Abstract
We describe a series of fluorocarbon surfactant polymers designed as surface-modifying agents for improving the thrombogenicity of ePTFE vascular graft materials by the reduction of platelet adhesion. The surfactant polymers consist of a poly(vinyl amine) backbone with pendent dextran and perfluoroundecanoyl branches. Surface modification is accomplished by a simple dip-coating process in which surfactant polymers undergo spontaneous surface-induced adsorption and assembly on PTFE/ePTFE surface. The adhesion stability of the surfactant polymer on PTFE was examined under dynamic shear conditions in PBS and human whole blood with a rotating disk system. Fluorocarbon surfactant polymer coatings with three different dextran to perfluorocarbon ratios (1:0.5, 1:1 and 1:2) were compared in the context of platelet adhesion on PTFE/ePTFE surface under dynamic flow conditions. Suppression of platelet adhesion was achieved for all three coated surfaces over the shear-stress range of 0–75 dyn/cm2 in platelet-rich plasma (PRP) or human whole blood. The effectiveness depended on the surfactant polymer composition such that platelet adhesion on coated surfaces decreased significantly with increasing fluorocarbon branch density at 0 dyn/cm2. Our results suggest that fluorocarbon surfactant polymers can effectively suppress platelet adhesion and demonstrate the potential application of the fluorocarbon surfactant polymers as non-thrombogenic coatings for ePTFE vascular grafts.
Keywords: Platelet adhesion, polytetrafluoroethylene, expanded polytetrafluoroethylene, dextran, fluorocarbon surfactant polymers
1. Introduction
Expanded polytetrafluoroethylene (ePTFE) is a widely used biomaterial for small and medium-sized vascular grafts, due to its mechanical strength, chemical inertness and non-adhesiveness [1–5]. ePTFE vascular grafts have exhibited reasonable success for medium-sized vessels (inner diameter (ID) approx. 6–8 mm). However, for smaller diameters (ID < 5 mm), graft performance has been drastically restricted by pathophysiological problems like thrombotic occlusion, thromboem-bolism, intimal hyperplasia and infection [1–5]. A particular clinical challenge for small diameter ePTFE grafts is thrombotic occlusion, which is initiated by protein and platelet interaction with the graft surface [3, 5]. Platelets are known to play a key role in the surface-induced thrombosis. Upon adhesion on a foreign material surface and subsequent activation, platelets accelerate thrombosis by secretion of the granule contents, generation of microparticles, formation of fibrinogen-mediated platelet aggregate and acceleration of thrombin production [6].
Platelet adhesion on synthetic materials is mediated by plasma protein adsorption [7]. Specifically, adsorbed fibrinogen [8] and von Willebrand factor (vWf) [9] are known to adhere and activate platelets in vitro. One strategy for improving blood material interfacial compatibility is to suppress the protein and platelet adhesion by incorporating hydrophilic chain molecules like PEO [10, 11] or oligosac-charide [12]. However, surface modification on PTFE, which generates highly hydrated surfaces consisting of individual chain molecules, generally involves high-energy surface treatment such as radio frequency glow discharge [13, 14], chemical etching [15, 16], iron-beam treatment [17], or photoexcited electron-transfer reactions [18]. The main drawback for such approaches is the undefined surface chemistry.
An alternative approach is to utilize physicochemical methods. Tae et al. recently reported using physisorption of fluoroalkyl-terminated poly(ethylene glycol) to modify PTFE through hydrophobic interaction [19]. Similar approaches have been utilized by Larendo et al. to modify carbon-coated PTFE graft with silyl–heparin [20]. There are other reports about combining the physisorption of phos-phorycholine polymer and PEO tri-block polymer with cross-linking by gamma irradiation [21, 22]. The approaches have met with only limited success. For example, PEO-containing triblock co-polymer adsorbed on ePTFE showed about 38% reduction of platelet adhesion [22]. PEG glow discharge coating on ePTFE membrane reduced the platelet pseudopod formation, but did not decrease the platelet adhesion [23]. Most of the above systems employ a simple AB or ABA type molecular structure, which only allows for 1 (AB type) or 2 (ABA type) hydrophobic chains to interact with the substrate. The adhesion stability of the surfactant can be improved by adopting a comb-like graft polymer structure, which allows for multiple hydrophobic chains (>150) along a polymer backbone to interact with the surface and with each other. From that perspective, we developed a series of surfactant polymers based on poly(vinyl amine) (PVAm) backbone with pendent dextran or oligomaltose and hydrophobic hexanoyl groups, which have shown to suppress protein adsorption on graphite [24–26], and platelet adhesion and activation on polyurethane and polycarbonate [26, 27]. However, the hydrocarbon surfactant polymers do not have strong affinity to the PTFE surface. To address this problem, surfactant polymers with fluorocarbon branches have been designed to increase the adhesion between surfactant polymer and PTFE [28]. The fluorocarbon surfactant polymers described here are based on poly(vinyl amine) with pendent hydrophilic dextran and hydrophobic perfluoroundecanoyl branches (Fig. 1).
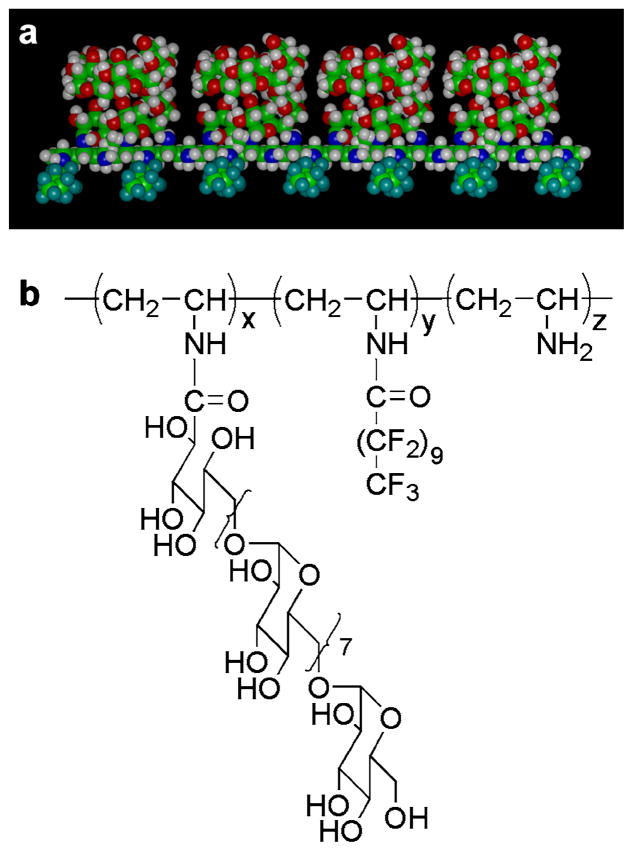
Figure 1.
