Abstract
Attentional control has been conceptualized as executive functioning by neuropsychologists and as working memory capacity by experimental psychologists. We examined the relationship between these constructs using a factor analytic approach in an adult lifespan sample. Several tests of working memory capacity and executive function were administered to over 200 subjects between the ages of 18-90 years old, along with tests of processing speed and episodic memory. The correlation between working memory capacity and executive functioning constructs was very strong (r = .97), but correlations between these constructs and processing speed were considerably weaker (r's ≈ .79). Controlling for working memory capacity or executive function eliminated age effects on episodic memory, and working memory capacity or executive function accounted for variance in episodic memory beyond that accounted for by processing speed. We conclude that tests of working memory capacity and executive function share a common underlying executive attention component that is strongly predictive of higher-level cognition.
Theories of cognitive control typically include an executive component that is responsible for coordinating goal-directed behavior (Baddeley & Hitch, 1974; Balota, Law, & Zevin, 2000; Braver, Gray, & Burgess, 2007; Engle & Kane, 2004; Hasher & Zacks, 1988; Jacoby, Bishara, Hessels, & Toth, 2005; Logan, 2003; Miyake et al., 2000; Posner & DiGirolamo, 1998; Shallice & Burgess, 1993). This executive control mechanism has been conceptualized in different ways, with experimental psychologists typically studying the working memory system (Baddeley, 1986) and neuropsychologists typically studying frontal lobe, or executive, functioning (Fuster, 1997). Historically there have been notable differences in the way executive functioning and working memory has been conceptualized. In the current study we investigated the relation between these constructs, and specifically examined the extent to which they shared common variance at the latent variable level, in a lifespan sample of adults between the ages of 18-90 years old.
Executive Functioning
The concept of executive functioning (EF) occupies a central role in neuropsychological theories of behavior control (Ferrier, 1886; Luria, 1973; Stuss & Knight, 2002). Although models of EF differ considerably, generally speaking, EF includes processing related to goal-directed behavior, or the control of complex cognition, especially in non-routine situations (Banich, 2009; Lezak, 1995; Fuster, 1997). Executive functions include control functions related to the inhibition of prepotent responses, shifting mental sets, monitoring and regulating performance, updating task demands, goal maintenance, planning, working memory, and cognitive flexibility, among others.
The term executive functioning (EF) has often been used synonymously with the term frontal-lobe functioning when describing the cognitive functions associated with the voluntary control of behavior (Carlson, 2005; Salthouse, Atkinson, & Berish, 2003; Stuss, & Knight, 2002). Though it is certainly the case that any task or ability recruits many disparate brain areas, EF tasks share the common characteristic of recruiting frontal areas (Alvarez & Emory, 2006), and thus we will use these terms interchangeably with this caveat in mind. Thus, sensitivity to frontal functioning may be considered a necessary, but not sufficient, criterion for a task to be considered an EF task. Related to the issue, we note too that frontal-lobe functioning is also related to many other functions, such as social functioning, including impulse control, emotion regulation, and personality (Gray, Chabris, & Braver, 2003). However, most neuropsychological measures of frontal or executive function have been aimed at assessing the fluid abilities associated with the frontal lobes, and share the characteristic that they recruit the dorsolateral prefrontal cortex, whereas social functions associated with the frontal lobes typically recruit orbital frontal areas, and thus, can be dissociated on anatomical, in addition to behavioral, grounds (Phillps & Della Sala, 1998). Thus, for the purposes of this paper the terms “frontal” or “executive” functioning will refer specifically to this narrower, traditional use of EF as a fluid ability construct.
Whether EF should be conceptualized as a unitary construct or several diverse functions has been a matter of considerable debate (Duncan, Johnson, Swales, & Freer, 1997; Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001; Stuss & Alexander, 2000; Teuber, 1972). Recently, many have suggested that executive functions (EFs) are best conceptualized as distinct functions that are only loosely related, and many neuropsychologists consider working memory to be one of several disparate EFs that control cognitive performance (Blair, Zelazo, & Greenberg, 2005; Fletcher, 1996; Pennington, Benneto, McAleer, & Roberts, 1996; Pennington & Ozonoff, 1996; Rapport, Chung, Shore, Denney, & Isaacs, 2000; Zillmer & Spiers, 2001). Others have argued that all EFs share a common executive attention component (Blair, 2006; Duncan, Emslie, Williams, Johnson, & Freer 1996; Shallice & Burgess, 1993). Recently, many researchers have taken a position on this issue occupying the middle ground, with EF characterized as consisting of both unity and diversity of function (Banich, 2009; Friedman et al., 2008; Garon, Bryson, & Smith, 2008). We maintain a similar theoretical approach here, though our study focuses empirically on the unity of EF.
Because different researchers have traditionally considered EF as either being a unitary or diverse construct, the measurement of EF has become a complex issue. Traditionally, specific tasks have been aligned with specific executive functions, which reflects the idea that EF consists of a diverse set of loosely related constructs. However, others have noted that particular EF tasks likely measure multiple EFs. Thus, at the outset it is important to describe the different EFs that the tasks we used in our battery have been proposed to measure. In the current study, we used the Wisconsin Card Sorting test (Heaton, 1993), the verbal fluency test (Thurstone, 1938), the mental control test (Wechsler, 1997a), and the mental arithmetic test (Wechsler, 1997a), each of which have been aligned with multiple EFs. The Wisconsin Card Sorting test is often used to measure set shifting or mental flexibility (Ashendorf & McCaffrey, 2008; Rhodes, 2004), but is also believed to measure inhibition of previous task sets (Salthouse et al., 2003), problem solving (Greve et al., 2002), strategic updating of goals based on feedback (Bishara et al., in press), abstract thinking (Shad, Muddasani, & Keshavan, 2006), and concept formation (Cinan, 2006). The verbal fluency test is believed to measure inhibitory functioning (Mahone, Koth, Cutting, Singer, & Denckla, 2001), but also is believed to measure memory monitoring (Rosen & Engle, 1997), and switching between retrieval strategies (Troyer, Moscovitch, & Winocur, 1997). The Mental Arithmetic test has been used to measure response selection (Deschuyteneer & Vandierendonck, 2005), but also is also believed to measure updating (or working memory; Deschuyteneer, Vandierendonck, & Muyllaert, 2006) Finally, the Mental Control test has been used to measure maintenance of task set (Lamar, Swenson, Kaplan, & Libon, 2004), but also is believed to require strategic retrieval (Wechsler, 1997a). Thus, the approach of aligning specific tasks with specific EFs appears to oversimplify issues related to measuring EFs, given that no EF tasks appear to be “process pure” (see Jacoby, 1999), an issue we return to below.
The foregoing analysis of the specific tasks used in our study highlights the issue of task impurity that has been problematic in previous research, as well as highlighting that most EF tasks require multiple distinct EFs. We also hope that this task analysis convinces readers that the tasks we used to measure EF assess multiple distinct EFs. As a point of comparison, Miyake and colleagues (Miyake et al., 2000; Friedman et al., 2006; Friedman et al., 2008) have used multiple tests of each of three distinct EFs - shifting, updating, and inhibition - and many other research groups have also used this approach (Garon et al., 2008; Hull, Martin, Beier, Lane, & Hamilton, 2008; Van der Sluis, De Jong, & Van der Leij, 2007). Certainly the EF battery we used measures these three EFs, and thus, we believe it provides a valid assessment of at least several EFs that have been thoroughly studied previously. As another point of comparison, other EF batteries have attempted to measure more than just a few EFs. For example, the Delis-Kaplan EF battery purports to measure several EFs, including: (1) flexibility of thinking, (2) inhibition, (3) problem solving, (4) planning, (5) impulse control, (6) concept formation, (7) abstract thinking, and (8) creativity (Delis, Kaplan, & Kramer, 2001; Homack, Lee, & Riccio, 2005). The four tasks we used to measure EF appear to measure most of these, including flexibility of thinking, inhibition, problem solving, impulse control, concept formation, and abstract thinking. Thus, we believe that the EF tasks we employed in the current study are representative of most of the EFs that have been studied extensively in the literature thus far, though like other studies, it does not provide an exhaustive sampling of all EFs.
The tests we used to measure EF in the current study also have several potential advantages not shared by other potential EF test batteries. First, the tasks we used included four of five tasks included in a battery that has been used extensively in previous research (Butler, McDaniel, Dornburg, Price, & Roediger, 2004; Glisky, Polster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001; McDaniel, Glisky, Rubin, Guynn, & Routhieaux, 1999; Roediger & Geraci, 2007; Van Petten et al., 2004). Second, this task battery has been used in younger and older adult samples (Chan & McDermott, 2007; Glisky & Kong, 2008). Third, the battery of tasks has been shown to have a reliable factor structure in these previous studies (Glisky & Kong, 2008; Glisky et al., 1995). Fourth, the battery of EF tasks we used has been shown to be related to episodic memory performance in previous studies, which is the outcome measure we used in the current study (discussed below). Finally, the tasks we used did not use differences in response times as the outcome measure, which are psychometrically problematic in cognitive aging research (see Faust, Balota, Spieler, & Ferraro, 1999).
With respect to the role of aging in EF, the frontal aging hypothesis has been developed (Moscovich & Winocur, 1992; Rodríguez-Aranda & Sundet, 2006; West, 1996; West & Schwarb, 2006), which is an explanation similar to the working memory aging hypothesis (described in the next section), but more closely associated with neuropsychology and cognitive neuroscience. According to this hypothesis, aging leads to structural and functional declines in the frontal lobes, and these changes lead to ubiquitous effects on complex cognition by affecting executive control functions (Phillips & Della Sala, 1998; West, 1996). Indeed, there is considerable support for the idea that age has a larger effect on changes in the frontal cortex as compared to many other brain areas (Raz, 2005), although it should be noted that not all frontal areas decline at similar rates with advancing adult age (Phillips & Della Sala, 1998). Specifically, evidence indicates that the dorsolateral prefrontal cortex is more acutely affected than the orbital frontal areas (Backman, Ginovart, & Dixon., 2000; Li & Lindenberger, 2002). Other frontal brain areas, such as orbital frontal areas, are associated with social and emotional functioning, and do not show dramatic age-related declines (Philips & Della Sala, 1998). Thus, there is support for the specificity of age-related structural declines in prefrontal cortex that is consistent with the frontal aging hypothesis.
Working Memory Capacity
The concept of working memory has become central to many theories of the control of thought and action in cognitive psychology (Cowan et al., 2005; Engle & Kane, 2004; Hasher, Lustig, & Zacks, 2007; Oberauer, 2005). Although there is disagreement among researchers about the specific definition of working memory, the working memory system is typically described as the system responsible for active maintenance and manipulation of information over brief time periods (Miyake & Shah, 1999). This system is viewed as a part of larger memory architecture, in which information is perceived, attended to, and retrieved (Baddeley, 1986; Cowan, 2005; Unsworth & Engle, 2007a). Historically, the most influential model of the working memory system has been the multiple component model, which divides the system into modality specific rehearsal buffers, i.e., the phonological loop and visuospatial sketchpad, and a modality-independent central executive component (Baddeley, 1986; Baddeley & Hitch, 1974). The central executive is responsible for controlled processing in working memory, including but not limited to, directing attention, maintaining task goals, decision making, and memory retrieval. Notably, other models of working memory also posit a central executive, or a common attentional control mechanism similar to the central executive (Cowan, 1999; Engle, Tuholski, Laughlin, & Conway, 1999; O'Reilly, Braver, & Cohen, 1999).
A great deal of recent research has been devoted to examining individual differences in working memory capacity (WMC), which is conceptualized as the efficiency of the central executive component of the working memory system, i.e., the coordination of multiple cognitive functions (Engle et al., 1999; Salthouse, 1990). By far the overwhelming majority of studies examining individual differences in WMC have used complex span tasks (e.g., reading span; Daneman & Carpenter, 1980) to measure WMC. A plethora of studies have shown that WMC, as measured by complex span task performance, is related to higher-level cognition, including measures of episodic memory (Kane & Engle, 2000; McCabe & Smith, 2002; McCabe, Smith, & Parks, 2007; Park, Lautenschlager, et al., 2002; Park, Smith, et al., 1996), reasoning (Barrouillet, & Lecas, 1999; Kyllonen & Christal, 1990), reading comprehension (Daneman & Carpenter, 1980; Lustig, May, & Hasher, 2001), and fluid intelligence (i.e., Engle et al., 1999; Colom, Rebollo, Palacios, Juan-Espinosa, & Kyllonen, 2004), to name but a few.
