental reduction in T-tubules (discussed above) suggest that reduced T-tubular organization and/or density also likely contributes to the reduction in Ca2+ transient amplitude in heart failure.
Figure 4.
Dyssynchronous Ca2+ transients in failing cardiomyocytes. (a) Simultaneous imaging of T-tubules stained with di-8-ANEPPS and Ca2+ transients in fluo-4-AM loaded myocytes using confocal line scans. The position of the line scan is indicated as a vertical dotted line in T-tubule images, and T-tubules appear as horizontal lines in line-scan images. In myocytes from mice with congestive heart failure (CHF) following myocardial infarction, Ca2+ release was delayed in regions lacking T-tubules (right panel), but was synchronous in sham-operated controls (left panel), from [44]. (b) Regions of delayed Ca2+ release were also observed in cardiomyocytes from spontaneously hypertensive rats with heart failure (SHR/HF), but not in controls (Wistar-Kyoto (WKY) rats). From [47] Copyright (2006) National Academy of Sciences, USA. All figures are reproduced with permission.
2.3. Ca2+ Current and Gain of Ca2+ Release
The concept of orphaned ryanodine receptors, a spatial mismatching of Ca2+ channels and ryanodine receptors (Figure 1(b)), was initially proposed by Gómez et al. [82] to account for reduced gain of Ca2+ release in failing myocytes. Recent direct observation of divergent T-tubule and ryanodine receptor localization supports this hypothesis [43, 47]. However, reduced efficiency of the Ca2+ release trigger may also have other underlying mechanisms. There is a general consensus that L-type Ca2+ current density is unchanged in heart failure when measured during voltage-clamp steps (for review, see [29]). However, prolongation of the action potential and loss of an early repolarization notch in failing cells reduce the driving force for Ca2+ entry, resulting in decreased peak Ca2+ current [65, 83, 84]. In addition, the time course of Ca2+ entry is prolonged during the failing action potential, which reduces efficiency for triggering Ca2+ release [65, 84]. Such alterations in Ca2+ current desynchronize Ca2+ release, as has been demonstrated by switching a voltage-clamped action potential stimulus from a normal human action potential to a failing human action potential [83, 84]. Importantly, this dyssynchronous Ca2+ release pattern is variable from beat to beat [83], which distinguishes it from the consistent pattern maintained across beats caused by alterations in T-tubule structure [44, 47]. We believe that slowed, dyssynchronous Ca2+ release in failing cells likely results from a combination of alterations in T-tubules and action potentials.
An alterative proposal put forward to explain reduced gain of Ca2+ release in failing myocytes is an expansion of the dyadic cleft, resulting in a greater distance between Ca2+ channels and ryanodine receptors [60, 82]. To our knowledge, there is not yet direct evidence based on imaging to support this hypothesis. However, we have observed that failing myocytes exhibit a longer delay between the upstroke of the action potential and the upstroke of the Ca2+ transient [44], which is consistent with this notion. Importantly, this delay was observed at all locations across the cell, not simply at those locations where large gaps between neighboring T-tubules had led to the formation of orphaned ryanodine receptors. We have proposed that a more subtle drift of T-tubules may occur throughout the cell, which less dramatically increases the T-tubule to SR distance (Figure 1(b)), yet is sufficient to delay CICR and reduce gain [44]. This hypothesis is supported by the findings of Xu et al. [60], who also observed that failing cells exhibited a slowed response of RyRs to the opening of a single Ca2+ channel. This was associated with reduced CICR efficiency, since there was a greater chance that RyRs would not open, and Ca2+ release which was desynchronized across the cell. Further work employing high resolution imaging and detailed examination of local control of EC coupling is required to confirm the hypothesis that expansion of the dyadic cleft impairs cross-talk between L-type Ca2+ channels and RyRs.
While L-type Ca2+ current density is generally observed to be unchanged in heart failure [29], the number of Ca2+ channels is reportedly reduced [70, 85]. However, increased single channel activity appears to maintain normal Ca2+ current density [85, 86], and this may result from increased phosphorylation of the Ca2+ channel by protein kinase A and/or CaMKII [86, 87]. Since Ca2+ channels are concentrated in the T-tubules, a decrease in Ca2+ channel number would, in fact, be expected in failing myocytes if T-tubules are lost. Indeed, experimentally promoting loss of T-tubules results in decreased Ca2+ current density [53, 88]. The consequence of reduced Ca2+ channel number for EC-coupling in failing cells is unknown. Litwin et al. [81] observed that reduced Ca2+ current density in cardiomyocytes isolated from the border zone of post-infarction rabbit promoted dyssynchronous Ca2+ transients. It is unclear whether loss of Ca2+ channels in failing cardiomyocytes from non-infarcted myocardium might also promote dyssynchrony, but this almost certainly depends on the localization of remaining channels. Are they redistributed between T-tubules and the surface sarcolemma? Some dyads are clearly disrupted by T-tubule reorganization (Figure 1(b)), but do those which remain intact have a normal composition of Ca2+ channels and ryanodine receptors? This issue could be addressed by the technique of Hayashi et al. [5], who recently employed electron tomography to estimate the number of RyRs in each dyad based on the calculated dyadic volume. In addition, the number of Ca2+ channels present in a couplon could be calculated by detailed analysis of Ca2+ sparks [89, 90]. To date, more rudimentary analyses of Ca2+ sparks have indicated that sparks in failing myocytes have similar characteristics to those from normal cells [57, 60].
Even without alterations in T-tubular structure, L-type Ca2+ current characteristics would be expected to be altered in heart failure. The reduced amplitude and slowed time course of Ca2+ release would be predicted to reduce Ca2+-dependent inactivation. However, alterations in Ca2+ current kinetics have often not been observed in failing myocytes [87, 91]. Bito et al. [87] have suggested that this apparent discrepancy may result from superimposition of NCX current over Ca2+ currents, which impairs detection of altered Ca2+ current kinetics. On the other hand, at least at high stimulation frequencies, greater Ca2+ current inactivation might be expected in failing myocytes, as lowered SERCA function promotes cytosolic Ca2+ accumulation [87, 92–94]. Such alterations may contribute to the negative force-frequency response which is a characteristic of human heart failure [56]. Finally, facilitation of Ca2+ current might be altered since this phenomenon is tightly regulated by SR-derived Ca2+ release [95], which is reduced in heart failure, and CaMKII [96] which is upregulated [97]. Importantly, loss of T-tubules may reduce facilitation, since Ca2+ current is preferentially modulated by SR Ca2+ release at the T-tubules rather than at the surface sarcolemma [88]. Indeed, studies conducted to date have shown decreased facilitation in failing human atrial and ventricular myocytes [88, 98, 99].
