Abstract
Background
The capacity for insulin synthesis in islets is important for islet transplantation to succeed. We developed a micro assay that evaluates the potency of human islets by measuring changes in glucose induced insulin gene (INS) expression using a single islet in octuplicate samples.
Methods
Poly (A)+ mRNA was purified from a set of single handpicked human islets. Glucose induced mature (post-spliced) and premature (pre-spliced) insulin mRNA were quantified by RT-PCR using several insulin mRNA primers designed at different locations including, intron, exon, and an exon-intron junction.
Results
The synthesis of premature INS mRNA was significantly increased in islets exposed to high glucose for 16 hours (vs. 4-hour, p<0.01), whereas mature INS mRNA showed no difference. Glucose-induced premature INS mRNA synthesis was attenuated in heat-damaged islets. Stimulation index (SI) calculated by normalizing premature by mature INS mRNA (SI_INS mRNA) positively correlated with SI of insulin release (SI_16h-insulin) from the same set of islets during 16-hour incubation in high or low glucose media, as well as SI of glucose-mediated insulin release obtained from the same islet lot in a perifusion system (N=12). Furthermore, linear multiple regression analysis using SI_INS mRNA and SI_16h-insulin predicted islet transplantation outcome in NODscid mice (N=8).
Conclusion
The measurement of glucose induced premature INS mRNA normalized by mature INS mRNA can be used to assess the functional quality of human islets and may predict islet function after transplantation in type 1 diabetic patients.
Keywords: islet quality, human islet, islet transplantation, Preproinsulin mRNA, insulin synthesis
INTRODUCTION
Insulin, the major hormone maintaining glucose homeostasis in mammals, is synthesized and secreted by β cells in the Islets of Langerhans of the pancreas. In type 1 diabetes, β cells are destroyed during the course of disease, thus, transplanting islets isolated from brain dead donors (1–3) or donors after cardiac death (4, 5) is considered an effective treatment. However, since viability and function of islets are reduced by post-mortem activation of proapoptotic and proinflammatory signaling pathways (6), as well as mechanical and chemical damages during islet isolation procedure, the quality of isolated islets is of prime concern for successful islet transplantation.
Transcription, mRNA stabilization, translation, post-translational processing, and secretion of insulin, in response to high glucose, are key factors in determining islet quality. Insulin is immediately secreted from the β cell in response to high glucose stimulation. This insulin release is compensated for by a corresponding increase in proinsulin biosynthesis, mainly at the translational level over a short period of time (< 2 hours) (7–9). Over longer periods (>12~24 hours), proinsulin biosynthesis is controlled, in part, by an increase in insulin gene transcription and preproinsulin mRNA stability (8, 10–12). The human insulin gene (INS) consists of three exons and two introns (13, 14), the splicing of which is regulated by glucose (15). Furthermore, studies by Evans-Molina et al. in human islets have shown that premature (pre-spliced) preproinsulin mRNA increases within 60 minutes following high glucose stimulation, which accurately reflects the acute transcriptional response of the insulin gene to glucose, whereas mature (post-spliced) preproinsulin mRNA did not increase until 48 hours after stimulation (16).
Based on these findings, we hypothesized that glucose-induced premature INS mRNA expression could be a marker for biosynthetic capacity of insulin in β cells and may reflect functional quality of transplanted islets. Short-term glucose induced insulin release is widely adapted for assessing islet quality. However, a method to measure INS mRNA has yet to be developed. To develop a method that can measure changes of INS mRNA using a small number of islets is important, especially for islets to be used in clinical transplantation, since the availability of more islets would results in a better transplant outcome. Compounding this challenge is that the β cell number varies substantially between islets, even among islets of similar size. Such variations between islets make statistical analysis of assay results extremely difficult. In the present study, we successfully overcame these technical difficulties and quantified glucose-induced INS mRNA from a set of single human islets. The results correlated well with those obtained through other islet quality assessments assays. We believe that this method will provide a valuable tool to predict function of transplanted islets in type 1 diabetic patients.