Engle and colleagues have investigated individual differences in WMC in young adults, and have argued that WMC is related to the ability to control attention, particularly under conditions of interference or distraction (see Engle & Kane, 2004 for a review). Importantly, in many situations in which cognition and behavior can be controlled under conditions that do not include distraction or interference, there are no differences in performance as a function of WMC (Kane, Bleckley, Conway, & Engle, 2001; Kane & Engle, 2003). Data have generally supported the controlled attention framework, and suggest that control is particularly important in situations that place a premium on active maintenance of task goals in the face of distraction, or require the retrieval of information under conditions of response competition (Conway & Engle, 1994; Unsworth & Engle, 2007a). For present purposes, it is important to point out that complex span tasks share a common executive attention component that is related to higher-level cognition (Engle et al., 1999; Kane et al., 2004). Thus, unlike the debate in the EF literature regarding the unity or diversity of EFs, WMC (i.e., central executive functioning) has typically been conceptualized as a unitary executive attention construct related to many kinds of higher-level cognition.
The study of working memory has also benefited from studies examining the effect of adult aging on WMC. Findings indicate that aging leads to declines in working memory performance, and that these declines in WMC mediate the relationship between age and higher-level cognition (Park et al., 1996; Park et al., 2002). Although the nature of explanations of age-related differences in working memory differ (e.g., see Hasher & Zacks, 1988; McCabe & Smith, 2002; Park et al., 2002; Salthouse, 1996), all of the explanations share the common idea that individual differences in WMC reflect individual differences in attentional processing. Other similar explanations of age differences in attentional processing have been offered as well (e.g., Balota et al., 1999; Hasher & Zacks, 1988; West & Bowry, 2005), and each explanation suggests that age-related declines in attentional processing will have wide-ranging effects on higher-order cognition. Moreover, each of the explanations suggests that age-related declines in frontal lobe functioning are related to the observed age-related declines in working memory task performance, which is consistent with research focused on the neural substrates of WM functions (Braver et al., 2007; O'Reilly et al., 1999).
The Relationship Between Working Memory Capacity and Executive Function
As described above, WMC and EF have been conceptualized very differently by researchers in neuropsychology and experimental psychology, though there appear to be commonalities with respect to the neuroanatomical substrates of WMC and EF, and age-related differences associated with them. In the current paper we take the position that there can be a common attentional control construct that underlies EF and WMC tasks, yet these tasks may also tap specific abilities that are not shared among different tasks. Thus, the current paper focuses on investigating the degree to which EF and WMC tasks share a common underlying attentional ability, which we label executive attention, following the framework of Engle, Kane, and colleagues (cf., Engle & Kane, 2004; Kane & Engle, 2002; McVay & Kane, 2009).
Though most theorists would acknowledge a relationship between EF and WMC, the extent to which these constructs share a common underlying ability remains unclear. In the present study we gained leverage on this issue with a two-pronged approach. First, across a lifespan sample of adults, we investigated the amount of variance that was common to WMC and EF, and examined whether the variance common to these two constructs was distinct from a general ability construct that is pervasive in the aging literature, namely, processing speed. Second, we examined whether WMC and EF showed a similar relationship with a key complex cognitive ability, namely, episodic memory. Related to this objective, we assessed the degree to which these constructs accounted for age-related variance in episodic memory performance.
To the extent that WMC and EF share a common underlying executive attention ability, we should find that (1) the correlation between the factors assessing WMC and EF is very high, (2) the correlation between WMC and EF factors is higher than the correlation with other general abilities, such as processing speed, and (3) structural equation models will show that both WMC or EF account for similar proportions of variance in episodic memory performance. Alternatively, if the two constructs do tap distinct abilities, at least in part, then we should find that (1) the correlation between WMC and EF should be moderate at best, (2) the correlation between WMC and EF may be similar to correlations with a general ability construct, such as processing speed, and (3) WMC or EF may account for unique variance in episodic memory performance that is distinct from the other factor.
Measurement of Executive Functioning and Working Memory Capacity
The way in which concepts like working memory capacity and executive function are operationalized has had considerable impact on our theoretical understanding of these concepts, but the best approach to measuring these constructs is unclear. With respect to the measurement of WMC, the development of complex span tasks in the early 1980s has provided a means by which to examine individual differences in the efficiency of the central executive component of the working memory system (Daneman & Carpenter, 1980), and this approach has become the generally accepted method to date (though see Cowan et al., 2005). Indeed, a great number of studies have used this approach of measuring performance on complex span tasks, often employing several tasks and using factor analytic approaches, and showing that complex span task performance is strongly related to higher-level cognition (see Engle & Kane, 2004 for a review). Although the exact nature of this correlation is a topic of debate, most explanations suggest that a common executive attention process underlies complex span tasks and higher-level cognition.
With respect to the measurement of executive function, the use of factor analytic techniques in recent years has helped to address issues of “task impurity” and task reliability to some extent (e.g., Miyake et al., 2000), but even within this factor analytic approach there are differences in the specific research strategies employed. Some have taken the approach of administering several measures of each of several distinct EF tasks to create distinct EF factors (e.g., Friedman et al., 2008; Hedden & Yoon, 2006; Miyake et al., 2000), whereas others have administered several measures of EF (or frontal) tasks to create a single EF factor (Albert, Blacker, Moss, Tanzi, & McArdle, 2007; Ettenhofer, Hambrick, & Abeles, 2006; Glisky et al., 1995; Salthouse et al., 2003; Wilson, Alderman, Burgess, Emslie, & Evans, 1996). The first approach, i.e., administering several measures of each of several EFs, has the benefit of allowing researchers to examine the relations between distinct EFs and various outcome measures, but has the shortcoming of potentially overlooking variance that is common to all EF tasks (note that correlating three distinct EF factors, e.g., Miyake et al., 2000, does not account for the variance overlapping all three factors). The second approach, i.e., administering several EF (or frontal) tasks to create a single EF factor, has the benefit of allowing researchers to examine the variance common to multiple EF tasks, thereby capturing the unity of EF, but it has the shortcoming of treating variance specific to individual EF tasks as measurement error. There seems to be growing consensus in recent years suggesting that a comprehensive understanding of executive functioning requires an understanding of both the unity and diversity of EFs (Friedman et al., 2008; Garon et al., 2008). As such, there is no “correct” approach to measuring EF, but rather, different approaches are suited to investigating the unity or diversity of EF. In the present study we were interested in gaining a better understanding of the unity of EF, and its similarity or dissimilarity as compared to WMC, and thus the common factor approach to measuring EF was most appropriate for our purposes. That said, we acknowledge that distinct EFs may exist, despite our focus on the unitary aspects of EF and WMC.
The Present Study
In the present study, we administered multiple tests of working memory capacity and executive functioning to an adult lifespan sample, along with measures of other constructs of interest (e.g., processing speed, episodic memory). We estimated correlations between WMC and EF to determine the degree to which their variance was identical or distinct from one another. We also investigated relations of each construct to a general processing resource construct, i.e., processing speed, in order to show that WMC and EF were distinct from a general fluid ability construct. Finally, we examined whether WMC and/or EF accounted for age-related differences in episodic memory, in order to examine the predictive power of each construct.
As mentioned previously, the way in which each construct is operationalized is clearly of paramount importance when examining the validity of multiple constructs. We took the approach of using sets of tasks (i.e., test batteries) that have been used successfully in previous studies of cognitive aging. Specifically, we used a battery of complex working memory capacity tasks modeled after Park et al. (2002), and a battery of executive (or frontal) tasks that has been used extensively by Glisky and colleagues (Glisky, Polster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001) and other researchers (e.g., Butler et al., 2004; Henkel, Johnson, & DeLeonardis, 1998; Roediger & Geraci, 2007). Each test battery included a mix of verbal and visuospatial tests in an effort to reduce the influence of modality-specific variance on the common factors.
The Role of WMC and EF in Episodic Memory
One of the primary reasons that cognitive control constructs like WMC and EF are useful and interesting is because they are related to higher-level cognitive abilities, which can reveal information about how the cognitive system operates. With respect to using an individual differences approach to understanding cognitive control, a construct is only useful to the extent that it relates to higher-level cognitive abilities. Thus, in addition to examining the relation between WMC and EF in the current study, we also examined whether each of these constructs was related to performance on tests of episodic memory.
WMC and EF have both been central to theorizing about episodic memory, and empirically both constructs are strongly related to episodic memory performance. Indeed, one of the defining characteristics of episodic memory is that recollective experiences associated with episodic remembering are dependent on attention demanding encoding and retrieval processes. For example, Oberauer (2005) found that WMC predicts an estimate of recollection, which is an attention-demanding retrieval process, whereas WMC was unrelated to familiarity, which is a retrieval process that does not require controlled attention (see also, Delaney & Sahakyan, 2007; Kane & Engle, 2000; McCabe & Smith, 2002; McCabe et al., 2007; Rosen & Engle, 1998; Watson, Bunting, Poole, & Conway, 2005). Moreover, WMC has been shown to mediate the relationship between aging and episodic memory (Park et al., 1996; Park et al., 2002). EF has also been closely linked to episodic memory performance. For example, EF is important for strategic encoding and retrieval processes, including organization and monitoring, which are involved in recall tasks. And tests of EF are related to performance on several types of attention demanding episodic memory tasks, and have been found to the mediate age-related differences in episodic memory (Bugaiska et al., 2007; Ferrer-Caja, Crawford, & Bryan, 2002; McCabe, Roediger, McDaniel, & Balota, 2009; Taconnat, Clarys, Vanneste, Bouazzaoui, & Isingrini, 2007; Troyer, Graves, & Cullum, 1994). Thus, previous research suggests that both WMC and EF constructs should predict episodic memory, but it is unclear if either would predict unique variance in performance not predicted by the other construct.
The Role of Processing Speed in Cognitive Aging
The speed at which people can process information is believed to constrain performance on all cognitive tasks, and thus processing speed can be conceived as a general processing resource related to higher-level cognition (Salthouse, 1996). Various theories of how processing speed affects higher-level cognition, including age-related differences in cognitive performance, have been proposed (Faust et al., 1999; Fry & Hale, 1996, 2000; Salthouse, 1996). For example, Salthouse (1996) has suggested that processing speed constrains performance on episodic memory tasks through two mechanisms: limited time and simultaneity. The limited time mechanism operates by constraining the amount of time that elaborative rehearsal can be completed during study, or that search processes can be engaged during retrieval. Slower processing also limits the amount of information that will simultaneously be available for processing, limiting the number of associations that can be created at study or accessed during retrieval. Indeed, in some studies controlling for processing speed has accounted for nearly all the age-related variance in episodic memory (see Salthouse, 1996, for a review of early studies). Moreover, age-related declines in WMC are related to declines in processing speed (Park et al., 1996). In the present study, including processing speed allowed an examination of whether WMC and/or EF were distinct from processing speed. Moreover, the present study allowed us to investigate whether WMC and/or EF accounted for unique age-related variance in episodic memory beyond that accounted for by processing speed (cf., Park et al., 1996).
In summary, the present study addresses three major questions: First, to what extent do WMC and EF measures share common variance? Second, to what extent is the variance common to WMC and EF tasks distinct from processing speed? Third, what is the relation between the variance common to WMC and EF tasks and age-related differences in episodic memory?
Method
Participants
Two-hundred six adults (110 female and 77 males) between the ages of 18-90 participated in this study (approximately 30 people per decade). For purposes of clarity of presentation, demographic characteristics were broken down in to four age groups with roughly identical numbers of subjects: younger (18-35 years), middle-aged (36-55 years), younger-old (56-70), and older-old (71-90). These data are presented in Table 1. Subjects were recruited from the Volunteers for Health participant pool which is maintained at the Washington University in St Louis School of Medicine for purposes of screening and matching potential research participants with appropriate studies. There were no significant differences for age groups for percentage of female subjects (59%), self-reported health (4.20), or number of years of education (15.20; all F's < 1.09). Age was positively correlated with the number of medications participants took on a regular basis (r = .46), and with Shipley vocabulary scores (r = .20; p's < .01). All participants who were included in the analysis had a minimum of high school education and scored greater than 26 on the Mini Mental Status Exam (Folstein, Folstein, & McHugh, 1975; see Results section below for exclusion criteria).