2.4. Ryanodine Receptor Function
There is growing evidence that ryanodine receptor function is importantly altered in heart failure. In isolated bilayer experiments, Marx et al. observed greater RyR activity [69] and suggested that greater SR Ca2+ leak via RyRs contributes to reduced SR Ca2+ content. This finding has since been confirmed in intact myocytes [100, 101]. The Marks group proposed that increased leak results from “hyper-phosphorylation” of RyR by PKA, which causes dissociation of FKBP12.6 from RyR [69]. This destabilizes the RyR complex, and causes functional uncoupling of neighboring RyRs [102]. Thus, while arrays of RyRs normally tend to open and close together, RyR uncoupling in failing myocytes is proposed to result in a greater open probability during diastole. However, these findings remain controversial as other groups have not been able to document dissociation of FKBP12.6 from RyR in either healthy or diseased myocytes [103–105]. Instead, recent work has suggested that greater RyR activity in heart failure may result from phosphorylation by Ca2+/calmodulin-dependent protein kinase II [106] or increased relative expression of RyR regulatory proteins triadin and junctin [100]. Regardless of the underlying mechanism, increased SR Ca2+ leak is believed to be detrimental in heart failure since reduced SR content impairs contractility. In addition, increased Ca2+ leak is thought to be pro-arrhythmic since spontaneously released Ca2+ is extruded from the cell by NCX, resulting in afterdepolarizations which may trigger an extra action potential [29].
It is unclear if and how alterations in RyR function in heart failure are related to T-tubular changes. However, Meethal et al. recently reported that in failing cells, spontaneous Ca2+ sparks occur more frequently at the irregular gaps between T-tubules created by T-tubule loss [51]. Resting Ca2+ levels were also higher at these locations. These observations suggest that orphaned ryanodine receptors exhibit greater activity than those in intact dyads, and that this overactivity may importantly contribute to increased SR leak and arrhythmic potential in failing cells. Are groups of orphaned RyRs also functionally uncoupled? Perhaps, and if so, such alterations might theoretically exacerbate Ca2+ release dyssynchrony by slowing propagation of CICR into regions where T-tubules are absent. Similarly, altered SR content and β-adrenergic tone might also influence synchrony by affecting wave propagation speed [77].
2.5. Other Alterations in the Dyadic “Neighborhood”
The neighborhood of proteins controlling local Ca2+ concentration in the dyad is complex, and includes many more proteins besides L-type Ca2+ channels and ryanodine receptors. As described in the introduction, Ca2+ regulation is closely linked to the activity of Na+-handling proteins. NCX expression and activity are increased in heart failure, and reverse-mode function is enhanced due to action potential prolongation, a reduced Ca2+ transient, and elevation of intracellular [Na+] (for review see [107]). Depending on the precise arrangement of the dyadic neighborhood, greater NCX-mediated Ca2+ entry might play an increased role in triggering Ca2+ release in heart failure. However, such a role depends on the relative proximity of NCX, ryanodine receptors, and the Na+ channel [30], and these measurements have not yet been reported in failing myocytes. Regardless, increased NCX-mediated Ca2+ entry is believed to support the Ca2+ transient in heart failure, partially counteracting reductions in SR content resulting from SERCA downregulation [107]. However, Fowler et al. [108] showed that Na+ accumulation was not translated into SR loading when cells were detubulated, and that this resulted in the development of a negative force-frequency relationship. Therefore, loss of NCX molecules along with T-tubules in failing myocytes may have significant consequences, especially at physiological frequencies. Confirmation of this hypothesis awaits a detailed examination of NCX distribution in failing cells.
Increased Na+ levels in heart failure [109–111] appear to result, at least in part, from reduced expression and activity of the Na+-K+ ATPase [28, 45]. We have observed that the Na+-K+ ATPase α2 isoform is preferentially localized in the T-tubules, where it regulates local [Na+] near NCX [24]. Therefore, down-regulation of α2 isoform in heart failure impairs crosstalk between the Na+–K+ ATPase and NCX, which may locally elevate [Na+] and impair NCX-mediated Ca2+ extrusion [28, 45]. There is also growing evidence that increased late Na+ current [112–114] and increased Na+-H+ exchanger activity [111, 115] additionally contribute to Na+ accumulation in failing cells.
Interestingly, although T-type Ca2+ channels are normally expressed at very low density in ventricular cells, a number of studies have reported that their expression is up-regulated in hypertrophy and heart failure (for review see [116]). In myocytes from rats with pressure overload, Martínez et al. [117] observed that T-type Ca2+ channel entry was one-third that carried by L-type channels, and thus a significant contributor to overall Ca2+ influx. It is unknown if T-type channels are expressed in the dyad or T-tubules of failing cells, but if so it is possible that they may importantly modulate local [Ca2+], or even serve as triggers for Ca2+ release. However, in possible contradiction to this notion, a recent study employing T-type channel over-expression showed preferential targeting of these channels to the surface sarcolemma [118]. Nevertheless, increased T-type Ca2+ channel expression is not without consequence, as it has been shown to play an important role in triggering of pathological cellular hypertrophy [119], and may be pro-arrhythmic [120].
Although the PMCA is believed to be only a minor contributor to Ca2+ removal during the decline of the Ca2+ transient [20], data from the NCX knockout mouse suggest that this Ca2+ pump can have a surprisingly large capacity to extrude Ca2+ when challenged [121]. Similarly, we have suggested that PMCA up-regulation might help maintain diastolic function following SERCA knockout [28, 122]. Therefore, with reduced SERCA function and impaired NCX-mediated Ca2+ extrusion (due to [Na+]i accumulation) in failing cells, the PMCA may theoretically become a more important Ca2+ extrusion pathway. However, a recent study by Mackiewicz et al. [123] showed that PMCA function is, in fact, progressively reduced during heart failure progression in rats following myocardial infarction. To our knowledge, no other data on PMCA function in heart failure are currently available, and protein localization is unknown. Based on available information, it appears that PMCA function in heart failure is most importantly involved with signaling pathways underlying cellular hypertrophy [124, 125] and not maintenance of the Ca2+ transient.