RESEARCH DESIGN AND METHODS
Primer design
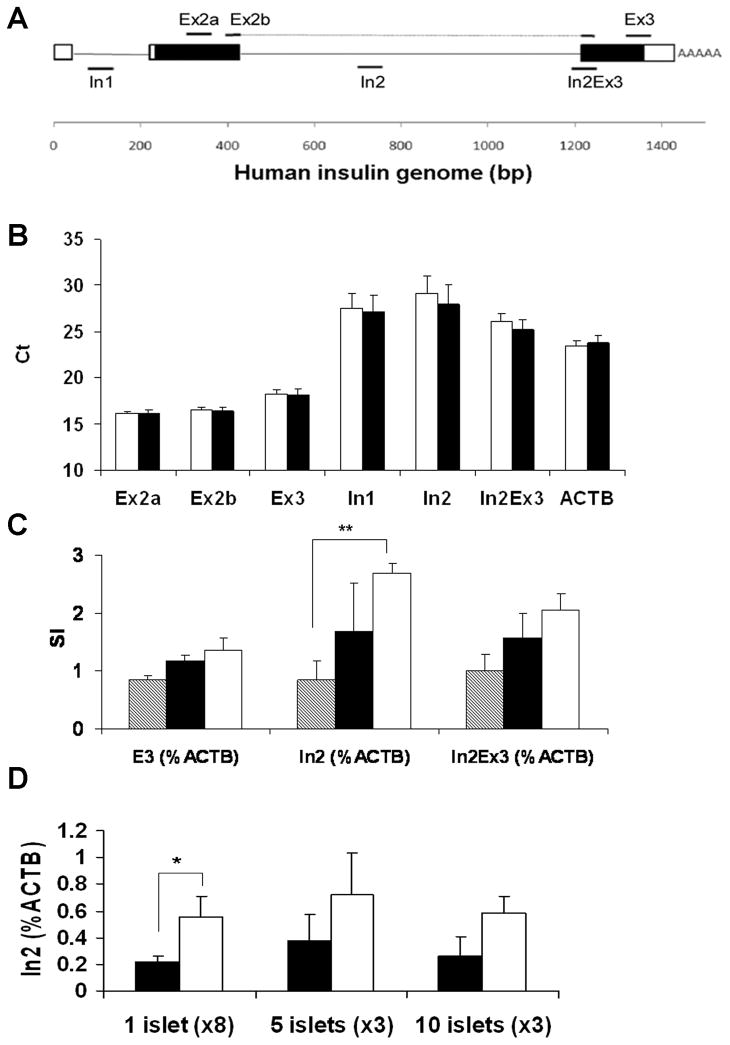
Human insulin mRNA (NM_000207) and genomic DNA (NG_007114) were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index.html). Primers were designed using Primer Express (Applied Biosystems, Foster City, CA) at different locations in exon, intron, and an exon-intron junction as shown in Figure 1A. Primers located in intron (In1, In2) and the intron-exon junction (In2Ex3) were use to identify premature pre-splicing poly(A)+ mRNA. Primers located in 2 exons (Ex2b) were used to quantify mature post-splicing poly(A) + mRNA, and primers located within a single exon (Ex2a, Ex3) were used to identify both pre- and post-splicing mRNA. Primer sequences are summarized in “Supplemental Digital Content: supplemental Table 1”, and oligonucleotides were synthesized by IDT (Coralville, IA).
FIGURE 1.
Amplification of INS using several primer pairs. A: Human INS primers were designed at different locations in exon (non-coding exon: white box, coding exon: black box), intron (black line), and exon-intron junction. PCR amplicon is shown by black solid line. B: The amount of total INS mRNA amplified by different primer pairs from islets cultured in the medium containing low (3.3 mmol/L) glucose (white bar) or high (17 mmol/L) glucose (black bar) for 16 hours (single islet/sample, octuplicate, n=4). C: Time dependent increase in premature INS detected from the sets of single islets. The islets were cultured in either low or high) glucose medium for 4, 8, 16 hours (4 hours: striped bar, 8 hours: black bar, and 16 hours: white bar). Mature plus premature (Ex3) INS and premature (In2 and In2Ex3) INS expression were first normalized by ACTB. Stimulation index (SI) was calculated as the fold increase of INS in high glucose as compared to that in low glucose. (Single islet/sample, octuplicate, n=4). D: The measurements of premature INS mRNA from octuplicate single islet/sample (total 8 islets/reaction), triplicate 5 islets (total 15 islets) or triplicate 10 islets (total 30 islets) cultured in low (black bar) or high (white bar) glucose for 16 hours. The figure shows representative data of two consecutive experiments. Results are shown by mean ± standard error (* p< 0.05, ** p< 0.01).