Table 1.
Demographic Characteristics of the Participants
| Age Group | ||||
|---|---|---|---|---|
| Variable | 18-35 | 36-55 | 56-70 | 71-90 |
| N | 49 | 53 | 45 | 55 |
| Age | 28.7 (5.9) | 45.2 (5.7) | 61.6 (4.2) | 79.4 (5.2) |
| % Females | 59 | 68 | 53 | 55 |
| Education (High School = 12) | 14.9 (2.0) | 15.8 (2.5) | 15.0 (2.6) | 15.2 (2.9) |
| Self-Reported Health (max=5) | 4.3 (0.9) | 4.2 (0.9) | 4.2 (.7) | 4.1 (.7) |
| No. of Medications | 0.7 (1.1) | 1.1 (1.5) | 2.1 (1.7) | 3.3 (2.4) |
| Shipley Vocabulary (max=40) | 33.2 (4.2) | 33.4 (3.6) | 34.2 (3.5) | 34.6 (3.6) |
General Procedure
Participants were tested in two sessions, each lasting approximately 2.5 hours. The first session included individually administered tasks, whereas the second session included group administered tasks. There were three or fewer subjects tested at a time in the second session, and subjects tested in the same group never differed in age by more than 20 years. Sessions were at least one week apart, but never more than three weeks apart.
Working Memory Capacity
Working memory capacity was assessed using four complex span tasks, each of which required that participants concurrently maintain and manipulate information in working memory. The reading span task used the sentences from Stine and Hindman (1994; we thank E. A. L. Stine-Morrow for providing these stimuli). Participants read sentences that were presented one at a time on the computer screen, such as The four-footed animal that barks is the mouse, and were asked to decide whether the sentences were true or false. They verbally answered YES or NO (or TRUE or FALSE if they preferred) and the experimenter advanced the computer to the next screen/sentence. Participants were asked to commit the last word in each sentence (i.e., mouse) to memory, and recall the to-be-remembered words in serial order when they saw a series of question marks on the screen. The number of sentences per trial began with one sentence and proceeded through five sentences if the participant correctly recalled in order two of the three trials at the previous length. Thus, the procedure involved a stair-step presentation, such that more difficult (i.e., longer) trials were attempted if participants were successful with easier trials. Three trials were presented at each length, and the task was stopped after a participant missed two of three trials at trial lengths of three or more (i.e., trials through lengths of three were completed regardless of accuracy on the previous trials). The number of trials on which all words were correctly recalled in their serial order was the dependent measure. Note that because we used a stair-step procedure to administer the span tasks, and discontinued the task when they failed to correctly recall two of the three trials at a given length, there is little difference in the variability using the traditional scoring method that we used and partial scoring methods that have been used by others in recent studies that have employed a randomized presentation method (e.g., Conway et al., 2005; Unsworth & Engle, 2007b).
The other WMC tasks were structurally very similar to reading span. The computation span task, based on Salthouse and Babcock (1991), was identical except that the processing component involved solving arithmetic problems and participants recalled digits. Participants read equations involving addition or subtraction of single digit numbers (3 + 6 = 10?), verbally responded YES or NO indicating whether the equation was correct, and attempted to remember the middle number in each equation (i.e., 6) in serial order. The equations never involved the same numbers being added or subtracted, and the answer was never a negative number. Incorrect answers were always one digit higher or lower than the correct answer.
The letter rotation span task, based on Shah and Miyake (1996), was structurally identical to the other tasks as well, except that the processing component involved determining whether rotated letters were presented in their normal orientation or were mirror reversed. Participants recalled the locations of the tops of these letters in serial order. The letters used were R, F, and P, and the letter was rotated at an angle of 45, 90, 135, 180, 225, 270, or 315 degrees. Thus, determining whether the letter was mirror reversed required mental rotation of the letter. The same letter was used on a given trial for all the processing phases, with each letter being used for one trial at each trial length. Participants were also required to remember where on the screen the top of the letter was located, and these locations were recalled by having participants point to the locations in correct order on a “recall grid” that included eight possible locations.
The match span task was developed for the current study, and was designed to be structurally identical to the other working memory tasks, in terms of interleaving maintenance and processing tasks. The processing component involved determining whether two digits “matched” in terms of being odd or even, and participants recalled digits that were presented for one second following the completion of each processing component. Thus, for example, for a trial of length two, participants would see “47” and say “no”, then see an “8” that they would try to commit to memory, then they would see a “62” and say “yes”, and then they would see a “4” that they would try to commit to memory. Finally, they would see a series of question marks prompting serial recall of the digits in serial order after presentation of the final digit.
Executive Functioning
The EF factor was based on four of the five measures used by Glisky and colleagues to measure frontal or executive function (Glisky et al., 1995; Glisky & Kong, 2008; Van Petten et al., 2004). We did not include the backward digit span measure from the Glisky et al. EF battery because it is a span task, and may have inflated the correlation between the EF and WMC factors because all of the WMC tasks were span tasks. The tasks from the EF battery will briefly be described here. The Wisconsin Card Sorting Test (WCST; Heaton, 1993) involves sorting cards based on one of three dimensions (i.e., color, shape, or number). After a participant has successfully sorted 10 cards consecutively based on one dimension, unbeknownst to the subject, the sorting rule changes, and cards must be sorted on another dimension. Participants received feedback after every trial indicating whether they were correct or incorrect. The number of categories achieved was used as the criterion measure by Glisky et al. (1995), but because this score showed ceiling effects for younger participants in the current study, we used the number of perseverative errors as the criterion measure (the sign for the correlations including this measure has been reversed to make it consistent with the other measures in the study). In the verbal fluency task subjects were given one minute to generate as many words as possible for a given letter. The letters used were F, A, and S (Thurstone, 1938). Mental Arithmetic involved completing a series of progressively more difficult arithmetic problems that were verbally spoken and had to be computed without aid of pen and paper, and the answer is given verbally (Wechsler, 1997a). A summary score is based on accuracy, with additional points given for faster answers. Mental Control required participants to quickly articulate various well learned categories of information (e.g., the days of the week; months of the year) in forward and reverse orders (Wechsler, 1997b), as well as switching between articulation of different categories (e.g., switching between saying days of the weeks and subtracting by 7's). A summary score is based on accuracy, with additional points given for faster answers.
Perceptual Speed
Perceptual speed was measured using the digit-symbol substitution task (WAIS-III; Wechsler, 1997a) and the letter and pattern comparison tasks (Salthouse & Babcock, 1991). Digit-symbol substitution requires subjects to quickly draw symbols below numbers according to a “look-up” key at the top of the page. The number of items completed in 90 seconds was used as the speed measure. The letter and pattern comparison tasks required subjects to do simple comparisons of letter strings and simple line drawings (i.e., patterns) to determine if they were the same or different. The number of items correctly completed in 30 seconds for each of two pages was used as the measure of processing speed for each task.
Vocabulary
Vocabulary measures were also included in the present study as a measure of general knowledge. Vocabulary was measured using the Synonym and Antonym tests (Salthouse, 1993), and the Shipley Institute Living Scale vocabulary test (Zachary, 1986). The Synonym test is a 10-item multiple-choice test in which subjects must select a synonym to a target word from among five possible answer choices. The Antonym test is identical, except that subjects must choose an antonym instead of a synonym. The Shipley vocabulary test is a 40-item multiple-choice test, in which subjects must choose a synonym of a target word from among four possible answer choices. For all tests the number of correct answers was the measure of vocabulary ability.
Episodic Memory
Episodic memory was based on three measures, each of which required immediate free recall of verbal stimuli. Because free recall is arguably the purest raw test of resource demanding retrieval processes (i.e., recollective ability), this construct was presumed to primarily assess episodic recollection. Tests included recall of a 40-word list, recall of a 16-word list, and recall of two prose passages. The 40-word list included four words from each of 10 “thematic” lists, taken from Roediger, Watson, McDermott, and Gallo (2001). These words were read aloud to participants at a rate of one word every three seconds, and participants recalled the words by writing them down on an answer sheet. The 16-word list included four words from each of four taxonomic categories. This list was the first list recalled from the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 2000). The list was read to participants at a rate of one word per second, and recalled auditorily as well. Prose recall was measured using the Logical Memory subtest from the Wechsler Memory Scales (WMS-III; Wechsler, 1997b), which measures recall of idea units from two brief stories that were read to participants by the experimenter and recalled aloud.
Results
The results will be divided into three sections. In the first, we discuss the characteristics of each of the measures in the study in terms of their overall level of performance, relation to age, other measures of the same factor, and reliability. In the second section, we calculate various measurement models and discuss the correlations among the constructs. In the third section we address the criterion validity of the constructs by examining the extent to which they mediate the relation between age and episodic memory, using structural equation models.
Descriptive Statistics and Reliability
Before calculating the descriptive statistics, four participants were removed from the analysis. We removed two participants from the analysis because their general cognitive ability was suggestive of possible dementia, i.e., a MMSE score of 26 or below. Two other participants were removed because they did not complete both sessions. Of the 202 participants left, no cases were identified as univariate or multivariate outliers.
Descriptive statistics are presented in Table 2 for each task used in the study, categorized by the construct each task measured. Although the sample was a continuous life span sample, for purposes of clarity of presentation, the data are divided into four age groups. All of the cognitive tasks were related to age except Mental Arithmetic and Antonyms. Age correlations for each task are presented in Table 3, along with the reliability for each measure. Most of the task reliabilities were computed for the present sample using coefficient alpha, except where noted in Table 3. Internal consistency reliability was .55 or greater for all tasks. Note too that factor analytic models measure error for each task within each model.
Table 2.
Descriptive Statistics for Cognitive Measures
| Young (18-35) | Middle-Age (36-55) | Young-Old (56-70) | Old-Old (71-90) | |||||
|---|---|---|---|---|---|---|---|---|
| Construct and Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Working Memory Capacity | ||||||||
| Computation Span | 7.37 | 2.18 | 7.55 | 2.60 | 6.93 | 2.04 | 6.13 | 2.05 |
| Reading Span | 8.06 | 2.06 | 7.83 | 2.80 | 7.23 | 1.59 | 6.64 | 1.75 |
| Match Span | 11.82 | 3.28 | 9.72 | 4.68 | 9.02 | 3.87 | 6.97 | 3.21 |
| Letter Rotation Span | 11.53 | 2.54 | 10.30 | 3.42 | 9.00 | 3.42 | 6.62 | 2.71 |
| Executive Function | ||||||||
| Mental Arithmetic | 13.18 | 2.57 | 13.60 | 3.28 | 14.04 | 2.76 | 12.31 | 3.02 |
| Mental Control | 30.73 | 4.10 | 29.30 | 5.57 | 28.64 | 4.75 | 25.75 | 5.01 |
| Verbal Fluency (FAS) | 46.69 | 12.09 | 43.70 | 10.43 | 40.84 | 13.55 | 40.60 | 10.37 |
| Wisconsin Card Sorting Test* | 16.98 | 6.95 | 23.65 | 12.72 | 25.73 | 13.25 | 40.09 | 15.69 |
| Processing Speed | ||||||||
| Letter Comparison | 26.00 | 5.13 | 23.21 | 4.00 | 19.20 | 3.33 | 15.85 | 3.94 |
| Pattern Comparison | 44.92 | 6.69 | 38.83 | 6.08 | 34.80 | 5.85 | 27.62 | 5.05 |
| Digit Symbol | 89.76 | 13.47 | 80.58 | 12.14 | 70.62 | 12.98 | 56.33 | 11.20 |
| Vocabulary (Gc) | ||||||||
| Shipley | 33.16 | 4.15 | 33.36 | 3.65 | 34.18 | 3.50 | 34.62 | 3.56 |
| Synonyms | 6.71 | 2.35 | 6.06 | 2.72 | 7.38 | 2.03 | 7.62 | 2.62 |
| Antonyms | 6.22 | 2.40 | 5.77 | 2.59 | 6.73 | 2.37 | 6.73 | 2.95 |
| Episodic Memory | ||||||||
| Prose Recall | 32.47 | 7.36 | 32.06 | 6.88 | 31.84 | 6.13 | 28.30 | 7.65 |
| Free Recall – 16 Words | 8.22 | 2.36 | 7.96 | 2.06 | 7.37 | 1.70 | 6.42 | 2.09 |
| Free Recall – 40 Words | 18.74 | 4.68 | 18.62 | 3.88 | 16.56 | 3.55 | 14.61 | 4.11 |
Table 3.