Another Ca2+ transporting protein which has recently come into focus in ventricular cardiomyocytes is the inositol 1,4,5-triphosphate receptor (IP3R). Initially thought to play only a minor role in ventricular cells since it is markedly outnumbered by the RyRs (50 : 1) [126], recent evidence shows that there is an important upregulation of IP3Rs in the junctional SR during hypertrophy and heart failure [127]. The authors showed that although Ca2+ fluxes through IP3Rs are smaller than through RyRs [128], enhanced Ca2+ release through IP3Rs in hypertrophic cardiomyocytes increases Ca2+ transient magnitude. Although such an effect might be thought beneficial, increased IP3R-mediated Ca2+ release is arrhythmogenic since the close proximity of IP3Rs and RyRs leads to RyR sensitization [127]. The presence of NCX nearby IP3Rs would promote early afterdepolarizations and thus extra-systolic Ca2+ transients. However, it remains to be determined how loss/disorganization of the T-tubules affects the distance between NCX and IP3Rs during heart failure. Ankyrin-B may be importantly involved, since this protein coordinates NCX, NKA and IP3R in a cardiac T-tubule/SR microdomain [129].
The above discussion illustrates that there are complex modifications in the dyadic neighborhood in heart failure, involving RyR, the L-type Ca2+ channel, and Na+ handling proteins such as the Na+-K+ ATPase and NCX. To fully understand the heart failure phenotype, it is critical that alterations in the distribution and function of these proteins are elucidated. This investigation will require a detailed analysis of T-tubule composition, and not simply overt changes in T-tubular structure as has largely been conducted to date.
3. Atrial Fibrillation
Atrial fibrillation is a common type of arrhythmia which is caused by local reentry circuits. This prevents coordinated atrial contraction, which compromises the heart's blood pumping capacity [130]. There is also an increased risk of stroke in this condition since blood pools and clots in the dysfunctional atria [131]. Recent evidence suggests that decreased contractile force of the atrial muscle [132] may, at least partly, result from pathological alteration of T-tubular structure. Lenaerts et al. [41] observed a 45% reduction in T-tubule density in right atrial cardiomyocytes following persistent atrial fibrillation. As in reports from failing myocytes described above, T-tubule loss during atrial fibrillation was associated with spatially dyssynchronous Ca2+ release but an intact ryanodine receptor distribution [41]. Therefore, the authors suggested that a reduced efficiency of EC coupling in this condition results from fewer Ca2+ channel-RyR couplings (more orphaned ryanodine receptors), and that this effect contributes to reduced Ca2+ transient and contraction magnitude.
While some myocyte alterations following atrial fibrillation may resemble those that occur in failing myocytes, there are also important differences. Unlike in heart failure, atrial myocytes following persistent fibrillation are widely reported to exhibit reduced L-type current density [41, 133–136]. Interestingly, L-type Ca2+ channel expression is reported to be reduced in both conditions [41, 70, 85, 133], while single channel function is increased [85, 86, 137]. It is unclear why this increase in function is sufficient to maintain normal current density in heart failure but not atrial fibrillation. Another important difference between the two conditions is that SR content is widely reported to be reduced in heart failure [55, 56] but may be normal in atrial fibrillation [41]. This might suggest that loss of T-tubules and associated Ca2+ channels is a primary defect in Ca2+ homeostasis in atrial fibrillation, but only one component of more complex alterations in heart failure. In both conditions, impaired function of the contractile machinery has also been proposed [138, 139].
Lenaerts et al. [41] were the first to examine T-tubule alterations in atrial fibrillation, but more investigation is required. For example, the importance of slowing of Ca2+ release has not yet been demonstrated in this condition, but would be expected to reduce contractile power. As discussed above, important pathological alterations in the dyadic neighborhood may include modified targeting and regulation of Ca2+- and Na+-handling proteins, and this issue also remains largely unexplored in atrial fibrillation.
4. Diabetic Cardiomyopathy
Many diabetic patients exhibit cardiac dysfunction [140]. In type-2 diabetes, reduced in vivo and cardiomyocyte contractile functions are modeled in the db/db insulin-resistant “diabetic” mouse [141–144]. Decreased contractility in these mice is associated with greater SR Ca2+ leak, which reduces SR Ca2+ content and the magnitude of the Ca2+ transient [142, 144]. In addition, the magnitude of the L-type Ca2+ current is reduced while single channel activity is increased [141]. This suggests that there are a decreased number of Ca2+ channels, similar to that reported in heart failure and atrial fibrillation. In a recent study, Stølen et al. [144] showed that cardiomyocytes from sedentary db/db mice exhibit reduced T-tubule density, which may account for Ca2+ channel loss. As expected, T-tubule loss was associated with dyssynchronous SR Ca2+ release and reduced Ca2+ transient amplitude. Although Ca2+ release kinetics were not reported in this study, an expected reduced rate of rise of the Ca2+ transient could contribute to the slowed contraction observed in vivo [142]. Another interesting observation in myocytes from db/db mice is a longer delay between the stimulus and the upstroke of the Ca2+ transient [144]. This is in agreement with our finding in failing post-infarction cardiomyocytes [44], which we have hypothesized reflects an expansion of the dyadic cleft (Figure 1(b)). Although such alterations together with T-tubule loss would be expected to reduce CICR efficiency, Pereira et al. [141] reported unaltered gain of EC coupling in db/db cardiomyocytes. The reason for this apparent discrepancy is unclear, but it may result from other unknown alterations in the dyadic neighborhood.
T-tubule alterations have also been reported in GK/Jcl and SDT/Jcl rat models of type II diabetes [145]. Although T-tubule loss was not observed in these models, the authors showed a disorganization of T-tubules and a decrease in the number of complete SR/T-tubule junctions. Ca2+ homeostasis was not investigated in this study, but dyssynchronous Ca2+ release would be expected. However, dyssynchronous Ca2+ transients have been reported in cardiomyocytes isolated from rats with streptozotocin-induced type-1 diabetes [146]. However, in this study dyssynchrony was attributed to a subpopulation of RyRs (37% of total RyR number) which were unresponsive to Ca2+. They proposed that some of these inactive RyRs may be localized in the dyad, preventing Ca2+ influx through L-type Ca2+ channels from triggering SR Ca2+ release. We suggest that Ca2+ channels in these inactive dyads could be described as “functionally orphaned”, a reversal of the situation that arises when ryanodine receptors are orphaned by removal of dyadic Ca2+ channels following T-tubule disruption. Interestingly, Shao et al. [146] observed that functional RyRs remaining in diabetic myocytes were hyperphosphorylated, which resulted in greater activity, increased SR Ca2+ leak, and reduced SR content [146]. Both Ca2+ transients and contractions were reduced in magnitude and exhibited a slowed rate of rise, as expected when SR content is reduced and SR Ca2+ release desynchronized. If and how modifications in T-tubular structure contribute to altered Ca2+ homeostasis in type-1 diabetes remains to be determined.