Molecular model and chemical structure of the fluorocarbon surfactant polymer. (a) Molecular model of a fluorocarbon surfactant polymer with pendent dextran and perfluoroundecanoyl chains along the poly(vinyl amine) backbone. (b) Chemical structure of the fluorocarbon surfactant polymer. This figure is published in colour at http://www.ingenta.com
This approach takes advantage of the thermodynamic compatibility between fluorocarbon chain and PTFE surface and the thermodynamic driving force that creates a dense surface coating of surfactant molecules. Fluorocarbon chains serve as physical binding ligands that constrain the polymer backbone to the fluorocarbon surface while dextran molecules in the surfactant polymer are designed to mimic the polysaccharide-rich cell-surface glycocalyx [29] to provide a highly hydrated steric barrier that prevents protein adsorption and cellular adhesion.
The effectiveness of the surfactant polymer coating in preventing protein adsorption and platelet adhesion is known to depend on several factors, including the packing density and the thickness of the hydrated dextran layer on PTFE [12, 30, 31]. To address the issue of optimal surface density, we examined the influence of hydrophilic/hydrophobic ratio in fluorocarbon surfactant polymers on the suppression of platelet adhesion. Fluorocarbon surfactant polymers with three different ratios of dextran to perfluoroundecanoyl side-chains were adsorbed on PTFE and examined for suppression of platelet adhesion under dynamic flow conditions. Coated PTFE and ePTFE surfaces were examined by epifluorescence microscopy for their ability to suppress the adhesion of platelets in human platelet-rich plasma and whole blood.
2. Materials and Methods
2.1. Surfactant Polymer Synthesis and Fluorescence Labeling
The synthesis and characterization of fluorocarbon surfactant polymers were described in a previous report [28]. Briefly, the fluorocarbon surfactant polymers were synthesized by sequential attachment of dextran and perfluoroundecanoyl branches to the poly(vinyl amine) backbone. The dextran percentage in the fluorocarbon surfactant polymers was kept constant at 22 mol%, whereas the percentage of fluorocarbon branches varied from 15 to 45 mol% [28]. The fluorocarbon surfactant polymers were designated as PVAm(Dex:FC11) 1:0.5, 1:1 or 1:2, according to the ratio of dextran to perfluoroundecanoyl side-chains in the polymer structure (Fig. 1).
Fluorocarbon surfactant polymer, PVAm(Dex:FC11) 1:2, was labeled with fluorescein to help examine the coating by fluorescence microscopy. The procedure was described in a previous report [27].
2.2. Surfactant Coating on PTFE and ePTFE Surface
Polytetrafluoroethylene (PTFE, skived thin sheet) was purchased from Enflo (Bristol, CT, USA). Expanded PTFE (ePTFE, pore size approx. 30 μm) was purchased from Zeus Industrial Products (Orangeburg, SC, USA). Cleaning was accomplished by sonicating PTFE/ePTFE sequentially in acetone, DMSO and water for 10 min. PTFE and ePTFE were modified by immersing in surfactant solution (1–2 mg/ml) for 48 h. The surfactant solution was diluted several times with pure water before samples were removed and air-dried overnight.
2.3. Whole Blood and Platelet-Rich Plasma Collection
Whole blood from healthy human donors was drawn by an IRB-approved venipuncture protocol, with sodium citrate (1:9, v/v) as anticoagulant. All subjects enrolled in this research responded to an Informed Consent approved by the Committee on Human Research at the Case Western Reserve University. Platelet-rich plasma (PRP) was obtained by centrifuging whole blood at 800 × g for 15 min and the remaining fraction was further centrifuged at 1100 × g to obtain platelet-poor plasma (PPP). The platelet concentration in PRP samples was adjusted with PPP to 2 × 108/ml.
2.4. Analysis of Surfactant Polymer Stability by Fluorescence Microscopy
The fluorescently labeled surfactant polymer, PVAm(Dex:FC11) 1:2, was adsorbed onto PTFE disks, and the coated disk surface was imaged under an epifluorescence microscope (Nikon Diaphot 200) using a 40× oil immersion objective with a fixed camera exposure time. Surface-averaged fluorescence intensity values were obtained from the images using ImageJ software. The disks were then subjected to PBS or whole blood in the rotating disk (RD) apparatus under dynamic shear and imaged again with the same objective and microscope setting to determine the post-exposure fluorescence intensity. The set up of the rotating disk system (RDS) was described previously [28, 30].
2.5. Platelet Adhesion Under Static and Dynamic Conditions
For static conditions, human whole blood (250 μl) was pooled on the test surface for 30 min, following which platelets adherent to the surface were stained using fluorescently tagged antibodies and imaged with an epifluorescence microscope. For dynamic conditions, RDS was used to simulate laminar flow to test platelet adhesion on PTFE/ePTFE surface. A shear-stress range of 0–75 dyn/cm2 was typically used in the RDS to test in vitro platelet adhesion in PRP or whole blood.