Factor loadings for all five cognitive factors, along with reliability estimates and age correlations for each measure.
| Construct and Measures | WMC | EF | Speed | Vocab | EM | Reliability. | Age r |
|---|---|---|---|---|---|---|---|
| Working Memory Capacity | |||||||
| Computation Span | .59 | .69 | -.20 | ||||
| Reading Span | .63 | .65 | -.28 | ||||
| Match Span | .64 | .88 | -.41 | ||||
| Letter Rotation Span | .77 | .87 | -.54 | ||||
| Executive Function | |||||||
| Mental Arithmetic | .58 | .88a | -.11 | ||||
| Mental Control | .60 | .51a | -.35 | ||||
| Verbal Fluency (FAS) | .47 | .55 | -.18 | ||||
| Wisconsin Card Sorting Test | .66 | 72a | -.58 | ||||
| Processing Speed | |||||||
| Letter Comparison | .88 | .86 | -.70 | ||||
| Pattern Comparison | .88 | .93 | -.74 | ||||
| Digit Symbol | .87 | .84a | -.71 | ||||
| Vocabulary | |||||||
| Shipley | .86 | .76 | .20 | ||||
| Synonyms | .91 | .76 | .22 | ||||
| Antonyms | .85 | .77 | .13 | ||||
| Episodic Memory | |||||||
| Prose Recall | .65 | .78 | -.22 | ||||
| Free Recall – 16 Words | .67 | .82 | -.32 | ||||
| Free Recall – 40 Words | .80 | .85 | -.33 |
The reliability estimates for all measures were assessed in the present study using coefficient alpha or split-half reliability with the Spearman-Brown correction, except for mental arithmetic, mental control, digit-symbol substitution, and WCST. These reliability estimates were taken from the norms provided with those tests, and were assessed using test-retest reliability for mental arithmetic, mental control, and digit symbol substitution (reported in Wechsler, 1997a, 1997b), and the intraclass correlation for WCST (reported in Tate, Perdices, & Maggiotto, 1998).
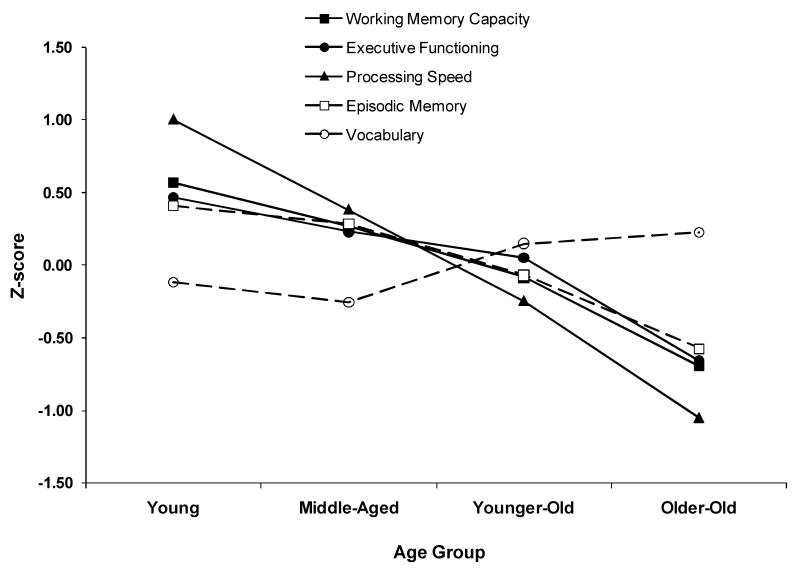
We also conducted a confirmatory factor analysis for the five factors included in the study to ensure reasonably strong factor loadings for each measure on the factor it was intended to measure. Using a minimum criterion for acceptability of fit as a CFI of .90 (Hu & Bentler, 1995), and a RMSEA of <.10 (Browne & Cudeck, 1993), the fit of the model was acceptable, χ2(109, N = 202) = 226.3, CFI = .931, RMSEA = .073). The factor loadings from this model are displayed in Table 3, and the intercorrelations among the factors are reported in Table 4. All of the factors were positively correlated (p < .01) except for Processing Speed and Vocabulary. Importantly, the correlation between WMC and EF in this model was .96, indicating that the latent variables for each factor very strongly correlated, an issue we discuss at length in the next section. Age was then correlated with each of the constructs, with all of these age correlations being negative, except for Vocabulary, which was positively correlated with age. This pattern of age relations is consistent with most previous studies of this type (e.g., Park et al., 2002; Salthouse et al., 2003). Figure 1 displays the age effect on each factor in the study. These factor scores were computed separately for each factor and then the average factor scores were plotted as a function of age group for the purposes of illustrating age effects.
Table 4.
Correlations between latent variables for all five cognitive factors and age.
| Construct and Measures | WMC | EF | Speed | Vocab | EM |
|---|---|---|---|---|---|
| Working Memory Capacity | - | ||||
| Executive Function | .96 | - | |||
| Processing Speed | .78 | .78 | - | ||
| Vocabulary | .27 | .45 | .08 | - | |
| Episodic Memory | .73 | .75 | .52 | .38 | - |
| Age | -.59 | -.56 | -.82 | .22 | -.41 |
Values in italics represent non-significant correlations; all other correlations were significant (p < .01)
Figure 1.
Age-related differences in performance on each factor score for each cognitive domain. There were significant age-related declines in performance on all factor scores, except vocabulary, which showed an age-related increase.
Because the EF battery we used was created using a sample of older adults (Glisky et al., 2001), and has only recently been extended to use with younger adults (Chan & McDermott, 2007; Glisky & Kong, 2008), we also examined the factor loadings for the EF battery separately for younger adults (ages 18-54; N = 100) and older adults (ages 55-90; N = 102), in order to confirm that the tasks shared substantial variance for younger adults in addition to older adults. The factor loadings for the EF tasks were .48 or greater for each task, and were similar for both age groups, with factor loading for younger and older adults (respectively) of .61 and .61 for Verbal Fluency, .81 and .81 for Mental Arithmetic, .78 and .73 for the WCST, and .78 and .69 for Mental Control. Thus, the average factor loadings for the younger and older adults in our sample were not appreciably different, with an average deviation of .03. Note too, that in all cases, for both age groups, the factor loadings were as large or larger than in the original Glisky et al. (1995) paper (though Glisky et al. partialled out age from their factor analysis, which may have reduced the magnitude of their factor loadings). Hence, these results clearly converge on the utility of the EF battery originally developed by Glisky et al., and recently replicated and extended by Glisky and Kong (2008).
Factor Analytic Models Examining Working Memory Capacity and Executive Function
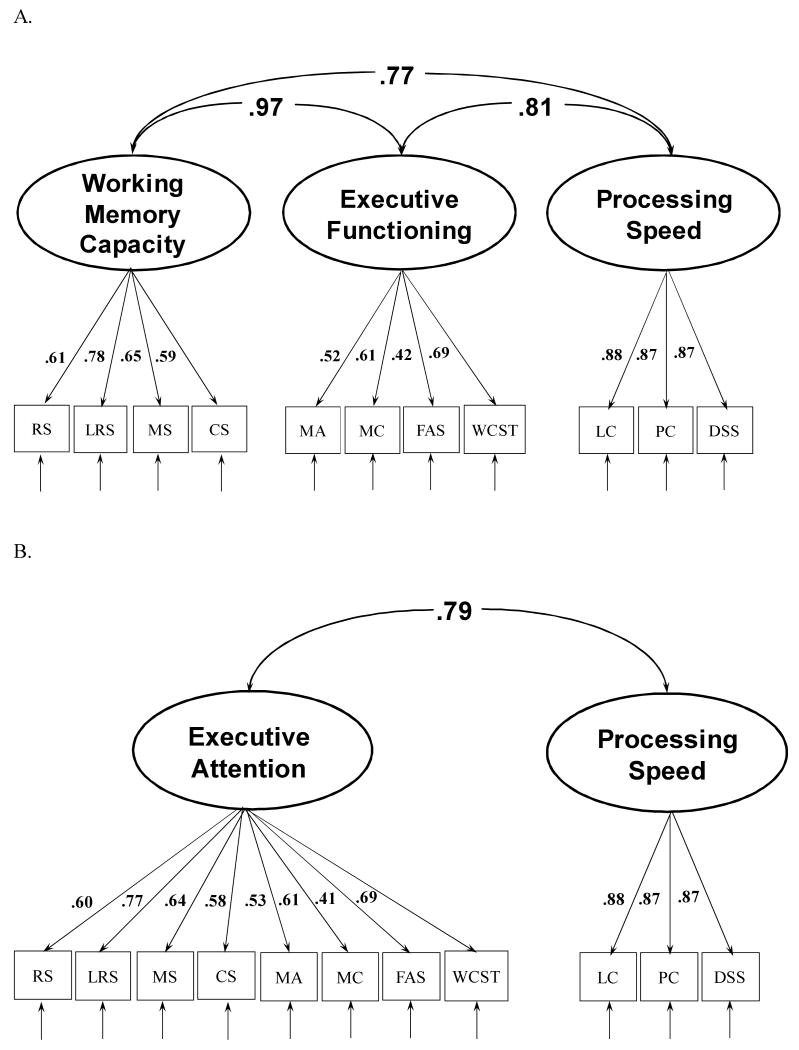
One of the primary purposes of the present study was to investigate relations among WMC and EF, and to examine whether these constructs were distinct from Processing Speed. Accordingly, the next analysis focused on the subset of these three constructs. The resulting model, Model 1, with factor loadings and factor correlations, is presented in Figure 2 (Panel A), χ2(41, N = 202) = 126.7, CFI = .915, RMSEA = .099). The model fit statistics for this model (Model 1) are presented in Table 5 as well. Remarkably, the correlation between the WMC and EF factors was nearly 1.0 (i.e., .97). In contrast, the correlations between each of these factors and Processing Speed was substantially lower (i.e., .77 and .81, respectively). Thus, although there is strong evidence to suggest that WMC and EF tasks measure a common underlying cognitive construct, tests of Processing Speed appear to measure a construct that is strongly related to WMC and EF, but is nonetheless distinct from them (i.e., less than two-thirds of the variance in Processing Speed was shared with WMC or EF).
Figure 2.
Model A: Factor analysis examining the relation between the working memory capacity, executive functioning, and processing speed constructs. Model B: Factor analysis with a single executive attention construct defined by the working memory capacity and executive function measures. Circles represent the latent variables, boxes represent each observed variable. RS = Reading Span; LRS = Letter Rotation Span; MS = Match Span; CS = Computation Span; BDS = Backward Digit Span; MA = Mental Arithmetic; MC = Mental Control; FAS = Letter Fluency; WCST = Wisconsin Card Sorting Test; RAPM = Raven's Advanced Progressive Matrices; SR = Space Relations; LS = Letter Sets
Table 5.