Human islet culture
Human islets isolated from 12 different donor pancreata approved for research use were obtained from the Southern California Islet Cell Resources (SC-ICR) Center, Beckman Research Institute of the City of Hope (Duarte, CA) 1 to 3 days after isolation. Demographic and clinical information of donors used in this study are summarized in “Supplemental Digital Content: supplemental Table 2”. The use of human islets in this study was approved by the Institutional Review Board of the City of Hope. Islets between 150 μm to 300 μm in diameter (medium size islet), were handpicked by experienced personnel under a dissection microscope without staining. For testing the influence of islet size on INS mRNA synthesis, handpicked islets less than 150 μm in diameter were considered “small” islets and islets larger than 300 μm were considered “large” islets. Each handpicked islet was cultured individually in a non-tissue culture treated 96 well plate (Sarstedt, Newton, NC) containing 100μL of RPMI 1640 medium supplemented with 5% fetal bovine serum, 12 mM HEPES and either low (3.3 mmol/L) or high glucose (17 mmol/L) for 16 hours. In the parallel experiments, islets were incubated at 50°C for 2 min prior to culture to induce cellular damage.
Quantification of insulin mRNA
After culture, islets were transferred to a 96-well filter plate (Hitachi Chemical Research Center-HCR, Irvine, CA) and centrifuged at 2000 × g for 5 min at 4°C to trap islets on filter membranes (17). Fifty microliters of Lysis Buffer (HCR), containing a cocktail of specific reverse primers (mixture of oligonucleotides for INS and β-actin mRNA, ACTB) was applied to the filter plate. The resultant cell lysates were transferred to oligo(dT)-immobilized microplates (GenePlate, HCR) for poly(A)+ mRNA purification (18). Poly(A) + mRNA was used to distinguish between pre- and post-splice mRNA.
The cDNA was directly synthesized with 30μL of solution in each well: specific primer-primed cDNA in the liquid phase and oligo(dT)-primed cDNA in the solid phase (17). The cDNA in the solution was diluted by adding 30 μL nuclease-free water, with 4 μL of the diluted cDNA used for SYBR Green PCR (BioRad, Hercules, CA) (19). Each gene was amplified individually. The cycle threshold (Ct) was determined using analytical software (SDS, Applied Biosystems, Foster City, CA). Differences in Ct between the target and control mRNA (ΔCt) are used to quantify the relative amount of eachtarget, calculated as 2−ΔCt.
To assess glucose induced INS expression, the stimulation index (SI) of INS mRNA expression (SI_INS mRNA) was calculated by dividing the fold increase of mRNA in high glucose-cultured islets by that of low-glucose cultured islets. Using the first 4 islet preparations, we confirmed that islets can be frozen at −80°C following glucose stimulation until testing without altering results (“Supplemental Digital Content: Supplemental Figure 1.”). Therefore, islets from the 8 subsequent preparations were frozen-stored after glucose stimulation.
Measurement of total insulin release
The medium supernatant was collected from each well after 16 hours islet culture. The medium samples were briefly centrifuged and the supernatants were frozen-stored. Insulin contents in the culture supernatants were measured using an Enzyme-Linked ImmunoSorbent Assay (ELISA) kit for human insulin (Mercodia Inc., Winston Salem, NC) following the manufacturer’s protocol. The stimulation index (SI_16h-insulin) was calculated by dividing the average insulin amount released from the islets cultured in high-glucose medium by the average insulin amount released from the islets cultured in low-glucose medium over a 16 hour culture period.
Insulin release assay in a perifusion system
Five hundred islet equivalents (IEQ) were placed in a minicolumn and perifused at 37°C with Krebs-Ringer’s buffer containing 1% human serum albumin and 3.3 mmol/L glucose as basal buffer followed by 17 mmol/L glucose as stimulation buffer at a rate of 0.7 mL/min. Effluent was collected every minute and insulin content of each effluent was measured as described above. The stimulation index (SI_perifusion) was calculated by dividing the total insulin amount released in stimulation buffer by the total insulin amount released in basal buffer for the same period of time (15 minutes).
Analysis of β cell apoptosis by Laser Scanning Cytometry
Paraffin sections of islets were stained for terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the Apop Tag plus Fluorescein In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) followed by immunostaining for insulin using a guinea pig anti-human insulin primary antibody (DAKO Carpinteria, CA) and a Cy5-conjugated secondary antibody (Jackson Immuno-Research, West Grove, PA). All sections were counterstained for DNA with 4′-6-Diamidino-2-phenylindole (DAPI, Sigma). To evaluate β cell apoptosis, slides were scanned using the iCys laser scanning cytometer and the iCys 3.2.5 software (Compucyte, Cambridge, MA) as previously described (20). Cells co-staining for insulin and TUNEL were designated as apoptotic β cells and their percentage was obtained from the histogram. The percentage of apoptotic β cells was calculated by dividing the insulin/TUNEL double-positive cell number by the total number of insulin-positive cells.