2.6. Platelet Staining and Fluorescence Microscopy
Platelet staining and fluorescence microscopy were performed as described in a previous report [27], with some modifications. Briefly, adherent platelets on test surfaces from static or dynamic studies were stained with fluorescein isothiocyanate (FITC) anti-human CD41a (emission approx. 530 nm, green fluorescence) which binds to platelet integrin GP IIb–IIIa and R-phycoerythrin (R-PE) anti-human CD62p (emission approx. 570 nm, orange-red fluorescence) which binds to platelet P-selectin. Test surfaces were then examined under an inverted epifluorescence microscope (Nikon Diaphot 200) with 40× and 100× oil immersion lenses. A filter cube containing a 450–490 nm excitation filter, a 510 nm dichromatic mirror and a 520–560 nm bandpass filter was used for capturing FITC fluorescence, while a filter cube containing a 510–560 nm excitation filter, a 575 nm dichromatic mirror and a 610 nm longpass filter was used to capture PE fluorescence. For qualitative analysis of platelet adhesion and activation, FITC and PE fluorescence images (hence expression of GP IIb–IIIa and P-selectin on platelets) were captured in the same field using alternate filter cubes in a filter slider. For quantitative assessment of platelet adhesion from dynamic rotating disk (RD) experiments, only FITC fluorescence images were captured at 1 mm incremental radial distances from the center of each disk sample and analyzed using Metamorph®.
2.7. Data Analysis
Statistically significant differences (P < 0.05) between multiple means were determined by the method of analysis of variance (ANOVA). The two-tailed Student’s t -test was used to determine statistically significant differences (P < 0.05) between pairs of means. The statistical analysis was completed using Minitab.
3. Results
3.1. Analysis of Surfactant Polymer Stability in PBS
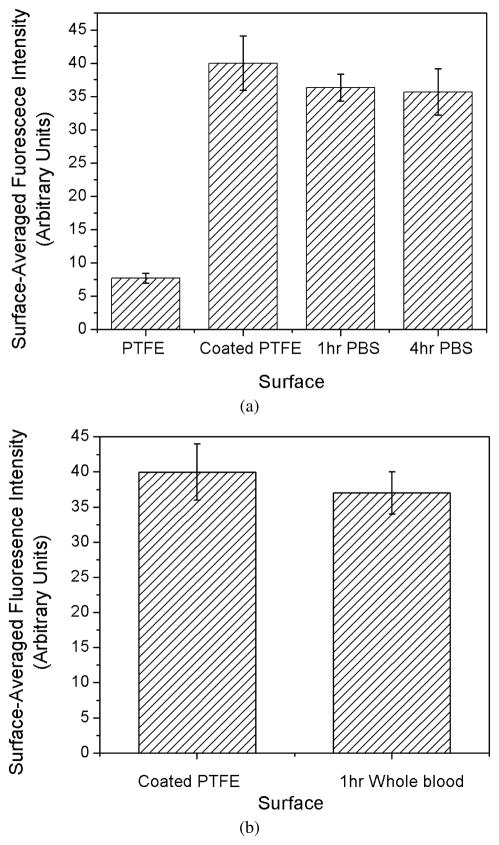
We examined the fluorocarbon surfactant polymer adhesion stability on PTFE using XPS under dynamic flow conditions in PBS in a previous report [28]. In this report, we further tested the surfactant polymer stability on PTFE under dynamic shear conditions with fluorescence microscopy. We labeled a fluorocarbon surfactant polymer, PVAm(Dex:FC11) 1:2, with fluorescein, and examined the surface before and after exposure to PBS in the RD experiment. Figure 2a shows the surface-averaged fluorescence intensity on PTFE before, 1 h after and 4 h after exposure to PBS at 100 dyn/cm2. There is a slight drop in fluorescence intensity between the initial coating and 1 h experiment (P = 0.06); however, there is no statistical difference (P = 0.65) in fluorescence intensity between the 1 h and 4 h experiment.
Figure 2.
Stability of the fluorocarbon surfactant polymer, PVAm(Dex:FC11) 1:2, on PTFE under dynamic flow conditions in PBS and whole blood. (a) Surface-averaged fluorescence intensity (gray value, measured in ImageJ) of uncoated PTFE, coated PTFE, 1 h and 4h after exposure to PBS at the shear-stress level of 100 dyn/cm2. (b) Surface-averaged fluorescence intensity of PTFE disk before and 1 h after exposure to human whole blood in the RD experiment at the shear-stress level of 75 dyn/cm2.
3.2. Analysis of Surfactant Polymer Stability in Human Whole Blood
The human blood contains many surface-active proteins that may exchange with the surfactant polymer on the surface. To get a direct assessment of the surfactant polymer coating stability on PTFE, we labeled a fluorocarbon surfactant polymer, PVAm(Dex:FC11) 1:2, with fluorescein, and imaged the coating before and after exposure to human whole blood in the RD experiment. Figure 2b shows the surface-averaged fluorescence intensity on PTFE before and after exposure to human whole blood at 75 dyn/cm2 for 1 h. The fluorescence intensity shows no significant difference before and after the human whole blood RD experiment (P = 0.08).