Models Examining Relations Among Working Memory Capacity, Executive Function, and Episodic Memory.
| Factor Correlations | |||||||
|---|---|---|---|---|---|---|---|
| Model | 1 | 2 | 3 | χ2 | df | CFI | RMSEA |
| Model 1: Three-Factors | 126.7 | 41 | .915 | .099 | |||
| (1) Working Memory Capacity | - | ||||||
| (2) Executive Function | .97 | - | |||||
| (3) Processing Speed | .77 | .81 | - | ||||
| Model 2: Two-Factors | 127.8 | 43 | .916 | .099 | |||
| (1) Executive Attention (WMC/EF) | - | ||||||
| (2) Processing Speed | .79 | - | |||||
| Model 3: One-Factor | 209.0 | 44 | .837 | .137 | |||
| Model 4: Three-Factors, Controlling for Age | 156.7 | 49 | .912 | .086 | |||
| (1) Working Memory Capacity | - | ||||||
| (2) Executive Function | .95 | - | |||||
| (3) Processing Speed | .62 | .70 | - | ||||
| Model 5: Two-Factors Controlling Age | 158.4 | 52 | .913 | .083 | |||
| (1) Executive Attention (WMC/EF) | - | ||||||
| (2) Processing Speed | .66 | - | |||||
| Model 6: One-Factor Controlling Age | 258.0 | 54 | .833 | .121 | |||
Model 2 examined the fit of the model when WMC and EF were collapsed in to one factor, which we refer to as Executive Attention, but Processing Speed was still a separate factor (see Figure 2, Panel B). The model fit was similar to the three-factor model, Δχ2(2, N = 202) = 1.01, ns, but the latter model is more parsimonious given that fewer factors are computed in Model 2. Finally, we examined a model in which all of the measures loaded on one-factor, Model 3, and that model had a significantly poorer model fit compared to Model 2, Δχ2(1, N = 202) = 81.2, p < .01. Thus, in terms of model fit and parsimony, Model 2, shown in Figure 2B, which collapsed WMC and EF into one factor, and kept processing speed as a separate factor, was the preferred model.
We also considered Models 4, 5 and 6, which were identical to Models 1, 2, and 3 (respectively), but the influence of Age on the factor intercorrelations was controlled by correlating Age with each latent variable in each model. The results are shown in Table 5. Notably, in the three-factor model, the strong correlation between WMC and EF was only changed slightly (.95), indicating that a common age relation was not driving the high correlation between these factors in Model 1. Controlling for Age also reduced the correlations between WMC and Processing Speed (from .77 to .62) and EF and Processing Speed (from .81 to .70). In Model 5, WMC and EF was collapsed into a single factor, and this did not reduce the model fit significantly, Δχ2(3, N = 202) = 1.7, ns. However, again, the one-factor model collapsing WMC, EF, and processing speed measures into a single model (Model 6), provided a significantly poorer fit than the two-factor model (Model 5), Δχ2(2, N = 202) = 99.6, p < .01, indicating that tests of Processing Speed measured a factor that was distinct from tests of WMC and EF, even when the influence of age was accounted for. In summary, at the latent variable level, it appears that the tests of WMC and EF administered in the current study measured a common underlying construct, but that the Processing Speed construct was distinctly different from WMC and EF.
Role of WMC and EF in Episodic Memory
Next, we investigated the role of WMC and EF in accounting for age-related variance in episodic memory using structural equation modeling. In each structural model the effect of age on Episodic Memory (EM) was examined after controlling for WMC, EF, or their common variance (i.e., Executive Attention). In all cases factor loadings in the structural models were within .05 of the loadings reported in Table 3 for the full measurement model, and thus the factor loadings are not reported for each model. The correlation matrix for all the measures is included in the Appendix to allow interested readers to recreate the exact models.
Fit statistics, and correlations between latent variables in each of the models, are presented in Table 6. The leftmost column in the table shows the latent variables being related in the model, and the correlations between them are presented in the second column from the left. Before examining the mediation models, a basic model examining the age effect on episodic memory was computed. This model revealed a moderate negative relationship between age and episodic memory performance (-.41), similar to findings from other factor analytic studies examining verbal episodic memory performance (e.g., Park et al., 1996; Salthouse, 1995). Fit statistics for the model are presented in the first row of Table 6. Note that in all subsequent models the significant direct age effect on episodic memory was reduced to a non-significant correlation.
Table 6.
Fit statistics and correlations for structural equation models including working memory capacity or executive function as mediators of the relation between age and episodic memory. EM: Episodic Memory; WMC: Working Memory Capacity; EF: Executive Function; EA: Executive Attention; PS: Processing Speed
| Model | Correlation | χ2 | df | CFI | RMSEA |
|---|---|---|---|---|---|
| Age Effect Model | 1.0 | 2 | 1.0 | 0.00 | |
| Age → EM | -.41 | ||||
| Model A1 | 41.8 | 18 | .95 | .081 | |
| Age → WMC | -.58 | ||||
| Age → EM | .01 | ||||
| WMC → EM | .73 | ||||
| Model A2 | 90.4 | 18 | .83 | .141 | |
| Age → EF | -.54 | ||||
| Age → EM | -.01 | ||||
| EF → EM | .73 | ||||
| Model A3 | 168.4 | 52 | .86 | .106 | |
| Age → EA | -.61 | ||||
| Age → EM | .05 | ||||
| EA → EM | .77 | ||||
| Model B1 | 70.8 | 41 | .97 | .062 | |
| Age → PS | -.81 | ||||
| PS → WMC | .75 | ||||
| PS → EM | -.08 | ||||
| Age → EM | -.04 | ||||
| WMC → EM | .78 | ||||
| Model B2 | 135.3 | 50 | .92 | .092 | |
| Age → PS | -.81 | ||||
| PS → EF | .78 | ||||
| PS → EM | -.25 | ||||
| Age → EM | -.08 | ||||
| EF → EM | .90 | ||||
| Model B3 | 212.6 | .86 | .91 | .086 | |
| Age → PS | -.81 | ||||
| PS → EA | .78 | ||||
| PS → EM | -.19 | ||||
| Age → EM | -.04 | ||||
| EA → EM | .86 |
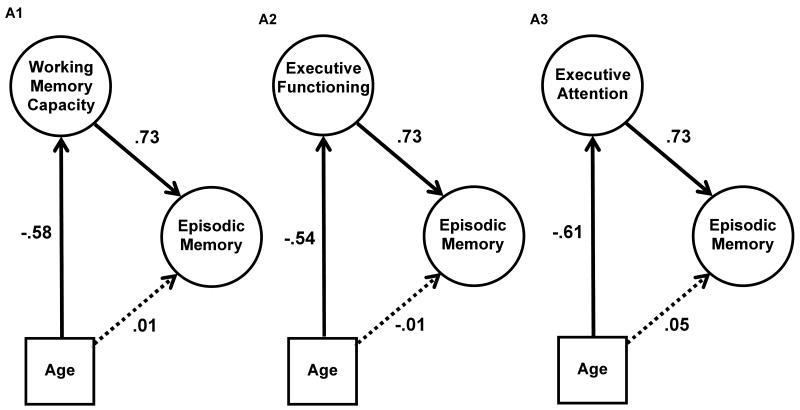
Several versions of Model A were computed to examine whether WMC and/or EF mediated the age effects on episodic memory. The first model, A1, included WMC as the mediator (see Figure 3, A1). This model revealed a moderate age-related decline in WMC (r = -.58), and a stronger correlation between WMC and episodic memory (r = .73). The fit of the model was acceptable (see Table 6 for fit statistics for all subsequent models), and thus, the model indicates that WMC is a plausible mediator of the age-episodic memory relation. Model A2 examined EF as the mediator and revealed correlations between latent variables that were similar to Model A1 (see Figure 3, A2). Age and EF were moderately correlated (r = -.54), and the correlation between EF and episodic memory was strong (r =.73). However, the fit of this model was relatively poor, with a RMSEA over .10. It is likely that the poor fit of this model is at least partly the result of small age effects on two of the four measures that were used to measure EF (see Table 3). For present purposes, it is worth noting that despite the poor model fit, the correlation between EF and episodic memory in Model A2 is identical to the correlation between WMC and episodic memory in Model A1, which is consistent with the factor analytic findings showing that these latent constructs were similar to one another. Finally, Model A3 was included to examine whether a single factor comprised of both the WMC and EF tasks was similar to the models with each of these as separate constructs. The measurement models provide an empirical basis for computing this structural model with one “executive attention” factor, because the best fitting measurement model collapsed these measures in to one construct (see Table 5). Note also that attempts to include WMC and EF as separate factors in models predicting episodic memory led to Heywood cases, which typically indicate that too many latent variables are included in the model (Bollen, 1989). As shown in Table 6, Model A3 fit somewhat better than the model with EF as the mediator (Model A2), and the correlation between this executive attention factor and episodic memory (.73) was similar to Models A1 and A2 (see Figure 3, A3), which is not surprising, given the results of the prior models.
Figure 3.
Structural equation models examining the relation between age and episodic memory with either working memory capacity (A1), executive functioning (A2), or executive attention (A3) as the mediator. Solid lines represent significant correlations (p < .01), dotted lines represent non-significant correlations.
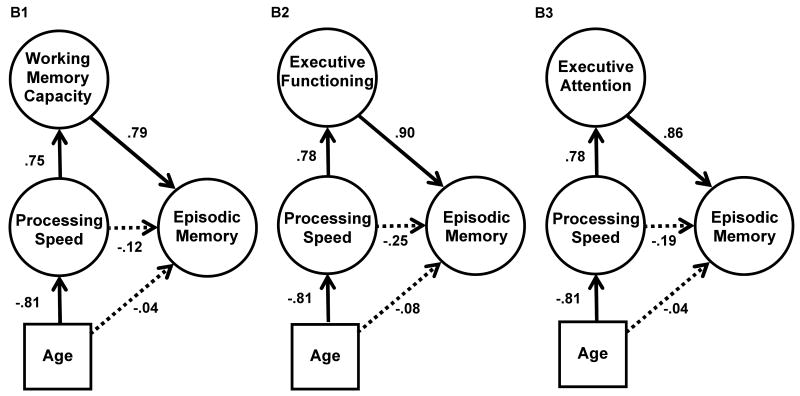
In the three versions of Model B, shown in Figure 4, Processing Speed was added as a mediator of the relationship between Age and cognitive control and Episodic Memory. Adding Processing Speed to the model allows an examination of whether WMC and/or EF accounts for additional variance above and beyond a more general explanatory construct (e.g., processing speed) for the age-episodic memory relation. Indeed, despite WMC and EF sharing considerable variance that was distinct from Processing Speed, that distinct variance may, or may not, be important with respect to predicting Episodic Memory performance. Models B1-B3 allowed an examination of whether the variance common to Episodic Memory and WMC and/or EF was distinct from Processing Speed, and/or whether Processing Speed accounted for unique variance after controlling for WMC and/or EF.
Figure 4.
Structural equation models examining the relation between age and episodic memory, with processing speed mediating the relationship between working memory capacity (B1), executive functioning (B2), or executive attention (B3), and episodic memory. Solid lines represent significant correlations (p < .01), dotted lines represent non-significant correlations.
Models B1, B2, and B3 were similar to models A1, A2, and A3, but Processing Speed mediated the relation between Age and WMC, Age and EF, or Age and Executive Attention. Because processing speed is believed to have a very general effect on constraining cognitive performance, any effect of WMC and/or EF above and beyond Processing Speed provides strong support for the notion that WMC and/or EF is an important mediator of the relation between Age and Episodic Memory. Stated another way, these models allowed an examination of whether WMC and EF had any explanatory power above and beyond age-related declines in processing speed.
In Model B1, WMC was the mediator. As shown in Figure 4, B1 there was a strong age effect on processing speed (r = -.81), and a strong correlation between processing speed and WMC (r = .75). However, the inclusion of processing speed did not reduce the strength of the correlation between WMC and episodic memory as compared to Model A1 (the path actually increased slightly from .73 to .79). Processing speed was not correlated with episodic memory after controlling for WMC (r = -.12). Thus, although the model indicates that it could be plausible that the age effect on WMC is mediated by processing speed because of the high correlations, the more important point is that the correlation between WMC and episodic memory is not due to the effects of processing speed on WMC.
Model B2 examined EF as the mediator of the age-episodic memory relationship. As with Model B1, there was a strong age effect on processing speed (r = -.81), and a strong correlation between processing speed and EF (r = .78; see Figure 4). However, the inclusion of processing speed did not reduce the strength of the correlation between EF and episodic memory, as compared to Model A2 (the path actually increased from .73 to .90). Moreover, processing speed was not correlated with episodic memory after controlling for EF (r = -.25). Unlike Model A2, Model B2 also provided an acceptable fit (see Table 6). Again, it is noteworthy that WMC and EF led to similar correlations with episodic memory, regardless of whether processing speed was included in the models or not, revealing their similarity as explanatory constructs.