5. Mechanisms Controlling T-tubular Structure and Growth
Our discussion thus far has described T-tubules and the dyadic neighborhood in health and disease, but not the mechanisms controlling their formation and maintenance. T-tubules are absent at birth, and develop thereafter by progressive invagination from the surface sarcolemma [2, 10]. Caveolae, which are small bulbous pockets at the cell surface, and the associated protein caveolin are believed to play an essential role in this process [147]. However, RyRs are present in the SR and aligned at the Z-disc very early in development [148]. The formation of dyads is therefore dependent on the later development of T-tubules. Ziman et al. [9] recently showed that dyad formation occurs as junctophilin-2 arrives with the maturing T-tubules. This protein spans the dyadic cleft, and anchors the T-tubular and SR membranes [149]. This role is believed critical for dyad formation, as junctophilin arrival at this location establishes efficient and spatially synchronous EC coupling [9].
Junctophilin alterations may promote loss of dyadic integrity in pathophysiological conditions. The interaction between junctophilin and caveolin-3 is down-regulated in cardiomyopathy [150]. In addition, junctophilin mutations are reported in cardiomyopathy patients [151, 152]. We hypothesize that abnormal junctophilin expression or function may promote drift of L-type Ca2+ channels from RyRs as the dyad becomes unanchored, resulting in reduced CICR gain. Indeed, decreased junctophilin expression in the triadin knockout mouse was recently reported to be associated with decreased colocalization of Ca2+ channels and RyRs [153].
A tubule-forming protein called amphiphysin-2 may also be involved in T-tubule maintenance. In skeletal muscle, this protein has been shown to be highly concentrated at the T-tubules [154], and Lee et al. [155] reported that expressing amphiphysin 2 in nonmuscle cells induces T-tubule-like invaginations in the plasma membrane. In addition, amphiphysin 2 mutation results in disorganized T-tubular structure in skeletal muscle [156]. Since this protein links the plasma membrane with submembranous cytosolic scaffolds [157], this finding suggests that unanchoring of the T-tubules from the cytoskeleton may lead to T-tubule drift. Although a role of amphiphysin 2 in T-tubule formation and maintenance is not yet established in cardiomyocytes, connections between the sarcolemma and cytoskeleton are well known [158]. Interestingly, failing cardiomyocytes show disorganization of cytoskeleton structure [158], and loss of T-tubules during cell culture has been linked to changes in actin [159]. Therefore, while T-tubule drift may occur if cytoskeletal anchors are disrupted, alterations in the structure of the cytoskeleton itself might also contribute to T-tubule changes in disease states.
During normal myocyte maintenance, lysosomes are known to degrade cellular organelles by autophagy [160, 161]. Interestingly, Meethal et al. [51] recently reported that in both normal and failing cardiomyocytes, lysosomes were present at gaps between T-tubules, suggesting that T-tubule degradation was occurring at these locations. They also observed that T-tubule loss in failing myocytes was associated with increased density of lysosomes. It is as yet unclear whether greater lysosome activity is the initiating event responsible for abnormal T-tubule degradation. Insight into this issue might be provided by an investigation of T-tubules in lysosomal storage diseases, although we are unaware of any such study to date.
The finding that heart failure, atrial fibrillation, and type-2 diabetes are all associated with T-tubule disruption, suggests that this is may be a common outcome triggered by hypertrophy. Interestingly, physiological hypertrophy resulting from training is not associated with T-tubule disruption [144]. This suggests that T-tubule disruption may result from activation of signaling pathways which are specifically involved in pathological hypertrophy.
6. Treatment/Future Perspectives
Based on what is now extensive evidence that altered T-tubular structure may contribute to the pathophysiology of several disease states, it is clear that T-tubules have the potential to serve as therapeutic targets. Preventing T-tubule loss and/or disorganization would be expected to improve Ca2+ release synchrony, leading to a more rapid rise of the Ca2+ transient and greater contractile power. To our knowledge, the only such intervention reported to date is exercise training. Stølen et al. [144] observed that db/db diabetic mice which underwent aerobic interval training avoided the T-tubule loss and Ca2+ release dyssynchrony present in sedentary mice. It is unknown which pathways activated by exercise training afforded this protection.
Development of more targeted treatment strategies will almost certainly rely on an improved understanding of the mechanisms underlying T-tubular maintenance as discussed above. Molecular targets may include components of the cytoskeleton and signaling molecules involved in triggering hypertrophy. Theoretically, promoting growth of new T-tubules could also be beneficial. For example, proliferation of longitudinal tubules, as has been observed in pathological conditions might provide greater opportunity for Ca2+ cycling across the sarcolemma, and also SR Ca2+ release if dyads form at these new tubules. Synchrony of Ca2+ release also might be increased by reducing the occurrence and size of delayed release regions by increasing the velocity at which Ca2+ propagates. Although the precise mechanisms controlling Ca2+ wave speed are only beginning to be elucidated, it appears that increasing SR Ca2+ content and β-adrenergic tone may promote greater Ca2+ release synchrony [77]. Altering T-tubule composition also has therapeutic potential. Strategies aimed at increasing expression of the L-type Ca2+ channel and NCX may be beneficial, as we have recently observed that enhanced transsarcolemmal Ca2+ cycling can remarkably compensate for impaired SR function [28, 122]. Finally, it may be possible to optimize the role of NCX as a Ca2+ release trigger in failing cardiomyocytes by precisely localizing other Na+ handling proteins in the dyad, or by direct molecular enhancement of NCX activity.