Assessment of in vivo islet function in diabetic NODscid mice
Male NODscid mice, ages 10–12 weeks, were obtained from the Animal Resources Center of Beckman Research Institute of the City of Hope and used as islet recipients. Mice were rendered diabetic by intraperitoneal injection of 50 mg/kg streptozotocin (Sigma-Aldrich, St. Louis, MO) on three consecutive days. Those that exhibited hyperglycemia (>350 mg/dL) for two consecutive days were used as recipients. Islets (800, 1200, or 1600 IEQ) were transplanted under the left kidney capsule of diabetic mice. Blood glucose levels were measured 2–3 times weekly. Recipient mice that maintained a blood glucose <200 mg/dL were considered to have reversed diabetes. At the end of each experiment, a nephrectomy was performed to confirm graft dependence. Four of the 12 islet preparations used in this study were not transplanted into NODscid mice due to low purity (<50%) or limited availability of islets. A total of 18 mice transplanted with 1200 IEQ from 8 islet preparations were used for correlation and Linear multiple regression analysis. Animal procedures followed protocols approved by the Institutional Animal Care and Use Committee of the City of Hope/Beckman Research Institute.
Data analysis
Data are presented as a mean ± standard error. The Correlation and Analysis of variance procedures were applied to assess the association between glucose induced premature insulin synthesis and other in vitro and in vivo test results. Linear multiple regressions were used to investigate the association between other in vitro quality control tests and in vivo islet transplant outcome in mice. Paired two-tailed Student’s t-test was used to compare the difference between the two groups. P value of less than 0.05 was considered significant. The data analysis was performed by statistical SAS 9.1 for Windows software package (SAS Institute Inc., Cary, NC).
RESULTS
Amplification of various regions of insulin mRNA
The INS mRNA was quantified from a single islet using several primer pairs designed at different regions (Fig. 1A). Figure 1B shows the result of the first 4 islet preparations presented by Ct obtained from each primer pairs in islets cultured with low- or high-glucose medium for 16 hours. In islets cultured with low-glucose medium, mature plus premature INS mRNA (Ex3) expression was 18.3 ± 0.43 (single islet/well, octuplicate), which is 34 folds more than that of control housekeeping ACTB mRNA (Ct=23.4 ± 0.6), and 220 folds (In2Ex3; Ct=26.1 ± 0.9) and 1837 folds (In2; Ct=29.1 ± 1.6) more than premature pre-splicing INS mRNA, respectively. Ct of mature and/or premature INS mRNA measured by all the primer pairs did not show a statistically significant difference between the islets cultured in low- or high-glucose medium for 16 hours. Figure 1C shows glucose-induced INS mRNA synthesis in islets cultured in either low- or high-glucose medium for 4, 8, or 16 hours. SI of premature INS mRNA (In2) normalized by ACTB mRNA was 2.7 ± 0.2 at 16 hours, which was significantly (p<0.01) higher than that at 4 hours (0.8 ± 0.3), whereas no difference was found in SIs between 8 and 16 hours. In contrast, the SI of mature plus premature INS (Ex3) and premature INS (In2Ex3) had no statistically significant difference between 4, 8, and 16 hour cultured islets (In2Ex3: 4 hours vs. 16 hours; p=0.05). Therefore, the primer pair for premature INS (In2) was used in the subsequent studies. We also compared the influence of islet number used per reaction on glucose response. As shown in figure 1D, single islet/well cultured in octuplicate (total 8 islets/reaction) identified a significant increase in response to high glucose (p<0.05), whereas 5 islets in triplicate (total 15 islets) or 10 islets in triplicate (total 30 islets) failed to detect such increases.