Finally, we also created an additional model, B3, based on all eight measures of WMC and EF that we label Executive Attention (see Figure 4, B3). Like Models B1 and B2 with each construct modeled separately, including processing speed in the model with one executive attention factor did not reduce the correlation between executive attention and episodic memory (the path increased from .77 to .86). Consistent with the models that had not included processing speed, the correlation between executive attention and episodic memory (.86) was similar to the correlations between WMC or EF and episodic memory (.78 and .90, respectively). Moreover, with Processing Speed included in Model B3, the CFI and RMSEA were acceptable (see Table 6), unlike Model A3, which included all eight executive attention measures but did not include Processing Speed.
General Discussion
The present study examined the relation between working memory capacity (WMC), a cognitive control construct borne out of the cognitive psychology tradition, and executive functioning (EF), a cognitive control construct developed from the neuropsychological tradition. The data were clear in showing that tasks intended to measure WMC and tasks intended to measure EF measured a construct with a high degree of similarity, which we refer to as executive attention. Furthermore this executive attention construct appears to be distinguishable from Processing Speed, a general cognitive ability construct. This conclusion was reached based on a consideration of the strong correlation between WMC and EF (r = .97; see Figure 2), the weaker correlations between WMC or EF and Processing Speed (r ≈ .79), as well as considering the pattern of correlations between WMC or EF and Episodic Memory.
Working Memory Capacity and Executive Function Tasks Measure a Common Attention Construct
The results of this study indicate that complex working memory span tasks and EF tasks we measured shared a common underlying cognitive ability, which we will refer to as executive attention (cf., Engle & Kane, 2004; Kane, Conway, Hambrick, & Engle. 2007; Posner & DiGirolamo, 1998). Many other terms have been used to describe the ability underlying performance on complex cognitive tasks, including executive control (Logan, 2003), attentional control (Balota et al., 1999), controlled attention (Engle et al., 1999), cognitive control (Depue, Banich, & Curran, 2006; Jacoby et al., 2005), and inhibitory control (Hasher et al., 2007), to name but a few. We chose to call this common factor executive attention for several reasons. First, the ability common to the tasks in each battery appears to be an attentional ability. Indeed, both theoretical and empirical considerations converge on this conclusion (Banich, 2009; Braver et al., 2007; Kane & Engle, 2004). Second, executive attention succinctly summarizes the functional nature of this construct; i.e., it is an attentional ability that is related to executive control functions. Third, using the term executive relates the construct to models of WM and (obviously) models of EF. Finally, the term executive attention has also been used by other researchers studying individual differences in WMC (Engle & Kane, 2004; Kane & Engle, 2007), and in cognitive neuroscience (Posner & DiGirolamo, 1998; Richards, 2008; Rueda, Posner, & Rothbart, 2005).
Our conceptualization of the term executive attention is similar to that of Engle, Kane, and colleagues (Engle & Kane, 2004; Engle et al., 1999; Kane et al., 2007). They have proposed a theory of executive attention that proposes that two functions of the central executive are measured by WMC tasks. The first is the ability to maintain a goal in an active state during task performance, an ability that has been proposed as crucial to EF as well (Banich, 2009; Braver et al., 2007; Duncan et al., 1996). The second is the ability to resolve interference, particularly when there is conflict between a prepotent response and task demands, an ability that has similarly been noted as important for EF (Braver et al., 2007; Norman & Shallice, 1986; Persson & Reuter-Lorenz, 2008).
The finding that WMC and EF constructs were so strongly related is even more surprising if one considers that they were created for different reasons, using different methods. Complex working memory span tasks, like the ones used in the current study, were originally developed to measure individual differences in the ability to concurrently store and process information (i.e., central executive functioning). Specifically, complex span tasks were believed to measure both the slave systems and central executive component of Baddeley's (1986) working memory model (Daneman & Carpenter, 1980). Thus, complex span tasks were theoretically motivated, deductively-derived tasks intended to measure functional differences in the efficiency of attentional allocation in the working memory system.
The executive function battery created by Glisky et al. (1995) was created using a method very different from the creation of WMC tasks, and for a different purpose. Glisky and colleagues were interested in assessing individual differences in functioning associated with the frontal lobes in older adults. Data and theory in neuropsychology suggested that age-related deficits in source memory were similar to those seen in patients with frontal lobe damage, motivating the creation of this test battery. Moreover, Glisky and colleagues administered several standardized tests to a sample of older adults, and the tasks that comprised the EF battery were the tasks that loaded together on a factor in an exploratory factor analysis. Thus, in contrast to the deductive method used to create WMC task batteries, the creation of the EF battery used in the current study was inductive in nature. Despite the differences in the way in which the batteries of WMC and EF tasks were created, the overlap in their common variance was extremely high.
Based on the finding that WMC and EF tasks shared substantial common variance, we argue that the current data provide evidence for reciprocal validity for both of those constructs. Reciprocal validity can be defined as a particularly strong form of construct validity, such that two constructs that are strongly empirically related to one another lend support to the theoretical reality of each other. Thus, the present results lend support not only to the idea that these measured constructs are similar, but provide support for some of the similar assumptions made by each theoretical approach. For example, people who have traditionally studied WMC and EF have argued that each of these constructs is closely associated with functioning of the frontal lobes of the brain (Kane & Engle, 2002; Shallice & Burgess, 1993). To the extent that there are strong data to back up this claim with respect to EF in neuropsychological patients, the current finding that the two constructs were so strongly correlated lends support to the notion that WMC is also related to frontal functioning in the brain. Similarly, fMRI data showing that working memory capacity tasks requiring simultaneous maintenance and processing activate prefrontal cortical areas (Osaka et al., 2003) lends support to the idea that EFs are related to these brain areas.
Implications for Models of Working Memory Capacity
The finding that WMC as measured by complex span tasks were so strongly correlated with EF tasks lends support to the idea that the functioning of the central executive component of the multiple component model (Baddeley, 1986) is captured by complex span tasks. Indeed, Baddeley (1986) has conceptualized the central executive as the supervisory attentional system proposed in Shallice and colleagues' model of executive functioning (Norman & Shallice, 1986; Shallice & Burgess, 1993), and our data provide support for the idea that a common executive attention component is involved in working memory.
The data from the current study are also consistent with other approaches suggesting that individual differences in complex span tasks primarily measure attentional abilities, such as inhibitory control (Hasher et al., 2007), goal maintenance (Braver et al., 2007), or the focus of attention (Cowan et al., 2005). Some of these approaches have taken a more fractionated view of the central executive, suggesting, for example, that WMC tasks measure multiple inhibitory processes (Hasher et al., 2007). The data here do not rule out this possibility, provided that one assumes that either the same set of inhibitory processes were common to the WMC and EF tasks that were used in the current study, or a single executive attention resource is common to multiple inhibitory processes. From the present data, the assumption that a single executive attention component underlies performance seems most parsimonious, but parsimony must be weighed against other factors such as the overall explanatory value of a theory, which is often a matter of debate, as in the current situation. Thus, the current data do not adjudicate between different explanations of individual difference in WMC, but rather, provide support for models that propose a unitary character to the central executive component of working memory.
Implications of the Current Study for Theories of Executive Function
The current results converge with other data suggesting that the ability to control attention during goal directed activity is common to many EF tasks (Diamond, 2006; Duncan et al., 1996; Wiebe, Espy, & Charak, 2008). This finding may seem at odds with the idea that there are several distinct executive functions, but even proponents of a distinct factor approach have acknowledged that there is a unitary nature to EFs as well (Miyake et al., 2000; Friedman et al., 2006; 2008). Thus, we do not view the current results as inconsistent with the notion that there are distinct EFs, but rather, that EF tasks reflect both unity and diversity in terms of the cognitive abilities they measure.
Some might argue that the present approach, in which several EF measures that had loaded on a common factor was used, stacked the deck in favor of uncovering a common executive attention factor. However, as noted in the Introduction, each of the tasks we used in the EF battery has been used as a measure of specific EFs other than working memory (e.g., set shifting, response selection, inhibition, etc.). Thus, from the perspective that different tasks should measure different EFs, our results are unexpected. Moreover, we believe that if one were to examine the task demands of the tests used in the EF battery post-hoc, after seeing the results, and then argue that it is obvious that the EF tasks require WM, they would be falling prey to a hindsight bias. The idea that each of the EF tasks requires an ability that is consistent with central executive function is the main point to draw from the current data, and we believe it is an important one, considering that many researchers would seem to predict otherwise (Heitz et al., 2006; Lehto, 1996; Pennington et al., 1996).
To put the matter another way, from a perspective focusing on the unitary nature of EF, it is assumed that it would be difficult to find several EF tasks that did not share a common executive attention component. Indeed, from the executive attention perspective of EF, any battery of disparate EF tasks administered to a sample (without a restriction of range in general abilities) should share considerable overlap with WMC tasks due to their common executive attention demands. That said, further research demonstrating a similar strong relationship between WMC and EF with a different EF battery (or batteries) will be necessary to provide converging evidence for the unitary nature of EF, but the battery we used contained several disparate EF tasks, and thus provides initial support for this claim.
Another point worth noting related to the issue of the unity and diversity of EF is that our approach of focusing on the variance that is common to EFs is as valid an approach to understanding the unitary nature of EF as focusing on variance that is distinct to different EFs is to understanding the diversity of EF. It is important to note that the data presented here do not suggest that each EF task only measures a single common factor, but simply that performance on each EF task is at least partly dependent on a single common factor, which we refer to executive attention, in addition to other factors.
In order to understand how a common executive attention factor, and distinct EF factors, can simultaneously coexist, it is important to consider how task performance was modeled in the current study. The variance that was common to all the EF measures treated the variance that is specific to each task as error. For example, Figure 2 shows a factor loading of .69 for the Wisconsin Card Sorting Task (WCST), indicated that 48% of the variance in performance (.69 × .69 = .48) is shared with the other four EF tasks used to measure that factor. This means that 52% of the variance in WCST performance is modeled as error. It is likely that some of this “error” in WCST performance includes EFs that are not shared with the other four tasks measuring the EF factor. For example, the ability to shift between task goals may be important for WCST performance (Miyake et al., 2000), but may not be important for performance on mental arithmetic. Thus, any specific cognitive process, like shifting, that is not shared by all of the tasks comprising the EF factor, will be modeled as measurement error in the common factor model we computed. This does not mean that the specific EFs that are not common to all of the tasks comprising the executive attention construct are unimportant to higher-level cognitive function; rather, it is simply the case that the approach taken in the current study investigated the variance common to disparate tasks, and did not investigate more specific EF constructs. If one were interested in examining the role of specific EFs in higher-level cognition, a more appropriate approach would be that of Miyake et al. (2000; see also Friedman et al., 2006; Salthouse et al., 2003), whereby multiple measures of each of several specific EFs, e.g., shifting, updating, or inhibition, are administered to a large sample of subjects, and factor analytic models are used to examine multiple EF constructs concurrently.
Complex working memory span tasks were created to tap the central executive component of working memory, and tasks that require concurrent maintenance and manipulation of information have succeeded in fulfilling this goal (see Engle & Kane, 2004 for a review). We would argue that span tasks that are structurally consistent with prototypical complex span tasks, like reading span and operation span, and are administered in a similar fashion (see Conway et al., 2005), should index WMC, and consequently, executive attention. Thus, there should be an “indifference of the indicator” (Spearman, 1927) in the measurement of WMC using complex span tasks, such that any complex span task could substitute for another in a factor analytic study, with little change in the measured construct. Of course, different tasks will rely more or less on central executive functioning and/or task specific abilities. As such, some measures may be better than others in terms of measuring this executive attention component, and will therefore have higher factor loadings.
