Summary
Background
Validated outcome measures in dermatology help standardize and improve patient care. A scoring system of skin disease severity in dermatomyositis known as the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) has been developed.
Objectives
To simplify and improve the tool for clinical research and care, we modified the CDASI and validated the new version, v2.
Methods
The original CDASI has four activity and two damage measures. The modified CDASI has three activity and two damage measures. The skin disease of 20 patients with dermatomyositis was evaluated by the same dermatologist using both the original and the modified CDASI. Global validation measures were implemented to assess overall skin disease state, skin disease activity and skin damage. Spearman's rho (rsp), adjusted for multiple observations on subjects, was used to determine the relationship between the two versions of the CDASI and their correlation with the physician global measures (PGMs).
Results
The total score and activity and damage subscores of the original and the modified CDASI correlated perfectly with each other (rsp = 0.99, 1.00, 1.00). The PGM-overall skin scale correlated with the total scores (rsp = 0.72, rsp = 0.76) and activity subscores (rsp = 0.68, rsp = 0.63) but not with the damage subscores (rsp = 0.14, rsp = 0.15) of the original and the modified CDASI, respectively. However, the PGM-activity and PGM-damage scales correlated with the activity (rsp = 0.76, rsp = 0.75) and damage subscores (rsp = 0.90, rsp = 0.90), respectively, of the original and the modified CDASI.
Conclusions
The modified CDASI is perfectly correlated with the original CDASI. It has equally good concurrent validity with the PGM-overall skin and PGM-activity scales. The CDASI subscores have equally good concurrent validity with the PGM-activity and PGM-damage scales. We suggest that PGMs of skin disease activity and damage should be assessed separately for greater specificity. The modified CDASI is a refined and equally as useful outcome measure.
Keywords: dermatomyositis, outcome measures
Validated outcome measures play important roles in clinical practice and trials. In the field of dermatology, cutaneous scoring systems help to standardize disease management and to assess therapeutic response.1 These validated tools also allow new therapies for various diseases to be studied systematically, as well as aiding in studies of disease natural history and development of correlative biomarkers. Such outcome measures for the autoimmune dermatoses, specifically cutaneous lupus erythematosus (CLE) and dermatomyositis (DM), have recently been developed for the purpose of clinical trials.2,3
Gaines and Werth emphasize that the design process is equally as important as the availability of cutaneous scoring systems.4 According to Singer et al., the ideal clinical outcome measure is credible, comprehensive, sensitive to change, accurate, biologically sensible and feasible.5 The elements used to measure disease activity must be well established and responsive to changes in disease severity over time. The tool must be easy to use, especially in a busy clinical setting. Once developed, outcome measures need to demonstrate validity and reliability. The Cutaneous Lupus Disease Area and Severity Index (CLASI), an outcome instrument for CLE, exemplifies the process of developing a thorough but not burdensome tool. Albrecht et al. first presented the CLASI in 2005 and three studies have subsequently shown the tool to be valid and reliable, and also to demonstrate clinical responsiveness and reliability across two subspecialties, dermatology and rheumatology.3,6,7
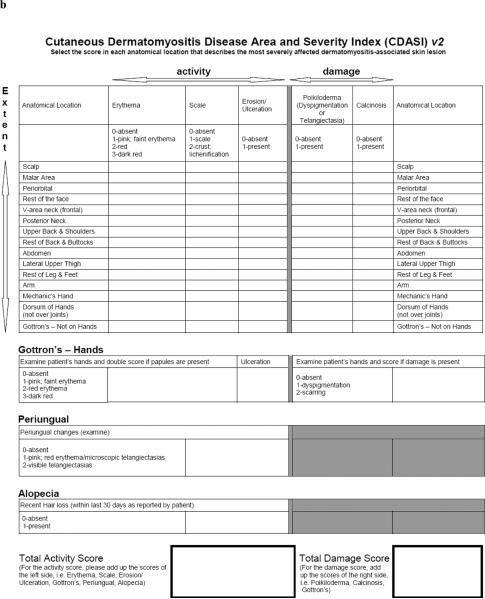
Leading academic dermatologists and rheumatologists with an expertise in DM have similarly developed a one-page outcome measure for the cutaneous manifestations of DM, the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI). The CDASI has four activity (erythema, scale, excoriation, ulceration) and two damage (poikiloderma, calcinosis) measures over 15 anatomical sites and measures separately three specific areas: hands, periungual and alopecia. A total score ranges from 0 to 148 with an activity subscore ranging from 0 to 116 and a damage subscore ranging from 0 to 32. A recent study demonstrated the reliability and partial validity of the CDASI.2 Inter-rater reliability (as measured by the intraclass correlation coefficient) was excellent: 0.84 for the activity subscore [95% confidence interval (CI) 0.75–0.95], 0.53 for the damage subscore (95% CI 0.32–0.73) and 0.83 for the total score (95% CI 0.72–0.94). Similarly, test–retest intrarater reliability was excellent: r = 0.86 for the activity subscore (95% CI 0.73–0.99), r = 0.87 for the damage subscore (95% CI 0.74–0.99) and r = 0.89 for the total score (95% CI 0.77–1.0). Concurrent validation with the physician global measure (PGM) overall skin scale revealed statistically significant correlations: Spearman's rho rsp = 0.77 for the activity subscore, rsp = 0.40 for the damage subscore and rsp = 0.76 for the total score. However, since that analysis, minor changes have been made to the original CDASI in order to improve the tool for clinical use (Fig. 1a). Here we seek to validate the modified version of the CDASI, v2 (Fig. 1b).
