Abstract
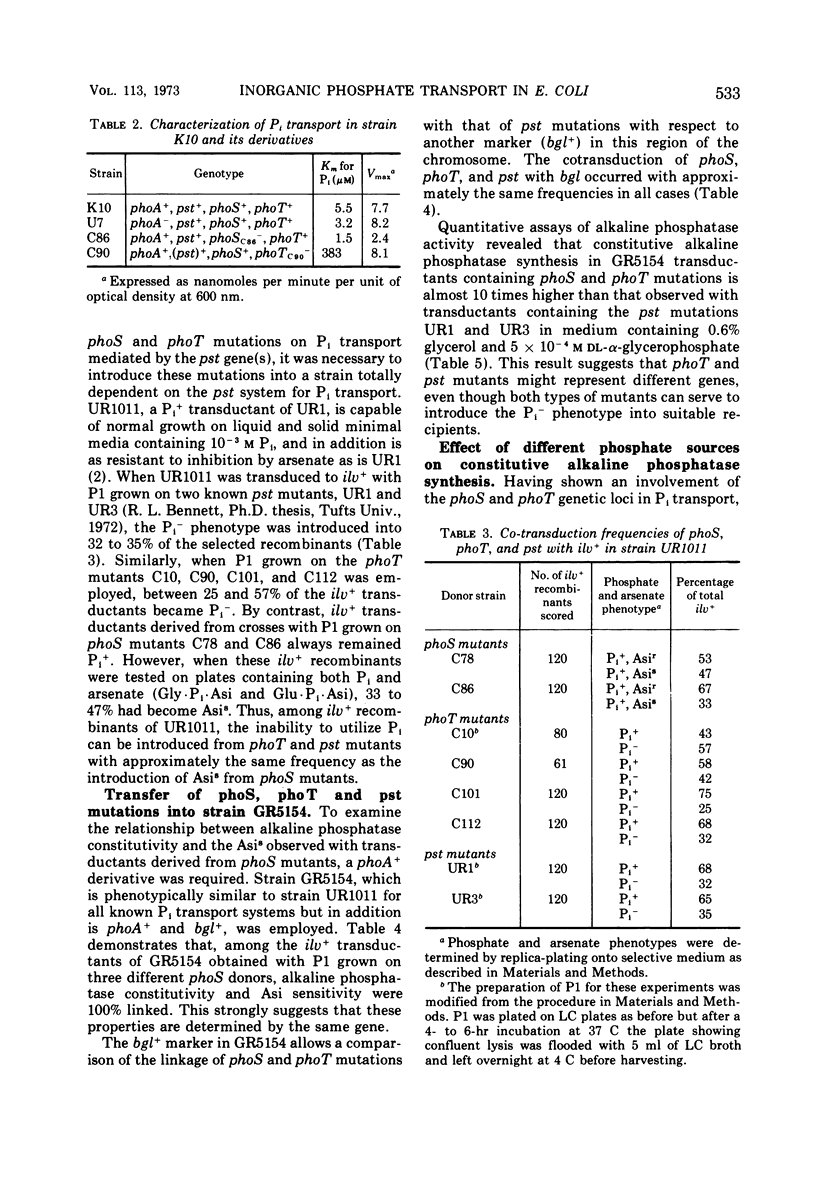
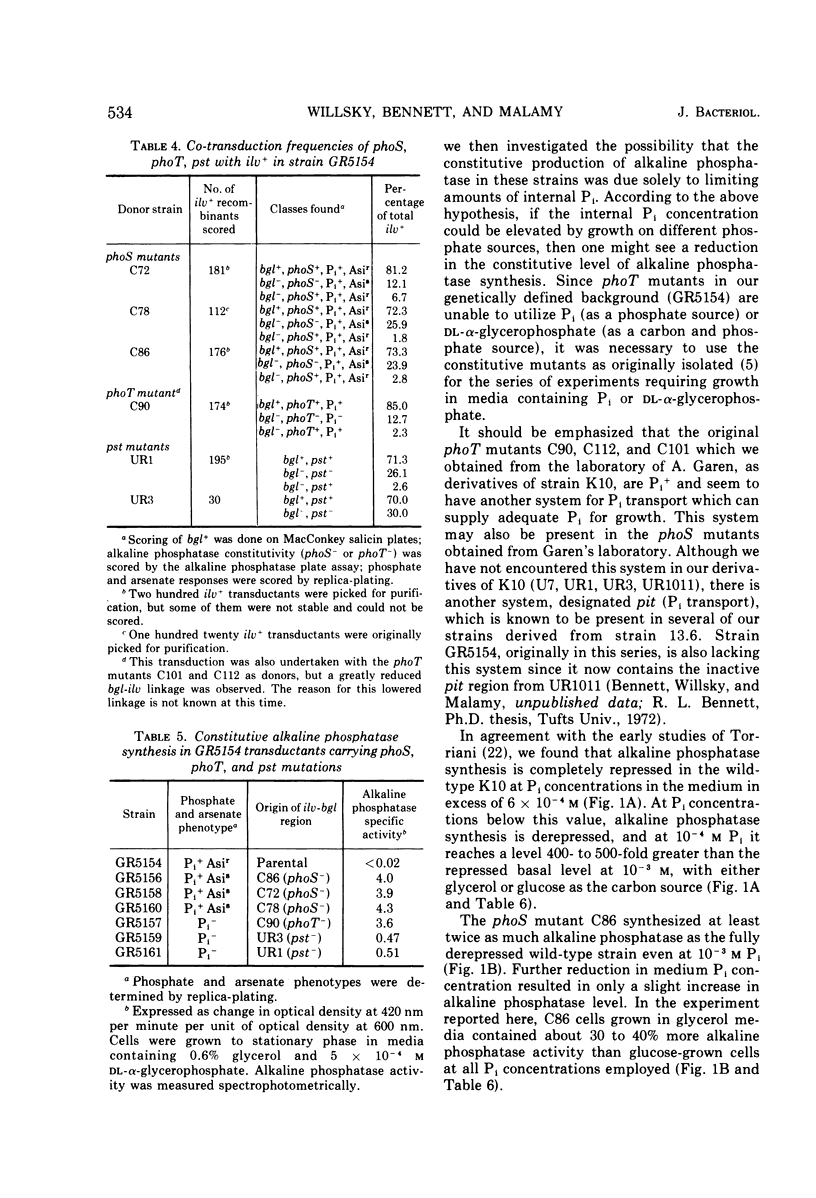
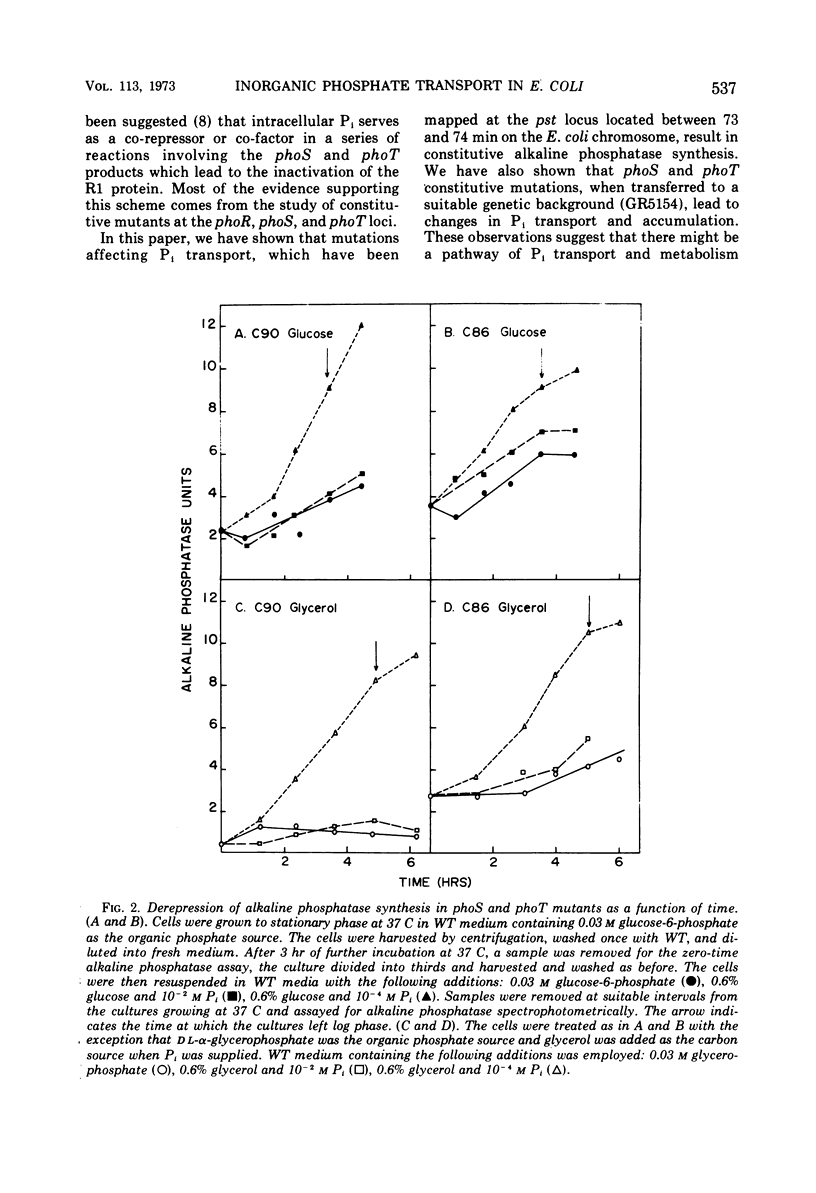
Two classes of alkaline phosphatase constitutive mutations which comprise the original phoS locus (genes phoS and phoT) on the Escherichia coli genome have been implicated in the regulation of alkaline phosphatase synthesis. When these mutations were introduced into a strain dependent on a single system, the pst system, for inorganic phosphate (Pi) transport, profound changes in Pi transport were observed. The phoT mutations led to a complete Pi− phenotype in this background, and no activity of the pst system could be detected. The introduction of the phoS mutations changed the specificity of the pst system so that arsenate became growth inhibitory. Changes in the phosphate source led to changes in the levels of constitutive alkaline phosphatase synthesis found in phoS and phoT mutants. When glucose-6-phosphate or l-α-glycerophosphate was supplied as the sole