3.3. Reduction of Platelet Adhesion on PTFE
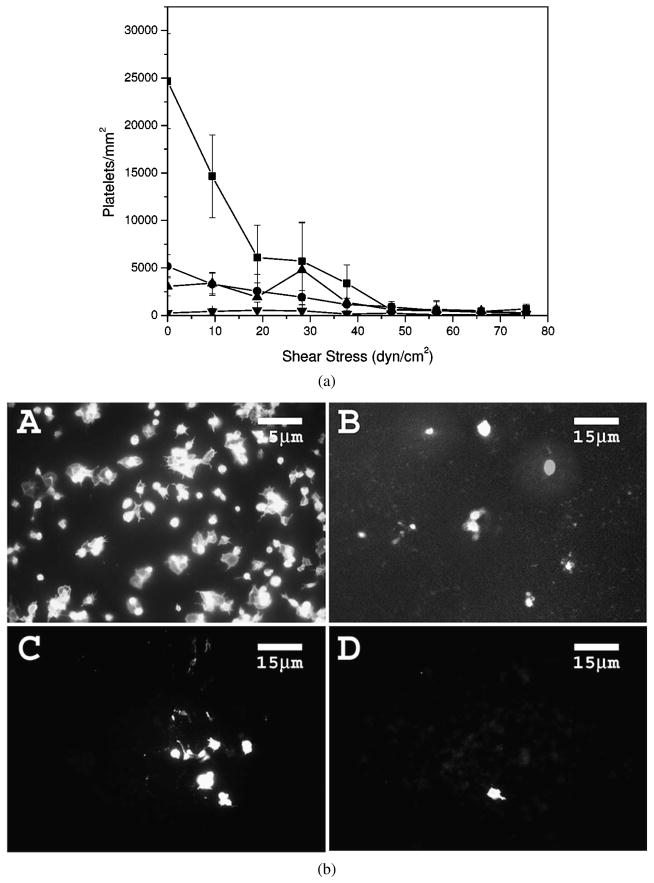
Platelet adhesion on PTFE was investigated using a RDS in PRP. The result for platelet adhesion on PTFE in PRP over the shear-stress range of 0–75 dyn/cm2 is shown in Fig. 3a. Platelet adhesion on uncoated PTFE decreases with increasing applied shear-stress level and becomes very low at shear-stress levels above 38 dyn/cm2, suggesting shear-induced detachment of platelets. Platelet adhesion on all fluorocarbon surfactant-coated PTFE is significantly lower than that on uncoated PTFE over the shear-stress range of 0–10 dyn/cm2, and is less shear-stress dependent. Among the fluorocarbon surfactant polymers, the platelet adhesion decreases in the order of PVAm(Dex:FC11) 1:0.5 < 1:1 < 1:2 at 0 dyn/cm2, and the differences are significant (P < 0.01). Over the shear-stress range of 0–10 dyn/cm2, PVAm(Dex:FC11) 1:2 is more effective at preventing platelet adhesion than PVAm(Dex:FC11) 1:1 or 1:0.5 (P < 0.01).
Figure 3.
Platelet adhesion on PTFE surfaces in PRP. (a) Platelet adhesion as a function of applied shear-stress level: (■) uncoated PTFE, (●) PVAm(Dex:FC11) 1:0.5 coated PTFE, (▲) PVAm(Dex:FC11) 1:1 coated PTFE and (▼) PVAm(Dex:FC11) 1:2 coated PTFE. (b) Representative epifluorescence images of platelets on PTFE surfaces at 0 dyn/cm2: (A) uncoated PTFE, (B) PVAm(Dex:FC11) 1:0.5 coated PTFE, (C) PVAm(Dex:FC11) 1:1 coated PTFE and (D) PVAm(Dex:FC11) 1:2 coated PTFE.
Platelet morphology is an important indicator of platelet activation [6, 32]. Activated platelets usually exhibit more spreading on the surface with extending pseudopodia. Figure 3b shows representative epifluorescence images of platelets on PTFE surfaces at 0 dyn/cm2. On the bare PTFE, platelets have an irregular shape and have spread on the surface, indicative of platelet activation. PVAm(Dex:FC11) 1:0.5 surfaces show less platelet adhesion than the PTFE control. PVAm(Dex:FC11) 1:1 surfaces exhibit even less platelet adhesion, and PVAm(Dex:FC11) 1:2 surfaces exhibit minimal platelet adhesion and the adherent platelets tend to be less activated than those on other surfaces, as indicated by the observed platelet morphology.
3.4. Reduction of Platelet Adhesion and Activation on ePTFE
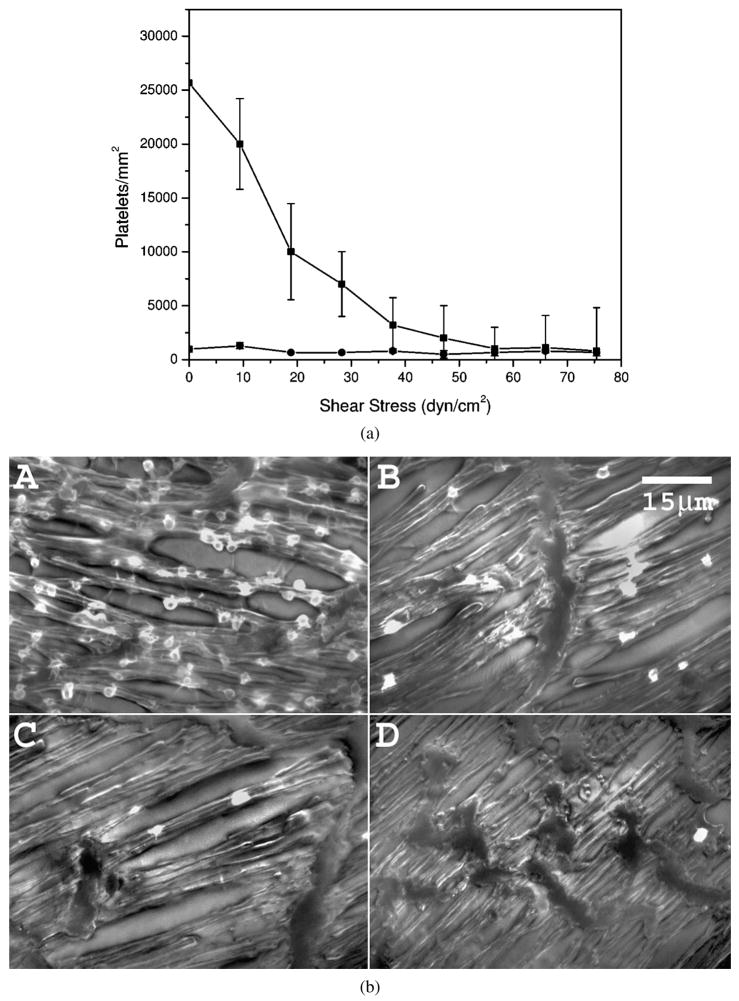
Platelet adhesion on ePTFE was investigated with the RD experiment in PRP. The surfactant polymer PVAm(Dex:FC11) 1:2 significantly reduces platelet adhesion, compared with uncoated ePTFE over the shear-stress range of 0–30 dyn/cm2 (P < 0.01), and the platelet adhesion on the coated ePTFE remains relatively constant over the shear-stress range of 0–75 dyn/cm2 (Fig. 4a).