Fig 1.
(a) Original Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI): changes were made to circled elements. (b) Modified CDASI, v2.
Materials and methods
In addition to simplifying and removing any redundancy in the tool, the following changes were made to the original CDASI to represent inflammatory activity more accurately: (i) activity measure `excoriation' removed; (ii) the word `erosion' added to the `ulceration' activity measure; (iii) the word `purple' removed from scoring system of `erythema' activity measure and `Gottron's – Hands' activity measure; (iv) scoring system of the `Gottron's – Hands' damage measure and `periungual' modified. The total score of the modified CDASI ranges from 0 to 132 and the activity and damage subscores range from 0 to 100 and 0 to 32, respectively.
This study was approved by the University of Pennsylvania Institutional Review Board. All patients gave written informed consent before inclusion in the study. The skin disease of 20 patients with DM was evaluated by the same dermatologist at each of their clinic visits between July and November 2008 using both the original and the modified CDASI. Using a 10-cm visual analogue scale (VAS), PGMs were implemented to assess overall skin disease state, skin disease activity and skin damage. Twenty patients were evaluated with the original CDASI for a total of 31 observations and with the modified CDASI for a total of 30 observations. During the study period, two patients were evaluated on three separate visits, seven patients on two separate visits, and 11 patients on one visit.
To assess concurrent validity, rsp was used: (i) to determine the correlation between the total score, activity subscore and damage subscore from both the original and modified CDASI; and (ii) to determine the correlation between the CDASI total and activity and damage subscores with the PGM measures. To estimate rsp while adjusting for multiple observations, GEE analysis (adjusted for repeated measures and employing an Exchangeable working correlation structure) was conducted on the standardized rank scores.
Results
Distribution of scores
Distribution of the total scores from the original and modified CDASI was almost identical: they ranged from 4 to 36 and from 4 to 34, the median was 23, and the middle 50% of scores was between 10 and 31 and between 11 and 30, respectively. Distribution of the activity subscores from the original and modified CDASI was also almost identical: the ranges were positively skewed from 2 to 34 and from 2 to 33, the medians were 17 and 18, and the middle 50% of scores was between 8 and 29 and between 8 and 27, respectively. In the current sample, distribution of the damage subscores was identical: the ranges were positively skewed from 0 to 9 and 40% of the scores were zero.
The PGM-overall skin scale ranged from 0 to 5 cm with the middle 50% between 1 and 5 cm and a median of 2 cm. The PGM-activity scale ranged from 0 to 5 cm with the middle 50% between 0.8 and 4 cm and a median of 1.6 cm. The PGM-damage scale ranged from 0 to 7 cm and 57% of the measurements were zero.
Validity
The correlation of the total, activity and damage scores between the original and the modified CDASI were rsp = 0.99, rsp = 1.00, and rsp = 1.00, respectively. Comparing the PGM-overall skin with the scores of the original and the modified CDASI, rsp = 0.72 (95% CI 0.49–0.94) and 0.76 (95% CI 0.54–0.98) for the total score, rsp = 0.68 (95% CI 0.43–0.92) and 0.63 (95% CI 0.33–0.92) for the activity subscore, and rsp = 0.14 (95% CI −0.11–0.38) and 0.15 (95% CI −0.11–0.41) for the damage subscore, respectively (Table 1). However, comparing the PGM-activity with the activity subscore of the original and the modified CDASI, the rsp values were 0.76 (95% CI 0.53–0.98) and 0.75 (95% CI 0.51–0.98), respectively. Similarly, comparing the PGM-damage scale with the damage subscore of each version, the rsp values were the same, 0.90 (95% CI 0.78–1.02).
Table 1.