Unlike WMC tasks, EF tests are not nearly as similar in terms of their structure or task demands, making their interchangeability less clear. That is, because EF tasks have been created to test many disparate abilities, and many different methods are used across EF tasks, it is not immediately apparent that they would share a common ability. However, we would argue that most, if not all, EFs do share common requirements for executive attention, perhaps owing to the goal maintenance requirements of these tasks (Duncan & Owen, 2000). Indeed, theoretically, all EF tasks should measure fluid abilities related to the completion of novel task goals (Burgess, 1997). Thus, we would argue that if other disparate EF tasks were included in a factor analytic study, the common ability underlying performance on the tasks would be executive attention, which would be indistinguishable from the executive attention required for performance on complex span tasks. We believe that the current study, which included verbal, numerical, and spatial tasks of both WMC and EF in order to tap general WMC and EF abilities, provides a better test of whether WMC and EF share an executive attention construct than examining correlation between individual tasks, or even factors that are underspecified (e.g., including only two indicators). We used four tasks to measure WMC and four tasks to measure EF, which assured that no one task had a disproportionate influence in defining either factor, and we used an adult life span sample which ensured a range of variability on general abilities.
We should be clear too that we are not arguing that individual EF and working memory span tasks are necessarily interchangeable. Instead, we contend that it is important that multiple measures of a construct be used to define it in order to avoid unreliable or conflicting results. Because each EF and WMC test measures task-specific skills (e.g., set shifting, or reading comprehension), as well as a common executive attention component, when single tests are used to define these constructs it is unclear if task-specific abilities are driving correlations with other measures, or if these correlations are due to the common executive attention component of the task. This claim seems particularly important in the case of EF tests, because they likely measure more specific EFs in addition to executive attention, but it has been common to associate specific EFs with a single task. This approach is precarious though, because it is not possible to determine which specific factors underlie the correlation between a single EF test and an outcome measure (i.e., whether it is the general attentional component or a task-specific EF component).
Implications of the Study for Cognitive Aging
The data reported in the current study lend support to both the working memory aging hypothesis and the frontal aging hypothesis. Specifically, each of these hypotheses has suggested that age-related declines in complex cognition are the result of age-related declines in frontal-lobe functioning, and to the extent that the current behavioral measures are related to frontal lobe integrity, the current data support this claim. In the present study, the higher-level cognitive function examined was episodic memory, which is arguably the most widely documented cognitive change associated with aging. The results were clear in showing that there were age-related declines in episodic memory associated with advancing adult age, and that when age-related declines in WMC and/or EF were accounted for, age differences in episodic memory were reduced or eliminated. Both WMC and EF behaved similarly in the models, regardless of whether speed was included in the models. Correlations between episodic memory and either WMC or EF ranged between .73-.90, indicating that the control functions measured by these predictors were important for episodic memory performance (see Figure 3 and 4). Combining all eight measures from both constructs in to an executive attention construct led to a very similar result. Thus, the working memory aging hypothesis and frontal aging hypothesis were well supported here.
Limitations of the Current Study
We would be remiss if we did not note limitations of the current study, particularly concerning investigations of the unity and diversity of EF. As mentioned previously, because the EF battery we used did not sample all EFs, it is unclear whether the results we report would generalize to other sets of EF tests. For example, none of the tasks we employed is considered a measure of planning, an important EF measured in previous studies, and thus, it is unclear if measures of planning (e.g., Tower of Hanoi; Arnet et al, 1997) tap the common ability measured by WMC and EF tasks in the current study. Given the lack of consensus regarding theory and measurement of EF, it is imperative to investigate whether the current results would replicate if a different set of EF tasks were used to measure EF.
The current study also does not allow firm conclusions regarding the specific nature of the ability that is common to WMC and EF. That is, we have labeled this common variance executive attention, but this term is somewhat underspecified, and is based on prior theorizing about WMC and EF. Indeed, the approach we employed (i.e., factor analysis) does not provide much specificity with regard to the nature of the overlapping ability measured by WMC and EF, and future research will be required to better understand whether this overlap is due to an ability such as goal maintenance, inhibitory control, resistance to interference, or some other factor. Nevertheless, we believe that the ability underlying WMC and EF is attentional in nature, based on theoretical and empirical considerations mentioned previously.
Another limitation of the current study is that despite the success of the EF construct in accounting for age differences in episodic memory, the models including the EF tasks often led to poor model fits, with fit statistics always being poorer than comparable models including WMC. This appears to at least be partly the result of some of the EF tasks being only weakly related to age. In fact, mental arithmetic was not significantly related to age (-.11), and the correlation between verbal fluency was weak (-.18; see Table 3). Thus, although EF was a plausible mediator of age differences in episodic memory, some of the EF tasks themselves were only weakly related to age, and performance on these tasks was probably influenced by task-specific crystallized abilities that remained stable or improved with age (e.g., vocabulary and arithmetic abilities), which would be expected to leave considerable variance in performance unexplained. The idea that these EF tasks are strongly influenced by crystallized abilities also helps explain why age differences are not always found in EF, or are often very small (see Chan & McDermott, 2007; Salthouse et al., 2003), and further underscores the importance of administering multiple EFs and using factor analytic techniques when examining EF and aging. Note, though, that age sensitivity should not be a criterion for determining if a task is a “good” EF task, especially because executive functions by definition operate on other cognitive operations that might be unaffected by, or even improve, with age (Miyake et al., 2000). Performance on EF tasks may be determined more by these task-specific abilities than the EF abilities the tasks are intended to measure, complicating their interpretation.
A final limitation that we note is that the episodic memory factor employed in the current study was limited to immediate free recall of verbal information. As such, it is not clear if the results of the current study would replicate using other types of episodic memory tasks (e.g., recognition, source memory), or for other types of materials (e.g., faces, visuospatial stimuli). Moreover, it is unclear if the results of the current study would generalize to other outcome measures, such as reading comprehension or fluid intelligence. Thus, future research should be aimed at investigating whether the results we report here would generalize to other measures of episodic memory, and/or other types of higher-level cognitive functioning.
Concluding Remarks
Our study is the first large, cross-sectional study to examine multiple measures of working memory capacity (as measured by complex span tasks) and executive functioning using a factor analytic approach. Our results show that working memory measures and executive function tasks share a large proportion of common variance. We suggest that work from these two traditions may be profitably wedded by focusing on a single underlying construct of executive attention, which we define as the common attention component required to maintain task goals and resolve interference during complex cognition. Our results also strongly support theories maintaining that a common ability, which we refer to as executive attention, is strongly related to complex cognition (i.e., episodic memory), and that age-related declines in executive attention account for age-related declines in episodic memory.
Appendix
Correlation Matrix for All of the Tests Included in the Study, as well as Chronological Age.
| Age | CS | RS | MS | LRS | MA | MC | BDS | FAS | WCST | LC | PC | DSS | SV | Syn | Ant | PR | FR16 | FR40 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||||||||||||||
| Computation Span | -.200** | 1 | |||||||||||||||||
| Reading Span | -.284** | .477** | 1 | ||||||||||||||||
| Match Span | -.409** | .424** | .462** | 1 | |||||||||||||||
| Letter Rotation Span | -.544** | .385** | .427** | .498** | 1 | ||||||||||||||
| Mental Arithmetic | -.107 | .414** | .264** | .275** | .430** | 1 | |||||||||||||
| Mental Control | -.353** | .380** | .331** | .307** | .479** | .449** | 1 | ||||||||||||
| Backward Digit Span | -.180* | .376** | .432** | .439** | .455** | .403** | .407** | 1 | |||||||||||
| Letter Fluency (FAS) | -.184** | .299** | .338** | .188** | .228** | .287** | .405** | .261** | 1 | ||||||||||
| Wisconsin Card Sorting Test | -.541** | .325** | .362** | .445** | .599** | .403** | .302** | .311** | .196** | 1 | |||||||||
| Letter Comparison | -.696** | .365** | .399** | .408** | .543** | .241** | .456** | .331** | .330** | .514** | 1 | ||||||||
| Pattern Comparison | -.736** | .273** | .337** | .428** | .530** | .218** | .434** | .252** | .241** | .538** | .786** | 1 | |||||||
| Digit Symbol Substitution | -.711** | .408** | .447** | .507** | .565** | .255** | .479** | .295** | .357** | .560** | .758** | .750** | 1 | ||||||
| Shiply Vocabulary | .201** | .210** | .261** | .065 | .133 | .421** | .180* | .294** | .310** | .172* | .124 | .046 | .040 | 1 | |||||
| Synonyms | .217** | .186** | .266** | .068 | .106 | .364** | .186** | .269** | .291** | .086 | .082 | .011 | .027 | .786** | 1 | ||||
| Antonyms | .127 | .259** | .266** | .085 | .195** | .392** | .231** | .273** | .267** | .167* | .141* | .083 | .057 | .719** | .775** | 1 | |||
| Prose Recall | -.219** | .304** | .325** | .209** | .376** | .458** | .254** | .291** | .196** | .361** | .291** | .269** | .323** | .342** | .333** | .317** | 1 | ||
| Free Recall-16 Words | -.324** | .218** | .354** | .289** | .355** | .319** | .248** | .282** | .273** | .314** | .250** | .292** | .340** | .168* | .185** | .194** | .424** | 1 | |
| Free Recall-40 Words | -.328** | .359** | .435** | .396** | .428** | .352** | .290** | .318** | .322** | .371** | .358** | .335** | .419** | .211** | .254** | .222** | .501** | .566** | 1 |
denotes significance at p<.01;
denotes significance at p<.05
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu.
Contributor Information
David P. McCabe, Colorado State University
Henry L. Roediger, III, Washington University in St Louis.