Effect of cellular damages on INS mRNA synthesis
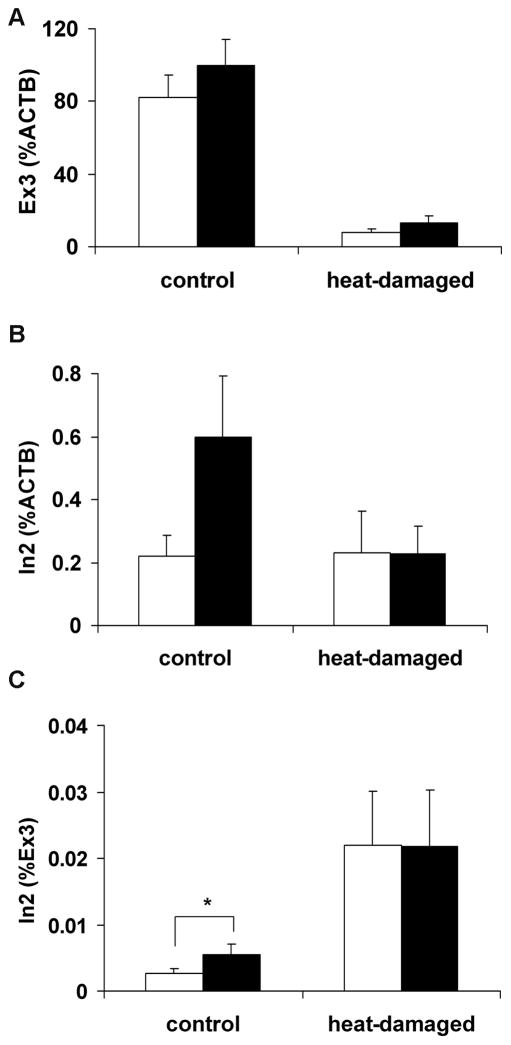
Heat-treated islets were used to examine the effect of cellular damage on glucose induced INS synthesis. Mature plus premature INS (Ex3) and premature INS (In2) normalized by ACTB and/or Ex3 were compared in non-heat treated control and heat-treated islets (Fig. 2). The In2 significantly increased in control islets during 16-hour high glucose culture when the Ct value was normalized by Ex3 (p<0.05) (Fig. 2C). This increase was attenuated in islets damaged by heat treatment. However, Ct of In2 normalized by ACTB did not show a significant increase (Fig. 2B). Since normalization of In2 by Ex3 gave more consistent results than normalization by ACTB, mature plus premature INS mRNA (Ex3) was used to normalize premature mRNA (In2) in the subsequent studies.
FIGURE 2.
The effect of cellular damages on INS mRNA synthesis. Heat-damaged and control islets were cultured in low (3.3 mmol/L) glucose (white bar) and high (17 mmol/L) glucose (black bar) media for 16 hours. A: mature plus premature (Ex3) INS mRNA synthesis normalized by ACTB mRNA, B: premature (In2) INS mRNA normalized by ACTB mRNA, and C: premature (In2) INS mRNA normalized by ACTB mRNA. The figure shows the representative data of consecutive experiments using two different islet preparations. Results are shown by mean ± standard error (* p< 0.05).
Comparison of glucose induced premature INS mRNA in Small, medium, and large islets
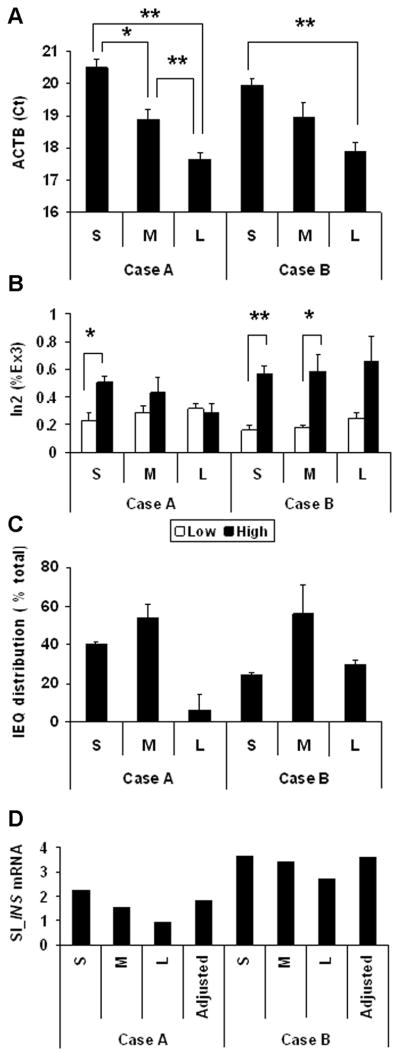
Glucose induced premature INS mRNA was compared in small (S), medium (M) and Large (L) islets from two different islet preparations. The Ct of ACTB in handpicked islets was significantly different between small, medium, and large islets in case A and Ct of each size islets were similar between cases A and B. These results show that estimation of islet size during handpicking was consistent in both cases (Figure 3A). Size-dependent glucose-induced INS mRNA synthesis was different between in case A and case B. Only small islets increased insulin synthesis in response to glucose stimulation in case A, while small and medium islets responded in case B. In addition, large islets in case A did not respond to high glucose, while those in case B clearly responded, but without statistical significance. Each islet preparation contains a different number of islets in each size group (Figure 3B), however the medium size islets constituted more than 50% of the total islet number in both cases (Figure 3C). SI of glucose induced INS mRNA synthesis (SI_INS mRNA) of medium islets were similar to adjusted SI_INS mRNA, which was calculated by the sum of SI_INS mRNA in each size multiplied by the percent distribution of all three sizes and divided by 100 (Figure 3D).