Figure 4.
Platelet adhesion on ePTFE surfaces in PRP. (a) Platelet adhesion as a function of applied shear-stress level on (■) uncoated ePTFE and (◆) PVAm(Dex:FC11) 1:2 coated ePTFE. (b) Reprehensive epifluorescence images of platelets on ePTFE surfaces in PRP: (A) uncoated ePTFE at 0 dyn/cm2, (B) PVAm(Dex:FC11) 1:2 coated ePTFE at 0 dyn/cm2, (C) uncoated ePTFE at 38 dyn/cm2 and (D) PVAm(Dex:FC11) 1:2 coated ePTFE at 38 dyn/cm2.
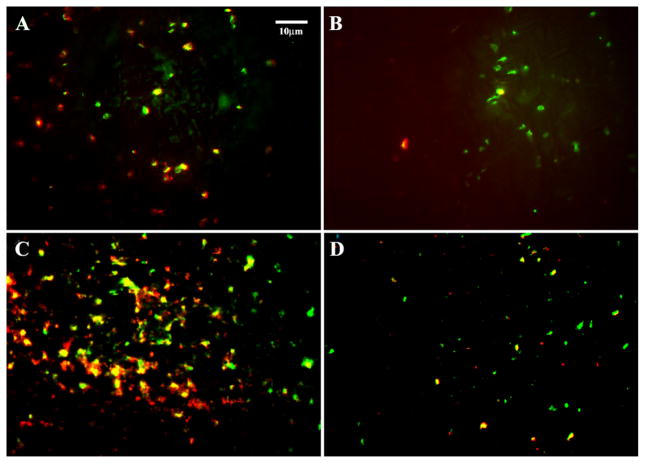
Formation of platelet pseudopodia is obvious on uncoated ePTFE (Fig. 4b). The platelets are much more rounded on the surfactant polymer coated ePTFE, suggesting a lower level of platelet activation (Fig. 4b). To confirm our observation, we performed a additional test on platelet activation by monitoring the expression of platelet P-selectin on ePTFE under both static and dynamic conditions. Figure 5 shows representative images of the adherent platelets double stained with FITC anti-human CD41a (GP IIb–IIIa) and PE anti-human CD62p (P-selectin) under the static and dynamic conditions. As seen from Fig. 5, the number of platelets adhering to the PVAm(Dex:FC11) 1:2 coated ePTFE under the static ‘pooled blood’ conditions is reasonably less than that on the uncoated ePTFE. More importantly, the level of PE fluorescence (hence P-selectin) from adherent platelets is considerably lower on the coated ePTFE than that on uncoated ePTFE, indicating a lower level of platelet activation on the coated surface. A similar trend was observed under the dynamic conditions, although the level of platelet activation is generally higher, especially on the uncoated ePTFE. There are numerous activated platelets adherent to the uncoated ePTFE as evidenced by the enhanced P-selectin (red) fluorescence (Fig. 5) emitted from aggregated platelets. Particles stained with P-selection (red) but not GP IIb–IIIa (green) are likely to be platelet microparticles released upon activation. On coated ePTFE there are significantly lower P-selectin (red) and GP IIb–IIIa (green) fluorescence intensity, and less platelet aggregation, indicating the surfactant polymer is effective preventing the platelet adhesion and activation.
Figure 5.
Epifluorescence images of platelet adhesion and activation on ePTFE surfaces in human whole blood: (A) uncoated ePTFE and (B) PVAm(Dex:FC11) 1:2 coated ePTFE under static conditions; (C) uncoated ePTFE and (D) PVAm(Dex:FC11) 1:2 coated ePTFE under dynamic conditions. Images show both platelet adhesion (green, FITC anti-human CD 41a) and platelet activation (red, PE anti-human CD62p). This figure is published in colour at http://www.ingenta.com
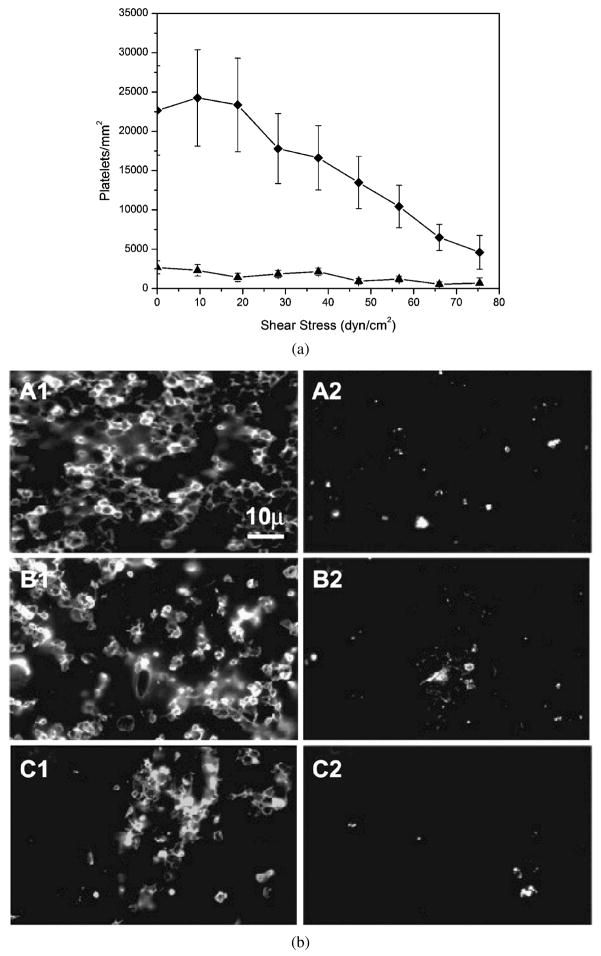
The RD experiments were repeated in human whole blood for a more stringent test of the fluorocarbon surfactant coating. Uncoated ePTFE and PVAm(Dex:FC11) 1:2 coated ePTFE were tested in human whole blood containing 3.8% sodium citrate over 0–75 dyn/cm2 at 37°C for 1 h. The quantitative data shown in Fig. 6a indicate platelets adhere on ePTFE at a much higher shear-stress level in human whole blood compared with PRP (Fig. 4a). Nevertheless, fluorocarbon surfactant polymer coatings maintain reduction of platelet adhesion on ePTFE by approx. 90% over the shear-stress range of 0–20 dyn/cm2. Figure 6b shows representative epifluorescence images of adherent platelets on uncoated and coated ePTFE surfaces at 0 dyn/cm2 (Fig. 6b, A1 and A2), 38 dyn/cm2 (Fig. 6b, B1 and B2) and 75 dyn/cm2 (Fig. 6b, C1 and C2). These results from whole-blood-based experiments provide further evidence of the non-thrombogenic nature of the fluorocarbon surfactant polymer coating.