7. Summary
In this review, we have described the dyadic neighborhood established by the close proximity and functional coupling of the T-tubular and SR membranes. This neighborhood allows for tight local control of [Ca2+]. It includes Ca2+ channels and RyRs, as well as proteins involved with Na+ homeostasis and maintenance of dyadic integrity. We have summarized data indicating that T-tubule loss and/or disorganization occurs in heart failure, atrial fibrillation, and diabetic cardiomyopathy. Resulting alterations in the dyadic neighborhood are associated with reduced Ca2+ release synchrony and impaired efficiency of CICR. Other modifications in EC coupling proteins which may be shared between these disease states include greater RyR leak, decreased Ca2+ channel number, and increased single Ca2+ channel activity. While the mechanisms responsible for disruption of the dyadic neighborhood remain largely unknown, recent evidence indicates that anchoring of the dyad may be importantly compromised. We suggest that further investigation of these mechanisms will reveal novel therapeutic targets for improving EC coupling in pathophysiological conditions.
References
- 1.Brette F, Orchard C. Resurgence of cardiac T-tubule research. Physiology. 2007;22(3):167–173. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- 2.Forbes MS, Sperelakis N. The presence of transverse and axial tubules in the ventricular myocardium of embryonic and neonatal guinea pigs. Cell and Tissue Research. 1976;166(1):83–90. doi: 10.1007/BF00215127. [DOI] [PubMed] [Google Scholar]
- 3.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circulation Research. 1999;84(3):266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 5.Hayashi T, Martone ME, Yu Z, et al. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. Journal of Cell Science. 2009;122(7):1005–1013. doi: 10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pásek M, Brette F, Nelson A, et al. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Progress in Biophysics and Molecular Biology. 2008;96(1–3):244–257. doi: 10.1016/j.pbiomolbio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Mottino G, Klitzner TS, Philipson KD, Frank JS. Distribution of the Na+/Ca2+ exchange protein in developing rabbit myocytes. American Journal of Physiology. 1995;268(5):C1126–C1132. doi: 10.1152/ajpcell.1995.268.5.C1126. [DOI] [PubMed] [Google Scholar]
- 8.Haddock PS, Coetzee WA, Cho E, et al. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circulation Research. 1999;85(5):415–427. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- 9.Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. Journal of Molecular and Cellular Cardiology. 2010;48(2):379–386. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Maio A, Karko K, Snopko RM, Mejía-Alvarez R, Franzini-Armstrong C. T-tubule formation in cardiacmyocytes: two possible mechanisms? Journal of Muscle Research and Cell Motility. 2007;28(4-5):231–241. doi: 10.1007/s10974-007-9121-x. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM, Stiffel VM. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. American Journal of Physiology. 1993;264(6):C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Annals of the New York Academy of Sciences. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 13.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophysical Journal. 1999;77(3):1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soeller C, Crossman D, Gilbert R, Cannell MB. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(38):14958–14963. doi: 10.1073/pnas.0703016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabiato A. Calcium induced release of calcium from the sarcoplasmic-reticulum of skinned fibers from the frog semitendinosus. Biophysical Journal. 1985;47(2):p. A195. [Google Scholar]
- 16.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 17.Niggli E, Shirokova N. A guide to sparkology: the taxonomy of elementary cellular Ca2+ signaling events. Cell Calcium. 2007;42(4-5):379–387. doi: 10.1016/j.ceca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H, Lederer WJ. Calcium sparks. Physiological Reviews. 2008;88(4):1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 19.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophysical Journal. 2000;78(1):334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassani RA, Bassani JWM, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. Journal of Physiology. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Despa S, Brette F, Orchard CH, Bers DM. Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophysical Journal. 2003;85(5):3388–3396. doi: 10.1016/S0006-3495(03)74758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negretti N, O’Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxations in rat ventricular myocytes. Cardiovascular Research. 1993;27(10):1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- 23.Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. Journal of Physiology. 1994;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+/K+-ATPase α2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovascular Research. 2007;75(1):109–117. doi: 10.1016/j.cardiores.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase α1 and α2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovascular Research. 2007;73(1):92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Despa S, Bers DM. Functional analysis of Na+/K+-ATPase isoform distribution in rat ventricular myocytes. American Journal of Physiology. 2007;293(1):C321–C327. doi: 10.1152/ajpcell.00597.2006. [DOI] [PubMed] [Google Scholar]
- 27.James PF, Grupp IL, Grupp G, et al. Identification of a specific role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Molecular Cell. 1999;3(5):555–563. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 28.Louch WE, Hougen K, Mørk HK, et al. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. Journal of Physiology. 2010;588(3):465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology. 2006;21(6):380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 30.Lines GT, Sande JB, Louch WE, Mørk HK, Grøttum P, Sejersted OM. Contribution of the Na+/Ca2+ exchanger to rapid Ca2+ release in cardiomyocytes. Biophysical Journal. 2006;91(3):779–792. doi: 10.1529/biophysj.105.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum: a comparison between L-type Ca2+ current and reverse-mode Na+-Ca2+ exchange. Circulation Research. 1997;81(6):1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- 32.Scriven DRL, Dan P, Moore EDW. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophysical Journal. 2000;79(5):2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Maio A, Ter Keurs HE, Franzini-Armstrong C. T-tubule profiles in Purkinje fibres of mammalian myocardium. Journal of Muscle Research and Cell Motility. 2007;28(2-3):115–121. doi: 10.1007/s10974-007-9109-6. [DOI] [PubMed] [Google Scholar]
- 34.Woo S-H, Cleemann L, Morad M. Diversity of atrial local Ca2+ signalling: evidence from 2-D confocal imaging in Ca2+-buffered rat atrial myocytes. Journal of Physiology. 2005;567(3):905–921. doi: 10.1113/jphysiol.2005.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forssmann WG, Girardier L. A study of the T system in rat heart. Journal of Cell Biology. 1970;44(1):1–19. doi: 10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayettey AS, Navaratnam V. The T-tubule system in the specialized and general myocardium of the rat. Journal of Anatomy. 1978;127, part 1:125–140. [PMC free article] [PubMed] [Google Scholar]
- 37.Kirk MM, Izu LT, Chen-Izu Y, et al. Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. Journal of Physiology. 2003;547(2):441–451. doi: 10.1113/jphysiol.2002.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotoh T. Quantitative studies on the ultrastructural differentiation and growth of mammalian cardiac muscle cells. The atria and ventricles of the cat. Acta Anatomica. 1983;115(2):168–177. doi: 10.1159/000145687. [DOI] [PubMed] [Google Scholar]