FIGURE 3.
Measurement of glucose induced premature INS mRNA from small (S), medium (M), and large (L) islets. A: ACTB expression in small, medium and large islets. B: Glucose induced premature (In2) INS mRNA normalized by mature plus premature (Ex3) INS mRNA in small, medium and large islets cultured in low-glucose medium (white bar) and high-glucose medium (black bar). C: Distribution of each size islets obtained from islet (IEQ) counting of the corresponding islet preparation. The percentage was calculated by dividing the IEQ of each size by the total IEQ. D: Stimulation index (SI) of glucose induced INS mRNA of S, M, L islets. Adjusted SI_INS mRNA was calculated by the sum of SI multiplied by percent IEQ distribution. Results are shown by mean ± standard error, * p< 0.001, ** p< 0.0001.
Association between stimulation index of glucose-induced premature INS mRNA (SI_INS mRNA), insulin released in medium over 16 hour-culture (SI_16hr insulin), and in vivo islet graft function
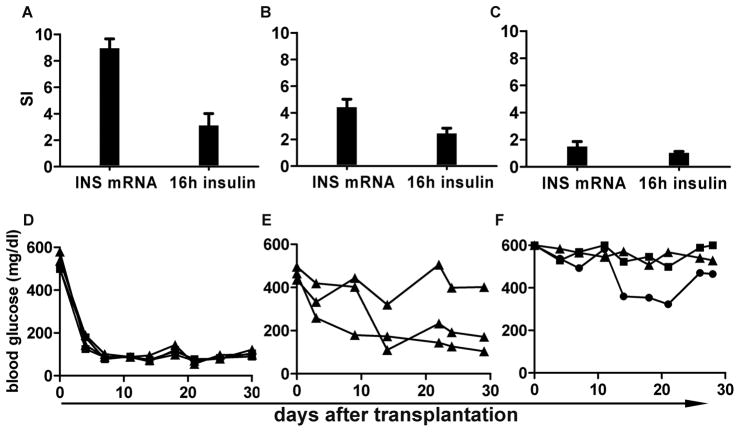
The association between the SI of glucose induced premature INS (In2) synthesis (SI_INS mRNA), insulin secretion over 16-hour high glucose culture (SI_16h insulin) and islet transplantation outcome was compared among three different islet preparations. As shown in Figure 4, islets with the highest SI_INS mRNA (9.0±0.7) released the highest amount of insulin (SI_16h insulin= 3.12±0.9) (Fig. 4A). These islets also reversed diabetes in all mice transplanted with 800 IEQ and 1200 IEQ (n=2, each) (Fig. 4D). Islets that had intermediate levels of SI_INS mRNA (4.4±0.6) released slightly less insulin (SI_16h insulin= 2.5±0.4) and reversed diabetes in 2 of 3 mice receiving 1200 IEQ (Fig. 4B, E). In contrast, the islets that had low SI_INS mRNA (1.5±0.4) failed to increase insulin secretion culturing in high glucose (SI_16h insulin= 1.0±0.1) and none of 3 mice receiving 800, 1200 and 1600 IEQ reversed diabetes (Fig. 4C, F). This study shows that the higher SI_INS mRNA and SI_16h insulin were associated with improved graft function in vivo.
FIGURE 4.
Association between SI of 16 hours premature INS mRNA synthesis (SI_INS mRNA), SI of insulin release over 16 hours from the same set of islets (SI_16h insulin), and islet transplantation outcome in STZ induced diabetic NODscid mice. A: Islets that had the highest SI_INS mRNA also had higher SI_16h insulin. All the mice transplanted with 800 IEQ (black square, n= 2) or 1200 IEQ (black triangle, n=2) became euglycemia after the transplantation (D). B: Islets that had higher SI_INS mRNA also had higher SI_16h insulin, and 2 out of 3 mice became euglycemia after transplantation with 1200 IEQ (E). C: Islets that had lowest SI_INS mRNA and SI.16h insulin did not reverse diabetes with 800 IEQ, 1200 IEQ or 1600 IEQ (black circle) (F).