Figure 6.
Platelet adhesion on ePTFE surfaces in human whole blood. (a) Platelet adhesion as a function of applied shear-stress level: (◆) uncoated ePTFE and (▲) PVAm(Dex:FC11) 1:2 coated ePTFE. (b) Representative epifluorescence images of platelets on ePTFE surfaces in human whole blood. (A1 and A2) Uncoated ePTFE (A1–C1) and surfactant-coated ePTFE (A2–C2) at 0 dyn/cm2, (B1 and B2) 38 dyn/cm2 and (C1 and C2) 75 dyn/cm2.
4. Discussion
We have developed a series of surfactant polymers based on poly(vinyl amines) backbone with pendent dextran and fluorocarbon branches to enhance the interaction between the surfactant polymer and PTFE/ePTFE substrate [28]. We investigated the hemocompatibility of the surfactant polymers in a dynamic shear environment, since the shear is integral part of physiological milieu. Reports have been variable in the context of the effect of shear-stress on platelet adhesion. Furukawa et al. reported increased platelet adhesion between 0 and 5 dyn/cm2 on ePTFE surface [33], whereas Chandy et al. reported a reduction in platelet adhesion from 0 to 1000 s−1 (12 dyn/cm2 for PRP) on PTFE surface [34]. The differences are likely caused by different experimental setup and the range of shear-stress investigated. In the systems reported above [33, 34], the shear-stress and the transport of platelets to the surface are related because increasing the shear-stress inevitably increases the flux of platelets to the surface due to the increased convection. On the other hand, increasing shear-stress may also dislodge platelets from the surface [27]. That may explain the increase of platelet adhesion with a slight increase of shear-stress at low shear-stress levels, and the reduction of platelet adhesion at higher shear-stress levels. A unique property of the rotating disk system is that the flux of platelets to the surface is a function of shear rate, and is, therefore, uniform across the surface, even though the shear-stress is a linear function of radial distance from the center of the disk [30, 35]. As a result, the platelet flux to the surface is the same at the center of the disk as that at the edge of the disk. This is consistent with our observation that platelet adhesion generally decreases along the radial of the disk due to higher shear-stress levels.
The rotating disk system employed to study in vitro platelet adhesion generally involves a shear-stress range of 0–75 dyn/cm2. This range is chosen to demonstrate the effect of the shear-stress since high shear-stress (>40 dyn/cm2) can dislodge platelets from the PTFE surface. However, platelet adhesion at lower shear-stress levels is more important, since the physiological shear-stress is relatively low (8–11 dyn/cm2) for small diameter vessels (4–6 mm) like femoral artery and common carotid artery [36]. Modification of PTFE/ePTFE surfaces with fluorocarbon surfactant polymers results in significant reduction in platelet adhesion over the shear-stress range of 0–10 dyn/cm2, as shown in the results (Figs 3, 4 and 6).
The effectiveness in suppressing platelet adhesion is related to the surfactant polymer composition. Fluorocarbon surfactant polymers were synthesized by sequential attachment of dextran and fluorocarbon branches to the poly(vinyl amine) backbone [28]. A benefit of the sequential synthesis of the surfactant polymer is that the dextran percentage in the fluorocarbon surfactant polymers is kept constant at 22 mol%, whereas the percentage of fluorocarbon branches varies from 15 to 21 to 45 mol% in the PVAm(Dex:FC11) 1:0.5, 1:1 and 1:2, respectively [28]. This allows us to systematically change the fluorocarbon branches density without affecting the hydrophilic dextran density along the PVAm backbone. Since the dextran density is kept constant, the differences between surfactant polymers in suppressing platelet adhesion are not due to the variation of the dextran density in the surfactant polymer, but are likely caused by the variation of surfactant polymer adsorption, i.e., surfactant molecular packing density on the surface. As shown in the previous report [28], the adsorption of fluorocarbon surfactant polymer on PTFE increases in the order of PVAm(Dex:FC11) 1:0.5 < 1:1 < 1:2, due to increased interaction between the fluorocarbon branches and the PTFE surface. Increased interaction between fluorocarbon branches and PTFE surface also leads to increased surfactant stability under dynamic flow conditions, as a result, the PVAm(Dex:FC11) 1:2 is the most stable surfactant polymer in the group [28]. Further increasing the fluorocarbon branch density is limited since it causes insolubility of the surfactant polymer. To further demonstrate the adhesion stability of the PVAm(Dex:FC11) 1:2 on PTFE, we labeled the surfactant with fluorescein and examined the adhesion stability in PBS and human whole blood under dynamic flow conditions. The shear-stress range in the test (0–100 dyn/cm2 in PBS and 0–75 dyn/cm2 in whole blood) is significantly higher than the physiological shear-stress (8–11 dyn/cm2) for small diameter vessels (4–6 mm) [36]. The high shear-stress puts the surfactant polymer in a vigorous dynamic flow environment and, thus, can better examine the stability of the polymer. The non-significant difference (P = 0.65) of surface fluorescence intensity between 1 h and 4 h exposure to PBS indicates the initial drop-off is more likely attributed to the loosely adhered polymers and the remaining surface-adsorbed polymers are stable under the test conditions. Surfactant polymer PVAm(Dex:FC11) 1:2 is also stable at 75 dyn/cm2 after 1 h in the human whole blood test, demonstrating the strong interaction between the fluorocarbon chains and the PTFE surface. These results suggest with the same dextran density along the PVAm backbone (22 mol%), the surfactant polymer with more fluorocarbon branches (from 15 to 45 mol%) is more effective in suppressing platelet adhesion and activation. This trend was observed on PTFE at the center of the disk (0 dyn/cm2, Fig. 3), where shear-induced detachment of platelets is minimal. As shear-stress increases, the difference in platelet adhesion between different fluorocarbon contents is rapidly diminished to a point of statistical in-significance. Previous studies [25, 26] have shown the ability of dextran surfactant polymers in suppressing plasma protein adsorption. The likely mechanism for surfactant polymer to suppress platelet adhesion is through the suppression of plasma protein adsorption [25, 26].