- 39.Tidball JG, Cederdahl JE, Bers DM. Quantitative analysis of regional variability in the distribution of transverse tubules in rabbit myocardium. Cell and Tissue Research. 1991;264(2):293–298. doi: 10.1007/BF00313966. [DOI] [PubMed] [Google Scholar]
- 40.Dibb KM, Clarke JD, Horn MA, et al. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circulation: Heart Failure. 2009;2(5):482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 41.Lenaerts I, Bito V, Heinzel FR, et al. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circulation Research. 2009;105(9):876–885. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- 42.Dolber PC, Bauman RP, Rembert JC, Greenfield JC., Jr. Regional changes in myocyte structure in model of canine right atrial hypertrophy. American Journal of Physiology. 1994;267(4):H1279–H1287. doi: 10.1152/ajpheart.1994.267.4.H1279. [DOI] [PubMed] [Google Scholar]
- 43.Heinzel FR, Bito V, Biesmans L, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circulation Research. 2008;102(3):338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 44.Louch WE, Mørk HK, Sexton J, et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. Journal of Physiology. 2006;574(2):519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swift F, Birkeland JAK, Tovsrud N, et al. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase α2-isoform in heart failure. Cardiovascular Research. 2008;78(1):71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 46.Lyon AR, MacLeod KT, Zhang Y, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song L-S, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinzel FR, Bito V, Volders PGA, Antoons G, Mubagwa K, Sipido KR. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circulation Research. 2002;91(11):1023–1030. doi: 10.1161/01.res.0000045940.67060.dd. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation-contraction coupling in cat atrial myocytes. Journal of Physiology. 2003;546(1):119–136. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. Journal of Cell Science. 2006;119(19):3915–3925. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- 51.Meethal SV, Potter KT, Redon D, et al. Structure-function relationships of Ca spark activity in normal and failing cardiac myocytes as revealed by flash photography. Cell Calcium. 2007;41(2):123–134. doi: 10.1016/j.ceca.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Brette F, Rodriguez P, Komukai K, Colyer J, Orchard CH. β-adrenergic stimulation restores the Ca transient of ventricular myocytes lacking t-tubules. Journal of Molecular and Cellular Cardiology. 2004;36(2):265–275. doi: 10.1016/j.yjmcc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Louch WE, Bito V, Heinzel FR, et al. Reduced synchrony of Ca2+ release with loss of T-tubules—a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovascular Research. 2004;62(1):63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 54.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Archives of Internal Medicine. 2008;168(4):418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 55.Piacentino V, III, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circulation Research. 2003;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 56.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circulation Research. 1999;85(1):38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 57.Gómez AM, Valdivia HH, Cheng H, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276(5313):800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 58.Sjaastad I, Bøkenes J, Swift F, Wasserstrom JA, Sejersted OM. Normal contractions triggered by ICa,L in ventricular myocytes from rats with postinfarction CHF. American Journal of Physiology. 2002;283(3):H1225–H1236. doi: 10.1152/ajpheart.00162.2001. [DOI] [PubMed] [Google Scholar]
- 59.Sjaastad I, Birkeland JA, Ferrier G, et al. Defective excitation-contraction coupling in hearts of rats with congestive heart failure. Acta Physiologica Scandinavica. 2005;184(1):45–58. doi: 10.1111/j.1365-201X.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 60.Xu M, Zhou P, Xu SM, et al. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biology. 2007;5(2, article e21) doi: 10.1371/journal.pbio.0050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vinereanu D, Lim PO, Frenneaux MP, Fraser AG. Reduced myocardial velocities of left ventricular long-axis contraction identify both systolic and diastolic heart failure—a comparison with brain natriuretic peptide. European Journal of Heart Failure. 2005;7(4):512–519. doi: 10.1016/j.ejheart.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Bøkenes J, Aronsen JM, Birkeland JA, et al. Slow contractions characterize failing rat hearts. Basic Research in Cardiology. 2008;103(4):328–344. doi: 10.1007/s00395-008-0719-y. [DOI] [PubMed] [Google Scholar]
- 63.Yoneyama A, Koyama J, Tomita T, et al. Relationship of plasma brain-type natriuretic peptide levels to left ventricular longitudinal function in patients with congestive heart failure assessed by strain Doppler imaging. International Journal of Cardiology. 2008;130(1):56–63. doi: 10.1016/j.ijcard.2007.07.171. [DOI] [PubMed] [Google Scholar]
- 64.Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation. 1995;92(9):2540–2549. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- 65.Mørk HK, Sjaastad I, Sejersted OM, Louch WE. Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in postinfarction mice. American Journal of Physiology. 2009;296(4):H1069–H1079. doi: 10.1152/ajpheart.01009.2008. [DOI] [PubMed] [Google Scholar]
- 66.del Monte F, O’Gara P, Poole-Wilson PA, Yacoub M, Harding SE. Cell geometry and contractile abnormalities of myocytes from failing human left ventricle. Cardiovascular Research. 1995;30(2):281–290. [PubMed] [Google Scholar]
- 67.Harding SE, Davies CH, Wynne DG, Poole-Wilson PA. Contractile function and response to agonists in myocytes from failing human heart. European Heart Journal. 1994;15:35–36. doi: 10.1093/eurheartj/15.suppl_d.35. [DOI] [PubMed] [Google Scholar]
- 68.Sen L, Cui G, Fonarow GC, Laks H. Differences in mechanisms of SR dysfunction in ischemic vs. idiopathic dilated cardiomyopathy. American Journal of Physiology. 2000;279(2):H709–H718. doi: 10.1152/ajpheart.2000.279.2.H709. [DOI] [PubMed] [Google Scholar]
- 69.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 70.He J-Q, Conklin MW, Foell JD, et al. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovascular Research. 2001;49(2):298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 71.Balijepalli RC, Lokuta AJ, Maertz NA, et al. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovascular Research. 2003;59(1):67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 72.Quinn FR, Currie S, Duncan AM, et al. Myocardial infarction causes increased expression but decreased activity of the myocardial Na+-Ca2+ exchanger in the rabbit. Journal of Physiology. 2003;553(1):229–242. doi: 10.1113/jphysiol.2003.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kostin S, Scholz D, Shimada T, et al. The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell and Tissue Research. 1998;294(3):449–460. doi: 10.1007/s004410051196. [DOI] [PubMed] [Google Scholar]
- 74.Cannell MB, Crossman DJ, Soeller C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. Journal of Muscle Research and Cell Motility. 2006;27(5–7):297–306. doi: 10.1007/s10974-006-9089-y. [DOI] [PubMed] [Google Scholar]
- 75.Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101(22):2586–2594. doi: 10.1161/01.cir.101.22.2586. [DOI] [PubMed] [Google Scholar]