Correlation analysis between SI_INS mRNA, SI_16 hr insulin, and other islet quality assessment results in 12 islet preparations
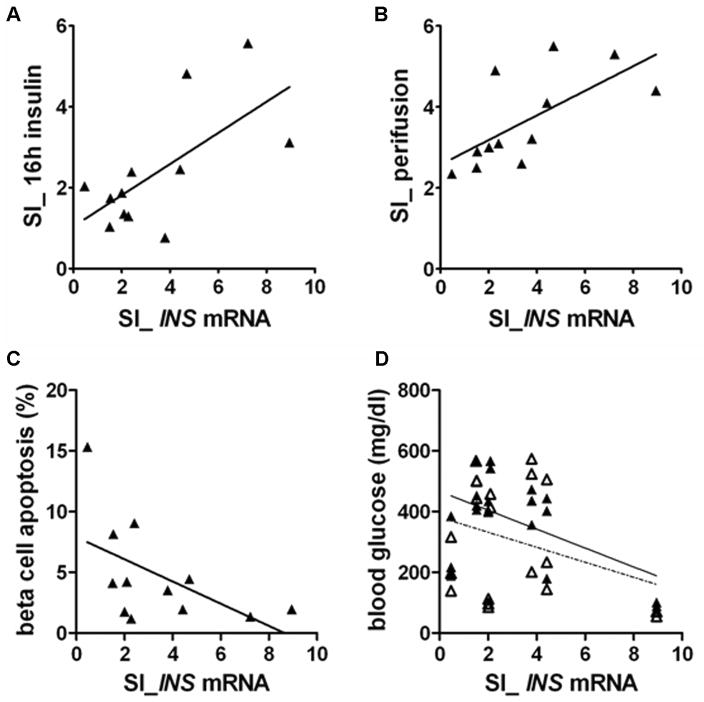
Further studies were conducted to investigate the correlation between SI_INS mRNA, SI_16h insulin, and other in vitro islet quality assessment results on islets derived from 12 different donors. SI_INS mRNA significantly correlated with SI_16h insulin obtained from the corresponding single islets (r=0.66, p=0.02) (Fig. 5A). SI_INS mRNA also significantly correlated with SI obtained by short-term glucose stimulated insulin release assay using a perifusion system (SI_perifusion) (r=0.67, p=0.02) (Fig. 5B). The percentage of β cell apoptosis obtained by LSC analysis of corresponding islet sections stained for TUNEL and insulin showed a moderate correlation with SI_INS mRNA (r=−0.55, p=0.06) (Fig. 5C). In vivo transplant results of higher SI_INS mRNA were closely associated with decreased blood glucose (mg/dL) levels 7 days after islet transplantation (r=−0.53, p=0.02). However, the correlation became weaker when analyzed with blood glucose levels 21 days post transplant (r=0.31, p=0.18) (Fig. 5D).
FIGURE 5.
Correlation between SI of glucose induced premature INS mRNA (SI_INS mRNA) and other in vitro and in vivo islet quality assessment results. A: Correlation between SI_INS mRNA and SI of insulin release over 16 hours (SI_16h insulin) obtained from the same set of single islets (r=0.66, p=0.02). B: Correlation between SI_INS mRNA and SI of glucose-stimulated insulin release using a perifusion system (r=0.67, p=0.02). C: Correlation between SI_INS mRNA and percent of β cell apoptosis obtained by TUNEL and insulin staining analyzed by Laser Scanning Cytometry (r=−0.55, p=0.06). In vitro assessment results were obtained from 12 different islet preparations. D: Correlation between SI_INS mRNA and blood glucose (mg/dl) level in STZ induced diabetic NODscid mice 7 days (black triangle and solid line, r=−0.53, p=0.02) and 21 days (white triangle and broken line) after islet transplantation (r=0.31, p=0.18). Statistical analyses used: Pearson’s correlation coefficient (r) and P value.
Linear multiple regression analysis using in vitro islet quality assessment test to predict islet transplantation outcome in mice
Linear multiple regression analysis was performed to examine the predictability of islet transplant outcome in STZ-induced diabetic NODscid mice using in vitro assessment results obtained from conventional assays, SI_perifusion and β cell apoptosis, with or without glucose induced premature INS mRNA synthesis (SI_INS mRNA) along with SI_16h insulin. As shown in Table 1, adjusted R-square indicates that the result of the conventional two in vitro tests, SI_perifusion and β cell apoptosis, can predict blood glucose levels on post transplantation day 7 with 51% variance (p=0.004, Adjusted R2=0.509). However, these predictors did not show a statistically significant relationship with blood glucose levels on day 21 (p=0.185, adjusted R2=0.11). Addition of SI_INS mRNA and SI_16h insulin didn’t improve the predictive values on day 21 (p=0.004, Adjusted R2=0.628 on day 7, p=0.187, Adjusted R2=0.188 on day 21). In contrast, when only SI_INS mRNA and SI_16h insulin were used, data could predict blood glucose variability on both days 7 and 21 (day 7; p=0.005 adjusted R2=0.44, day 21; p=0.028, adjusted R2=0.30).