The ePTFE surface represents a special challenge in that some platelets may become trapped within the fibrils and may not be detected by the fluorescence microscope. Consequently, the actual number of platelets on the surface may be underestimated. Figure 4 shows the platelet adhesion on ePTFE. The fibrils and nodes of the ePTFE can be clearly seen in the fluorescence image because of the autofluorescence of the substrate. Close analysis of related images (Figure 4b) indicates platelets preferentially sit along the ePTFE fibrils. As a result, most platelets still experience the shear-stress in the RD experiment. Higher shear-stress dislodges platelets from the surface and, thus, the adhesion of platelets decreases with the increasing shear-stress level over the range of 0–40 dyn/cm2. The reduction of platelet adhesion in whole blood is shown in Fig. 6. Compared with the results in PRP, platelet adhesion on ePTFE in human whole blood is less shear-stress sensitive, and there are still significant amounts of platelets on the surface, even at the shear-stress level of 75 dyn/cm2, whereas few platelets remain on the ePTFE surface over 55 dyn/cm2 in PRP. The enhanced platelet adhesion in whole blood may relate to the increased transport of platelets to the surface in the presence of red blood cells [37, 38]. ePTFE surface also shows increased adsorption of the blood component in whole blood, since ePTFE fibrils are not easily seen under the fluorescence microscope. Nevertheless, surfactant-coated ePTFE shows significant reduction of platelet adhesion compared with uncoated ePTFE over the shear-stress range of 0–75 dyn/cm2.
Besides the enhanced transport of platelets to the surface induced by the dynamic flow conditions (RDS), exposure to shear-stress activates platelets [39, 40]. Platelet activation on the biomaterial surface is thus the result of the intricate interaction of surface thrombogenicity and dynamic flow environment. Platelet ‘contact activation’ involves certain specific protein adsorption on the biomaterial surface that triggers platelet adhesion and activation through bimolecular signaling; whereas ‘shear activation’ involves interaction between vWf and platelet GP Ib/Ix and platelet GP IIb–IIIa, which triggers further bimolecular signaling of platelet morphological changes, adhesion and aggregation. We examined platelet activation caused by ‘contact activation’ under static conditions, and ‘shear activation’ under dynamic conditions. It is important to note that platelets experienced the same shear rate across the surface in the RD experiment. It is evident that platelet activation is much higher under dynamic conditions on uncoated ePTFE, indicating that under shear a significantly higher number of platelets are activated in contact with the uncoated surface and adhere to the surface. Platelet activation on coated ePTFE under dynamic conditions is low and comparable to that under static conditions. These results are consistent with our quantitative results obtained from RD experiments where coated PTFE/ePTFE surfaces have low and almost constant level of platelet adhesion. Uncoated PTFE/ePTFE surfaces in comparison have high platelet adhesion under physiological shear conditions. Given the promising in vitro performance by the fluorocarbon surfactant polymers, we plan to test the polymer as a potential non-thrombogenic coating for ePTFE graft in a in vivo porcine model and will report the result in future publications.
5. Conclusion
Fluorocarbon dextran surfactant polymers are effective in suppressing platelet adhesion and activation on PTFE and ePTFE surface in the platelet rich plasma or human whole blood under dynamic shear conditions. The effectiveness in suppressing platelet adhesion correlates with the surfactant polymer composition. The results suggest with the same dextran density (22 mol%), the surfactant polymer with more fluorocarbon branches (from 15 to 45 mol%) is more effective in suppressing platelet adhesion and activation at 0 dyn/cm2, and fluorocarbon surfactant polymer coating potentially can improve the thrombogenicity of the ePTFE vascular graft.
Acknowledgments
We gratefully acknowledge the financial support of the work provided by NIH SBIR grant R43 HL076915, and the facilities provided by the Center for Cardiovascular Biomaterials at Case Western Reserve University.