source of phosphate, phoT mutants showed a 3- to 15- fold reduction in constitutive alkaline phosphatase synthesis when compared to the maximal levels found in limiting Pi media. However, these levels were still 100 times greater than the basal level of alkaline phosphatase synthesized in wild-type strains under these conditions. The phoS mutants showed only a two- to threefold reduction when grown with organic phosphate sources. The properties of the phoT mutants selected on the basis of constitutive alkaline phosphatase synthesis were similar in many respects to those of pst mutants selected for resistance to growth inhibition caused by arsenate. It is suggested that the phoS and phoT genes are primarily involved in Pi transport and, as a result of this function, play a role in the regulation of alkaline phosphatase synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono H., Otsuji N. Genetic mapping of regulator gene phoS for alkaline phosphatase in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1182–1183. doi: 10.1128/jb.95.3.1182-1183.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. L., Malamy M. H. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970 Jul 27;40(2):496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Jones T. C. Genetic control of basal level of alkaline phosphatase in Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):91–100. doi: 10.1007/BF00445678. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of hexose phosphate uptake by Escherichia coli. Nature. 1969 Dec 27;224(5226):1261–1262. doi: 10.1038/2241261a0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971 Aug 13;241(2):494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- TORRIANI A., ROTHMAN F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961 May;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A. S. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):616–623. doi: 10.1128/jb.110.2.616-623.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