Mark A. McDaniel, Washington University in St Louis
David A. Balota, Washington University in St Louis
David Z. Hambrick, Michigan State University
References
- Albert M, Blacker D, Moss M, Tanzi R, McArdle J. Longitudinal cognitive change among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive functions and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Ashendorf L, McCaffrey RJ. Exploring age-related decline on the Wisconsin Card Sorting Test. The Clinical Neuropsychologist. 2008;22:262–272. doi: 10.1080/13854040701218436. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA. Age-related cognitive deficits mediated by changes in the striatal dopamine system. American Journal of Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford: Clarendon Press; 1986. [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Balota DA, Law MB, Zevin JD. The attentional control of lexical processing pathways: Reversing the word frequency effect. Memory & Cognition. 2000;28:1081–1089. doi: 10.3758/bf03211809. [DOI] [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, Yerys BE. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Banich MT. Executive function: The search for a integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Barrouillet P, Lecas J. Mental models in conditional reasoning and working memory. Thinking & Reasoning. 1999;5:289–302. [Google Scholar]
- Bishara AJ, Kruschke JK, Stout JC, Bechara A, McCabe DP, Busemeyer JR. Sequential learning models for the Wisconsin Card Sort Task: Assessing processes in substance dependent individuals. Journal of Mathematical Psychology. doi: 10.1016/j.jmp.2008.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. Toward a revised theory of general intelligence: Further examination of fluid cognitive abilities as unique aspects of human cognition. Behavioral and Brain Sciences. 2006;29:145–160. [Google Scholar]
- Blair C, Zelazo PD, Greenberg M. The assessment of executive function in early childhood: Prospects and progress. Developmental Neuropsychology. 2005;28:561–571. doi: 10.1207/s15326942dn2802_1. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: John Wiley & Sons; 1989. [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. Oxford: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Burgess PW. Theory and methodology in executive function research. In: Rabbitt P, editor. Methodology of Frontal and Executive Function. Hove, U.K.: Psychology Press; 1997. pp. 81–16. [Google Scholar]
- Butler KM, McDaniel MA, Dornburg CC, Price AL, Roediger HL. Age differences in veridical and false recall are not inevitable: The role of frontal lobe function. Psychonomic Bulletin & Review. 2004;11:921–925. doi: 10.3758/bf03196722. [DOI] [PubMed] [Google Scholar]
- Bugaiska A, Clarys D, Jarry C, Taconnat L, Tapia G, Vanneste S, Isingrini M. The effect of aging in recollective experience : The processing speed and executive functioning hypothesis. Cousciousness and Cognition. 2007;16:797–808. doi: 10.1016/j.concog.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Chan JCK, McDermott KB. The effects of frontal lobe functioning and age on veridical and false recall. Psychonomic Bulletin & Review. 2007;14:606–611. doi: 10.3758/bf03196809. [DOI] [PubMed] [Google Scholar]
- Cinan S. Age-related changes in concept formation, rule switching, and perseverative behaviors: A study using WCST with 12 unidimensional target cards. Cognitive Development. 2006;21:377–382. [Google Scholar]
- Colom R, Rebollo I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin and Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Engle RW. Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K.: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Cowan N. Working memory capacity. New York: Psychology Press; 2005. [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Delaney PF, Sahakyan L. Unexpected costs of high working memory capacity following directed forgetting and context change manipulations. Memory & Cognition. 2007;35:1074–1082. doi: 10.3758/bf03193479. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan executive function system. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. second. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Depue BE, Banich MT, Curran T. Suppression of emotional and non-emotional content in memory: Effects of repetition on cognitive control. Psychological Science. 2006;17:441–447. doi: 10.1111/j.1467-9280.2006.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschuyteneer M, Vandierendonck A. Are “input monitoring” and “response selection” involved in solving simple mental arithmetical sums? European Journal of Cognitive Psychology. 2005;17:347–370. [Google Scholar]
- Deschuyteneer M, Vandierendonck A, Muyllaert I. Does solution of mental arithmetic problems such as 2 + 6 and 3 × 8 rely on the process of “memory updating”? Experimental Psychology. 2006;53:298–208. doi: 10.1027/1618-3169.53.3.198. [DOI] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik F, editors. Lifespan cognition: Mechanisms of change. NY: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Johnson R, Swales M, Freer C. Frontal lobe deficits after head injury: Unity and diversity of function. Cognitive Neuropsychology. 1997;14(5):713–741. [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B, editor. The Psychology of Learning and Motivation. Vol. 44. NY: Elsevier; 2004. pp. 145–199. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Ettenhofer M, Hambrick DZ, Abeles N. Reliability and stability of executive function in a sample of older adults. Neuropsychology. 2006;20:607–613. doi: 10.1037/0894-4105.20.5.607. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Ferrer-Caja E, Crawford JR, Bryan J. A structural modelling examination of the executive decline hypothesis of cognitive aging through reanalysis of Crawford et al.'s (2000) data. Aging, Neuropsychology and Cognition. 2002;9:231–249. [Google Scholar]
- Ferrier D. The functions of the brain. 2nd. London: Smith, Elder; 1886. [Google Scholar]
- Fletcher JM. Executive functions in children: Introduction to a special series. Developmental Neuropsychology. 1996;12:1–3. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AF, Hale S. Processing speed, working memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7:237–241. [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biological Psychology. 2000;54:1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 3rd. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Greve KW, Love JM, Sherwin E, Mathias CW, Ramzinski P, Levy J. Wisconsin Card Sorting Test in chronic severe traumatic brain injury: Factor structure and performance subgroups. Brain Injury. 2002;16:29–40. doi: 10.1080/0269905011008803. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. New York: Oxford University Press; 2007. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York, NY: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Heaton RK. Wisconsin card sorting test: Computer version-2 research edition. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Hedden T, Yoon C. Individual differences in executive processing predict susceptibility to interference in verbal working memory. Neuropsychology. 2006;20:511–528. doi: 10.1037/0894-4105.20.5.511. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Redick TS, Hambrick DZ, Kane MJ, Conway ARA, Engle RW. WM, EF, and gF are not the same. Behavioral and Brain Sciences. 2006;29:135–136. [Google Scholar]
- Henkel LA, Johnson MK, DeLeonardis DM. Aging and source monitoring: cognitive processes and neuropsychological correlates. Journal of Experimental Psychology: General. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Homack S, Lee D, Riccio CA. Test Review: Delis-Kaplan executive function system. Journal of Clinical and Experimental Neuropsychology. 2005;20:117–128. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler P. Evaluating model fit. In: Hoyle RH, editor. Structural equation Mmodelin: Concepts, issues, and applications. London: Sage; 1995. pp. 76–99. [Google Scholar]
- Hull R, Martin RC, Beier ME, Lane D, Hamilton AC. Executive function in older adults: A structural equation modeling approach. Neuropsychology. 2008;22:508–522. doi: 10.1037/0894-4105.22.4.508. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Bishara AJ, Hessels S, Toth JP. Aging, subjective experience, and cognitive control: Dramatic false remembering by older adults. Journal of Experimental Psychology: General. 2005;134:131–148. doi: 10.1037/0096-3445.134.2.131. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of WM capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Hambrick DZ, Engle RW. Variation in working memory capacity as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 21–48. [Google Scholar]
- Kane MJ, Engle RW. WM capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Lamar M, Swenson R, Kaplan E, Libon DJ. Characterizing alterations in executive functioning across distinct subtypes of cortical and subcortical dementia. The Clinical Neuropsychologist. 2004;18:22–31. doi: 10.1080/13854040490507127. [DOI] [PubMed] [Google Scholar]
- Lehto J. Are executive functions tests dependent on working memory capacity? Quarterly Journal of Experimental Psychology. 1996;48A:29–50. [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Li KZH, Lindenberger U. Connections among sensory, sensorimotor, and cognitive aging: Review of data and theories. Neuroscience and Biobehavioral Reviews. 2002;26:777–783. doi: 10.1016/s0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Logan GD. Executive control of thought and action: In search of the wild homunculus. Current Directions in Psychological Science. 2003;12:45–48. [Google Scholar]
- Luria AR. The Working Brain. New York: Basic Books; 1973. [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Koth CW, Cutting LE, Singer HS, Denckla MB. Executive function in fluency and recall measures among children with Tourette Syndrome or ADHD. Journal of the International Neurology Society. 2001;7:102–111. doi: 10.1017/s1355617701711101. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA. Aging decreases veridical remembering but increases false remembering: Neuropsychological test correlates of remember/know judgments. Neuropsychologia. 2009;47:2164–2173. doi: 10.1016/j.neuropsychologia.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Smith AD. The effect of warnings on false memories in younger and older adults. Memory & Cognition. 2002;30:1065–1077. doi: 10.3758/bf03194324. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Smith AD, Parks CP. Inadvertent plagiarism in young and older adults. Memory & Cognition. 2007;35:231–241. doi: 10.3758/bf03193444. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Glisky EL, Rubin SR, Guynn MJ, Routhieaux BC. Prospective memory: A neuropsychological study. Neuropsychology. 1999;13:103–110. doi: 10.1037//0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York: Cambridge University Press; 1999. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Salthouse TA, Craik FIM, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Norman D, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and Self Regulation: Advances in Research and Theory. Vol. 4. New York: Plenum; 1986. pp. 1–18. [Google Scholar]
- Oberauer K. Control of the contents of working memory - a comparison of two paradigms and two age groups. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2005;31:714–728. doi: 10.1037/0278-7393.31.4.714. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Braver TS, Cohen JD. A biologically based computational model of working memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. New York: Cambridge University Press; 1999. pp. 375–411. [Google Scholar]
- Osaka M, Osaka N, Kondo H, Morishita M, Fukuyama H, Aso T, Shibasaki H. The neural basis of individual differences in working memory capacity: An fMRI study. NeuroImage. 2003;18:789–797. doi: 10.1016/s1053-8119(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson N, Smith AD, Smith P. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles J, Frieske D, Zwahr M, Gaines C. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11(4):621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bennetto L, McAleer O, Roberts RJ. Executive functions and working memory. In: Lyons GR, Krasnegor NA, editors. Attention, memory and executive function. Baltimore: Paul H. Brookes; 1996. pp. 327–348. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Persson J, Reuter-Lorenz PA. Gaining control: Training executive function and far transfer of the ability to resolve interference. Psychological Science. 2008;19:881–888. doi: 10.1111/j.1467-9280.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Della Sala S. Aging, intelligence, and anatomical segregation in the frontal lobes. Learning and Individual Differences. 1998;10:217–243. [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: The MIT Press; 1998. pp. 401–423. [Google Scholar]
- Rapport MD, Chung KM, Shore G, Denney CB, Isaacs P. Upgrading the science and technology of assessment and diagnosis: Laboratory and clinic-based assessment of children with ADHD. Journal of Clinical Child Psychology. 2000;29:555–568. doi: 10.1207/S15374424JCCP2904_8. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo. In: Cabeza R, Nyberg L, C D, editors. Cognitive neuroscience of aging. New York: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin Card Sorting Task: A meta-analytic review. Psychology and Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Rodríguez -Aranda C, Sundet K. The frontal hypothesis of cognitive aging: factor structure and age effects on four “frontal tests” among healthy individuals. Journal of Genetic Psychology. 2006;167:269–287. doi: 10.3200/GNTP.167.3.269-287. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Geraci L. Aging and the misinformation effect: A neuropsychological analysis. Journal of Experimental Psychology: Learning, Memory & Cognition. 2007;33:321–334. doi: 10.1037/0278-7393.33.2.321. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Watson JM, McDermott KB, Gallo DA. Factors that determine false recall: A multiple regression analysis. Psychonomic Bulletin & Review. 2001;8:385–407. doi: 10.3758/bf03196177. [DOI] [PubMed] [Google Scholar]
- Rosen V, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. Working memory capacity and suppression. Journal of Memory and Language. 1998;39:418–436. [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: contributions to the emergence of self regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Working memory as a processing resource in cognitive aging. Developmental Review. 1990;10:101–124. [Google Scholar]
- Salthouse TA. Speed and knowledge as determinants of adult age differences in verbal tasks. Journal of Gerontology: Psychological Sciences. 1993;48:29–36. doi: 10.1093/geronj/48.1.p29. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Processing capacity and its role on the relations between age and memory. In: Weinert FE, Schneider W, editors. Memory Performance and Competencies: Issues in Growth and Development. Hillsdale, N.J.: Erlbaum; 1995. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Shad M, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia – A pilot study. Psychiatry Research: Neuroimaging. 2006;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shah P, Miyake A. The separability of working memory resources for spatial thinking and language processing: An individual differences approach. Journal of Experimental Psychology: General. 1996;125:4–27. doi: 10.1037//0096-3445.125.1.4. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. Supervisory control of action and thought selection. In: Baddeley AD, Weiskrantz L, editors. Attention: Selection, awareness, and control. Oxford: Oxford University Press; 1993. pp. 171–187. [Google Scholar]
- Spearman C. The abilities of man, their nature and measurement. London: Macmillan; 1927. [Google Scholar]
- Stine EAL, Hindman J. Age differences in reading time allocation for propositionally dense sentences. Aging and Cognition. 1994;1:2–16. [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Introduction. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 1–7. [Google Scholar]
- Taconnat L, Clarys D, Vanneste S, Bouazzaoui B, Isingrini M. Aging and strategic retrieval in a cued-recall test: The role of executive functions and fluid intelligence. Brain and Cognition. 2007;64:1–6. doi: 10.1016/j.bandc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Teuber HL. Unity and diversity of frontal lobe functions. Acta Neurobiologiae Experimentalis. 1972;32:615–656. [PubMed] [Google Scholar]
- Thurstone LL. Primary mental abilities Psychometric Monographs, No.1. Chicago: University of Chicago Press; 1938. [Google Scholar]
- Troyer AK, Graves RE, Cullum KM. Executive functioning as a mediator of the relationship between age and episodic memory in healthy aging. Aging and Cognition. 1994;1:45–53. [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007a;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex spans and their relation to higher-order abilities. Psychological Bulletin. 2007b;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Van der Sluis S, de Jong PF, Van der Leij A. Executive functioning in children, and its relations with reasoning, reading, and arithmetic. Intelligence. 2007;35:427–449. [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Watson JM, Bunting MF, Poole BJ, Conway ARA. Individual differences in susceptibility to false memory in the Deese-Roediger-McDermott paradigm. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2005;31:76–85. doi: 10.1037/0278-7393.31.1.76. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. WAIS-III/WMS-III technical manual. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R, Bowry R. The aging of cognitive control: Studies of conflict processing, goal neglect, and error monitoring. In: Engle RW, Sedek G, von Hecker U, McIntosh DM, editors. Cognitive Limitations in Aging and Psychopathology: Attention, Working Memory, and Executive Functions. Cambridge, UK: Cambridge University Press; 2005. pp. 97–121. [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal status on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Charak D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology. 2008;44:575–587. doi: 10.1037/0012-1649.44.2.575. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. BADS: Behavioural Assessment of the Dysexecutive Syndrome Bury St. Edmunds, UK: Thames Valley Test Company; 1996. [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised Manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- Zillmer EA, Spiers MV. Principles of neuropsychology. Belmont: Wadsworth; 2001. [Google Scholar]