References
- 1.Boyce B. In: Biological and Synthetic Vascular Prosthesis. Stanley JC, editor. Grune & Stratton; New York, NY: 1982. p. 553. [Google Scholar]
- 2.Brewster DC. In: Vascular Surgery. Rutherford RB, editor. Vol. 1. Saunders; Philadelphia, PA: 2000. p. 559. [Google Scholar]
- 3.Ku DN, Allen RC. In: Biomedical Engineering Handbook. Bronzino JD, editor. CRC Press; Boca Raton, FL: 1995. p. 1871. [Google Scholar]
- 4.Kleed D, Hocker H. Adv Polym Sci. 2000;149:1. [Google Scholar]
- 5.Hanson SR. Cardiovasc Pathol. 1993;2:157S. [Google Scholar]
- 6.Adams GA, Brown SJ, McIntire LV, Eskin SG, Martin RR. Blood. 1983;62:69. [PubMed] [Google Scholar]
- 7.Lamba NMK, Copper SL. In: Hemostasis and Thrombosis, Basic Principles & Clinical Practices. 4. Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. p. 661. [Google Scholar]
- 8.Zucker MB, Vroman L. Proc Soc Exp Biol Med. 1969;131:318. doi: 10.3181/00379727-131-33866. [DOI] [PubMed] [Google Scholar]
- 9.Savage B, Saldivar E, Ruggeri ZM. Cell. 1996;84:289. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell KD. In: Poly(ethylene glycol): Chemistry and Biological Applications. Harris JM, Zalipsky S, editors. American Chemical Society; Washington, DC: 1997. p. 400. [Google Scholar]
- 11.Deible CR, Beckman EJ, Russell AJ, Wagner WR. J Biomed Mater Res. 1998;41:251. doi: 10.1002/(sici)1097-4636(199808)41:2<251::aid-jbm10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Osterberg E, Bergstrom K, Holmberg K, Schuman TP, Riggs JA, Burns NL, Vanals-tine JM, Harris JM. J Biomed Mater Res. 1995;29:741. doi: 10.1002/jbm.820290610. [DOI] [PubMed] [Google Scholar]
- 13.Baquey C, Palumbo F, Porte-Durrieu MC, Legeay G, Tressaud A, d’Agostino R. Nucl Instrum Methods Phys Res Sect B: Beam Interact Mater Atoms. 1999;151:255. [Google Scholar]
- 14.Dekker A, Reitsma K, Beugeling T, Bantjes A, Feijen J, Vanaken WG. Biomaterials. 1991;12:130. doi: 10.1016/0142-9612(91)90191-c. [DOI] [PubMed] [Google Scholar]
- 15.Costello CA, McCarthy TJ. Macromolecules. 1984;17:2940. [Google Scholar]
- 16.Costello CA, McCarthy TJ. Macromolecules. 1987;20:2819. [Google Scholar]
- 17.Koh SK, Park SC, Kim SR, Choi WK, Jung HJ, Pae KD. J Appl Polym Sci. 1997;64:1913. [Google Scholar]
- 18.Noh I, Chittur K, Goodman SL, Hubbell JA. J Polym Sci Part A: Polym Chem. 1997;35:1499. [Google Scholar]
- 19.Tae G, Lammertink RGH, Kornfield JA, Hubbell JA. Adv Mater. 2003;15:66. [Google Scholar]
- 20.Laredo J, Xue L, Husak VA, Ellinger J, Greisler HP. Am J Surg. 2003;186:556. doi: 10.1016/j.amjsurg.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Ofenloch JC, Yianni YP, Hanson SR, Lumsden AB. J Surg Res. 1998;77:119. doi: 10.1006/jsre.1998.5399. [DOI] [PubMed] [Google Scholar]
- 22.Kidane A, Lantz GC, Jo S, Park K. J Biomater Sci Polymer Edn. 1999;10:1089. doi: 10.1163/156856299x00702. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Wang C, Babukutty Y, Ohyama T, Kogoma M, Kodama M. J Biomed Mater Res. 2002;60:502. doi: 10.1002/jbm.1294. [DOI] [PubMed] [Google Scholar]
- 24.Qiu YX, Zhang TH, Ruegsegger M, Marchant RE. Macromolecules. 1998;31:165. [Google Scholar]
- 25.Holland NB, Qiu Y, Ruegsegger M, Marchant RE. Nature. 1998;392:799. doi: 10.1038/33894. [DOI] [PubMed] [Google Scholar]
- 26.Ruegsegger MA, Marchant RE. J Biomed Mater Res. 2001;56:159. doi: 10.1002/1097-4636(200108)56:2<159::aid-jbm1080>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Sen Gupta A, Wang S, Link E, Anderson EH, Hofmann C, Lewandowski J, Kottke-Marchant K, Marchant RE. Biomaterials. 2006;27:3084. doi: 10.1016/j.biomaterials.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Marchant RE. Macromolecules. 2004;37:3353. doi: 10.1021/ma030423w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. Garland; New York, NY: 1994. [Google Scholar]
- 30.Vacheethasanee K, Marchant RE. J Biomed Mater Res. 2000;50:302. doi: 10.1002/(sici)1097-4636(20000605)50:3<302::aid-jbm3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. Langmuir. 2001;17:5605. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 32.Salzman EW, Lindon J, McManama G, Ware JA. Ann NY Acad Sci. 1987;516:184. doi: 10.1111/j.1749-6632.1987.tb33040.x. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa KS, Ushida T, Sugano H, Tamaki T, Ohshima N, Tateishi T. ASAIO J. 2000;46:696. doi: 10.1097/00002480-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Chandy T, Das GS, Wilson RF, Rao GHR. Biomaterials. 2000;21:699. doi: 10.1016/s0142-9612(99)00231-8. [DOI] [PubMed] [Google Scholar]
- 35.Riddiford AC. In: Advances in Electrochemistry and Electrochemical Engineering. Delahay P, editor. Vol. 4. Interscience; New York, NY: 1966. p. 47. [Google Scholar]
- 36.Slack SM, Turitto VT. Cardiovasc Pathol. 1993;2:11S. [Google Scholar]
- 37.Aarts PAMM, Heethaar RH, Sixma JJ. Blood. 1984;64:1228. [PubMed] [Google Scholar]
- 38.Turitto VT, Weiss HJ, Baumgartner HR. J Rheol. 1979;23:735. [Google Scholar]
- 39.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Blood. 1996;88:1525. [PubMed] [Google Scholar]
- 40.Gorbet MB, Sefton MV. Biomaterials. 2004;25:5681. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]