- 76.Lipp P, Hüser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. Journal of Physiology. 1996;497(3):589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brette F, Despa S, Bers DM, Orchard CH. Spatiotemporal characteristics of SR Ca2+ uptake and release in detubulated rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2005;39(5):804–812. doi: 10.1016/j.yjmcc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+-Ca2+ exchange activity is localized in the t-tubules of rat ventricular myocytes. Circulation Research. 2002;91(4):315–322. doi: 10.1161/01.res.0000030180.06028.23. [DOI] [PubMed] [Google Scholar]
- 79.Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. Journal of Physiology. 1996;494(3):641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JHB. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. Journal of Physiology. 2001;531(2):301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Litwin SE, Zhang D, Bridge JHB. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circulation Research. 2000;87(11):1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 82.Gómez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction altered excitation-contraction coupling. Circulation. 2001;104(6):688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 83.Harris DM, Mills GD, Chen X, et al. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circulation Research. 2005;96(5):543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 84.Sah R, Ramirez RJ, Oudit GY, et al. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito) Journal of Physiology. 2003;546(1):5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schröder F, Handrock R, Beuckelmann DJ, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98(10):969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 86.Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circulation Research. 2002;91(6):517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 87.Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovascular Research. 2008;77(2):315–324. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- 88.Brette F, Sallé L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circulation Research. 2004;95(1):e1–e7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
- 89.Inoue M, Bridge JHB. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: involvement of clusters of L-type Ca2+ channels. Circulation Research. 2003;92(5):532–538. doi: 10.1161/01.RES.0000064175.70693.EC. [DOI] [PubMed] [Google Scholar]
- 90.Inoue M, Bridge JHB. Variability in couplon size in rabbit ventricular myocytes. Biophysical Journal. 2005;89(5):3102–3110. doi: 10.1529/biophysj.105.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103(11):1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 92.Altamirano J, Bers DM. Effect of intracellular Ca2+ and action potential duration on L-type Ca2+ channel inactivation and recovery from inactivation in rabbit cardiac myocytes. American Journal of Physiology. 2007;293(1):H563–H573. doi: 10.1152/ajpheart.00469.2006. [DOI] [PubMed] [Google Scholar]
- 93.Sipido KR, Stankovicova T, Flameng W, Vanhaecke J, Verdonck F. Frequency dependence of Ca2+ release from the sarcoplasmic reticulum in human ventricular myocytes from end-stage heart failure. Cardiovascular Research. 1998;37(2):478–488. doi: 10.1016/s0008-6363(97)00280-0. [DOI] [PubMed] [Google Scholar]
- 94.Li G-R, Yang B, Feng J, Bosch RF, Carrier M, Nattel S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. American Journal of Physiology. 1999;276(1):H98–H106. doi: 10.1152/ajpheart.1999.276.1.H98. [DOI] [PubMed] [Google Scholar]
- 95.Richard S, Perrier E, Fauconnier J, et al. ‘Ca2+-induced Ca2+ entry’ or how the L-type Ca2+ channel remodels its own signalling pathway in cardiac cells. Progress in Biophysics and Molecular Biology. 2006;90(1–3):118–135. doi: 10.1016/j.pbiomolbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nature Cell Biology. 2000;2(3):173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 97.Zhang R, Khoo MSC, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nature Medicine. 2005;11(4):409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 98.Piot C, Lemaire S, Albat B, Seguin J, Nargeot J, Richard S. High frequency-induced upregulation of human cardiac calcium currents. Circulation. 1996;93(1):120–128. doi: 10.1161/01.cir.93.1.120. [DOI] [PubMed] [Google Scholar]
- 99.Barrère-Lemaire S, Piot C, Leclercq F, Nargeot J, Richard S. Facilitation of L-type calcium currents by diastolic depolarization in cardiac cells: impairment in heart failure. Cardiovascular Research. 2000;47(2):336–349. doi: 10.1016/s0008-6363(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 100.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circulation Research. 2003;93(7):592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 102.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circulation Research. 2001;88(11):1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 103.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circulation Research. 2002;91(11):1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 104.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. Journal of Biological Chemistry. 2003;278(51):51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 105.Xiao B, Jiang MT, Zhao M, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circulation Research. 2005;96(8):847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 106.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circulation Research. 2005;97(12):1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 107.Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Annals of the New York Academy of Sciences. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- 108.Fowler MR, Dobson RS, Orchard CH, Harrison SM. Functional consequences of detubulation of isolated rat ventricular myocytes. Cardiovascular Research. 2004;62(3):529–537. doi: 10.1016/j.cardiores.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 109.Pieske B, Maier LS, Piacentino V, III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106(4):447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 110.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105(21):2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 111.Baartscheer A, Schumacher CA, van Borren MMGJ, Belterman CNW, Coronel R, Fiolet JWT. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovascular Research. 2003;57(4):1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- 112.Valdivia CR, Chu WW, Pu J, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. Journal of Molecular and Cellular Cardiology. 2005;38(3):475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 113.Sossalla S, Wagner S, Rasenack ECL, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. Journal of Molecular and Cellular Cardiology. 2008;45(1):32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 114.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. European Journal of Heart Failure. 2007;9(3):219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baartscheer A, Schumacher CA, van Borren MMGJ, et al. Chronic inhibition of Na+/H+-exchanger attenuates cardiac hypertrophy and prevents cellular remodeling in heart failure. Cardiovascular Research. 2005;65(1):83–92. doi: 10.1016/j.cardiores.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 116.Ono K, Iijima T. Cardiac T-type Ca2+ channels in the heart. Journal of Molecular and Cellular Cardiology. 2010;48(1):65–70. doi: 10.1016/j.yjmcc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 117.Martínez ML, Heredia MP, Delgado C. Expression of T-type Ca2+ channels in ventricular cells from hypertrophied rat hearts. Journal of Molecular and Cellular Cardiology. 1999;31(9):1617–1625. doi: 10.1006/jmcc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 118.Jaleel N, Nakayama H, Chen X, et al. Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circulation Research. 