Comparison of the original (v1) and modified (v2) Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) with the physician global measures (PGMs)
| CDASI | n | Mean ± SD | Median (range) | PGM-overall skin rsp (95% CI) | PGM-skin activity rsp (95% CI) | PGM-skin damage rsp (95% CI) |
|---|---|---|---|---|---|---|
| Activity score v1 (range 0–116) | 31 | 18.23 ± 10.60 | 17 (2–34) | 0.68 (0.43–0.92) | 0.76 (0.53–0.98) | |
| Activity score v2 (range 0–100) | 30 | 17.83 ± 10.14 | 18 (2–33) | 0.63 (0.33–0.92) | 0.75 (0.51–0.98) | |
| Damage score v1 (range 0–32) | 31 | 2.16 ± 2.66 | 1 (0–9) | 0.14 (−0.11–0.38) | 0.90 (0.78–1.02) | |
| Damage score v2 (range 0–32) | 30 | 2.27 ± 2.73 | 1 (0–9) | 0.15 (−0.11–0.41) | 0.90 (0.78–1.02) | |
| Total score v1 (range 0–148) | 31 | 20.39 ± 10.78 | 23 (4–36) | 0.72 (0.49–0.94) | ||
| Total score v2 (range 0–132) | 30 | 20.10 ± 10.25 | 23 (4–34) | 0.76 (0.54–0.98) |
n, number of patients; rsp, Spearman rank order correlation coefficient; CI, confidence interval.
Discussion
Successful outcome measures need to be tested and, if possible, improved with revision.4 Chren comments that they must be feasible to use in a clinical or research setting and be `user friendly – easy to understand, clear and unambiguous and generally not overly long'.8 Following the use of the original CDASI in a clinical setting with the appropriate patient population, it was recognized that further changes were necessary to improve the tool. Specifically, the `excoriation' column was removed because it is an indirect measure of itch, which is best directly measured on a patient-derived VAS. The word `erosion' was added to the `ulceration' column to a capture a greater spectrum of the disease. The word `purple' was removed from the scoring system because we did not believe it is synonymous with dark red, or the highest level of inflammatory erythema. Finally, based on biological mechanisms, we felt that pigment change was representative of less damage than scarring and thus adjusted the scoring of the damage section of `Gottron's – Hands.'
The modified CDASI correlates perfectly with the original CDASI. In addition, the total score and activity subscore of the modified CDASI have equally good concurrent validity with the PGM-overall skin and the damage subscore has a similarly low correlation with the PGM-overall skin as compared with the original CDASI. Furthermore, our validation results are almost identical to the study of Klein et al. which used multiple raters (n = 10) on a different sample of patients with cutaneous DM.2 This finding supports our notion that the changes made to the original CDASI were minor and merely served to refine and simplify the scoring system. In addition, we defined more accurately the elements of inflammatory activity and damage in the tool.
A PGM-overall skin was used as the gold standard in this study to capture overall disease state and to measure the validity of the CDASI. The total and activity subscore correlated well with the PGM-overall skin. However, the damage subscore correlated poorly. Of note, validity assessment of the original CDASI also demonstrated a low correlation (rsp = 0.4) between the global physician scale and the damage subscore of the CDASI.2 This finding may suggest that the physician places more emphasis on activity rather than damage when evaluating overall disease. Therefore, in this study, we also included two additional PGMs: physician assessment of skin disease activity and of damage. We calculated correlation of the PGM-activity and PGM-damage to the activity and damage subscores, respectively, of both the original and modified CDASI. The activity and damage subscores correlated well. Thus, in addition to assessing overall disease state, we suggest using PGMs that assess patient skin disease activity and damage separately for greater value.
The ultimate purpose of the CDASI is to serve as a tool in clinical trials and longitudinal patient assessment. Thus additional studies are required to evaluate the responsiveness, or ability to detect clinical change, of the CDASI. We suggest the use of the modified CDASI, designated v2, in any future application of this tool.
Acknowledgments
This study was supported in part by a Merit Review Grant from the Department of Veterans Health Administration, Office of Research Development, Biomedical Laboratory Research and Development and by the National Institutes of Health (NIH K24-AR 02207) to V.P.W.
Footnotes
Conflicts of interest None declared.
References
- 1.Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006;72:315–21. doi: 10.4103/0378-6323.26722. [DOI] [PubMed] [Google Scholar]
- 2.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159:887–94. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaines E, Werth VP. Development of outcome measures for autoimmune dermatoses. Arch Dermatol Res. 2008;300:3–9. doi: 10.1007/s00403-007-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer AJ, Thode HC, Hollander JE. Research fundamentals: selection and development of clinical outcome measures. Acad Emerg Med. 2000;7:397–401. doi: 10.1111/j.1553-2712.2000.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonilla-Martinez ZL, Albrecht J, Troxel AB, et al. The Cutaneous Lupus Erythematosus Disease Area and Severity Index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008;144:173–80. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krathen MS, Dunham J, Gaines E, et al. The Cutaneous Lupus Erythematosus Disease Area and Severity Index: expansion for rheumatology and dermatology. Arthritis Rheum. 2008;59:338–44. doi: 10.1002/art.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chren MM. Giving `scale' new meaning in dermatology: measurement matters. Arch Dermatol. 2000;136:788–90. doi: 10.1001/archderm.136.6.788. [DOI] [PubMed] [Google Scholar]