2008;103(10):1109–1119. doi: 10.1161/CIRCRESAHA.108.185611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiang C-S, Huang C-H, Chieng H, et al. The CaV3.2 T-type Ca2+ channel is required for pressure overload-induced cardiac hypertrophy in mice. Circulation Research. 2009;104(4):522–530. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- 120.Kuwahara K, Saito Y, Takano M, et al. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. The EMBO Journal. 2003;22(23):6310–6321. doi: 10.1093/emboj/cdg601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reuter H, Henderson SA, Han T, et al. Cardiac excitation-contraction coupling in the absence of Na+ -Ca2+ exchange. Cell Calcium. 2003;34(1):19–26. doi: 10.1016/s0143-4160(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 122.Andersson KB, Birkeland JAK, Finsen AV, et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. Journal of Molecular and Cellular Cardiology. 2009;47(2):180–187. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 123.Mackiewicz U, Maczewski M, Konior A, et al. Sarcolemmal Ca2+-ATPase ability to transport Ca2+ gradually diminishes after myocardial infarction in the rat. Cardiovascular Research. 2009;81(3):546–554. doi: 10.1093/cvr/cvn285. [DOI] [PubMed] [Google Scholar]
- 124.Hammes A, Oberdorf-Maass S, Rother T, et al. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circulation Research. 1998;83(9):877–888. doi: 10.1161/01.res.83.9.877. [DOI] [PubMed] [Google Scholar]
- 125.Wu X, Chang B, Blair NS, et al. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. Journal of Clinical Investigation. 2009;119(4):976–985. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moschella MC, Marks AR. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. Journal of Cell Biology. 1993;120(5):1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harzheim D, Movassagh M, Foo RS-Y, et al. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11406–11411. doi: 10.1073/pnas.0905485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. Journal of Physiology. 2004;555(3):607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biology. 2005;3(12, article e423) doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alpert JS, Petersen P, Godtfredsen J. Atrial fibrillation: natural history, complications, and management. Annual Review of Medicine. 1988;39:41–52. doi: 10.1146/annurev.me.39.020188.000353. [DOI] [PubMed] [Google Scholar]
- 131.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 132.Schotten U, Ausma J, Stellbrink C, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation. 2001;103(5):691–698. doi: 10.1161/01.cir.103.5.691. [DOI] [PubMed] [Google Scholar]
- 133.Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circulation Research. 1999;84(7):776–784. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- 134.Christ T, Boknik P, Wöhrl S, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110(17):2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 135.Carnes CA, Janssen PML, Ruehr ML, et al. Atrial glutathione content, calcium current, and contractility. Journal of Biological Chemistry. 2007;282(38):28063–28073. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 136.Greiser M, Halaszovich CR, Frechen D, et al. Pharmacological evidence for altered src kinase regulation of ICa,L in patients with chronic atrial fibrillation. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2007;375(6):383–392. doi: 10.1007/s00210-007-0174-6. [DOI] [PubMed] [Google Scholar]
- 137.Klein G, Schröder F, Vogler D, et al. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation: role of phosphatase 2A. Cardiovascular Research. 2003;59(1):37–45. doi: 10.1016/s0008-6363(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 138.Sun H, Gaspo R, Leblanc N, Nattel S. Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation. 1998;98(7):719–727. doi: 10.1161/01.cir.98.7.719. [DOI] [PubMed] [Google Scholar]
- 139.de Tombe PP. Altered contractile function in heart failure. Cardiovascular Research. 1998;37(2):367–380. doi: 10.1016/s0008-6363(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 140.Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. Journal of Cardiac Failure. 2008;14(7):615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 141.Pereira L, Matthes J, Schuster I, et al. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55(3):608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 142.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53(12):3201–3208. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 143.Kralik PM, Ye G, Metreveli NS, Shem X, Epstein PN. Cardiomyocyte dysfunction in models of type 1 and type 2 diabetes. Cardiovascular Toxicology. 2005;5(3):285–292. doi: 10.1385/ct:5:3:285. [DOI] [PubMed] [Google Scholar]
- 144.Stølen TO, Høydal MA, Kemi OJ, et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circulation Research. 2009;105(6):527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 145.McGrath KF, Yuki A, Manaka Y, Tamaki H, Saito K, Takekura H. Morphological characteristics of cardiac calcium release units in animals with metabolic and circulatory disorders. Journal of Muscle Research and Cell Motility. 2009;30(5-6):225–231. doi: 10.1007/s10974-009-9191-z. [DOI] [PubMed] [Google Scholar]
- 146.Shao C-H, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. Journal of Molecular and Cellular Cardiology. 2007;42(1):234–246. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 147.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. Journal of Cell Biology. 1997;136(1):137–154. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sedarat F, Xu L, Moore EDW, Tibbits GF. Colocalization of dihydropyridine and ryanodine receptors in neonate rabbit heart using confocal microscopy. American Journal of Physiology. 2000;279(1):H202–H209. doi: 10.1152/ajpheart.2000.279.1.H202. [DOI] [PubMed] [Google Scholar]
- 149.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Molecular Cell. 2000;6(1):11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 150.Minamisawa S, Oshikawa J, Takeshima H, et al. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochemical and Biophysical Research Communications. 2004;325(3):852–856. doi: 10.1016/j.bbrc.2004.10.107. [DOI] [PubMed] [Google Scholar]
- 151.Landstrom AP, Weisleder N, Batalden KB, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. Journal of Molecular and Cellular Cardiology. 2007;42(6):1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matsushita Y, Furukawa T, Kasanuki H, et al. Mutation of junctophilin type 2 associated with hypertrophic cardiomyopathy. Journal of Human Genetics. 2007;52(6):543–548. doi: 10.1007/s10038-007-0149-y. [DOI] [PubMed] [Google Scholar]
- 153.Chopra N, Yang T, Asghari P, et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Butler MH, David C, Ochoa G-C, et al. Amphiphysin II (SH3p9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. Journal of Cell Biology. 1997;137(6):1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee E, Marcucci M, Daniell L, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297(5584):1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 156.Razzaq A, Robinson IM, McMahon HT, et al. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes and Development. 2001;15(22):2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature Reviews Neuroscience. 2000;1(3):161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 158.Sharov VG, Kostin S, Todor A, Schaper J, Sabbah HN. Expression of cytoskeletal, linkage and extracellular proteins in failing dog myocardium. Heart Failure Reviews. 2005;10(4):297–303. doi: 10.1007/s10741-005-7544-2. [DOI] [PubMed] [Google Scholar]
- 159.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38(5):515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 160.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 161.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]