Table 1.
Linear multiple regressions using in vitro islet quality assessment tests to predict blood glucose levels 7 and 21 days after islet transplantation in STZ induced diabetic NODscid mice. The combination of two conventional factors, two new factors, and all four factors were compared.
| Day 7 |
Day 21 |
||||||
|---|---|---|---|---|---|---|---|
| Parameter estimate | P | Adjusted R2 | Parameter estimate | P | Adjusted R2 | ||
| Conventional factors | Intercept | 1263.442 | <0.001 | 1064.444 | 0.018 | ||
| SI_perifusion | −28.250 | 0.004 | −20.397 | 0.177 | |||
| β cell apoptosis | −229.783 | 0.001 | −193.959 | 0.072 | |||
| Total | 0.004 | 0.509 | 0.185 | 0.110 | |||
| All four factors | Intercept | 793.549 | 0.015 | 544.683 | 0.508 | ||
| SI_perifusion | −20.672 | 0.842 | 114.008 | 0.578 | |||
| β cell apoptosis | −22.738 | 0.029 | −3.842 | 0.834 | |||
| SI_INS mRNA | −47.307 | 0.042 | −44.411 | 0.294 | |||
| SI_16h insulin | −46.207 | 0.361 | −137.588 | 0.177 | |||
| Total | 0.004 | 0.628 | 0.187 | 0.188 | |||
| Two new factors | Intercept | 636.327 | <0.001 | 612.296 | <0.001 | ||
| SI_INS mRNA | −14.083 | 0.279 | −11.196 | 0.525 | |||
| SI_16h insulin | −120.187 | 0.017 | −136.156 | 0.042 | |||
| Total | 0.005 | 0.444 | 0.028 | 0.297 | |||
DISCUSSIONS
The assessment of functional potency of islets prior to transplantation is important to achieve success in islet transplantation. In vitro assessment methods of islets, including viability using membrane permeable fluorescence dye (21, 22), dynamic glucose stimulated insulin release assay using a perifusion system (23, 24), oxygen consumption assay (25, 26), assessment of ATP/ADP ratio and reactive oxygen species (27), islet cell composition and β cell apoptosis (28–30) are currently utilized by various islet transplant centers to evaluate the overall quality of isolated human islets. However, none of current assays measures insulin synthesis in β cells, including the transcriptional response of insulin gene to glucose stimulation. In this study, we developed an assay that measures glucose-induced newly synthesized premature INS mRNA using a set of single human islets. We have shown that the SI of premature INS mRNA synthesis and insulin release in high glucose medium over 16 hours from the same set of islets predicted blood glucose levels of diabetic NODscid mice 7 and 21 days after islet transplantation, while the conventional quality assessment results, such as SI of perifusion assay and percent β cell apoptosis, did not.
It is generally believed that glucose regulated preproinsulin mRNA transcription is controlled by long-term glucose stimulation balanced by both transcription and mRNA degradation rates (5; 8–10). However, transcriptional regulation coupled to short-term glucose stimulation is also reported in rodent (31), as well as human islets (16). Evans-Molina et al. showed an increase of premature INS mRNA after 8 hours of high-glucose stimulation (7 mmol/L and 25 mmol/L) as compared to basal glucose incubation (2.5 mmol/L) in human islets (16). Our study showed no significant transcriptional change in 4 and 8 hours stimulations, but a significant increase in premature INS mRNA after culturing for 16 hours in 17 mmol/L glucose medium compared to those cultured in 3.3 mmol/L glucose. The difference between these two results may be due to the different glucose concentrations used. We plan to evaluate glucose concentration and incubation time to further shorten the assay period in order to obtain the results before islet transplantation takes place.
The quantitation of basal INS mRNA and glucose induced newly synthesized INS mRNA in an islet reflects the number of functional β cells, as well as the viability of β cells existing in an islet. The production of glucose induced INS is reduced in damaged islets, as confirmed by attenuated INS synthesis caused by cellular damage induced by heat treatment (Fig. 2). The values obtained by mature or premature INS mRNA normalized by ACTB mRNA reflect the β cell content in an islet, as well as the actual function/viability of existing β cells. The normalization of premature INS mRNA by mature plus premature INS mRNA would represent the transcriptional capacity of INS in existing β cells better than normalization by ACTB mRNA. Since mature INS mRNA did not increase during 16 hour- high glucose stimulation, it is reasonable to use the mature INS mRNA as an indicator of existing β cell quantity. However, the fold difference (premature INS mRNA normalized by mature INS mRNA) will increase when the quantities of mature INS are reduced significantly due to cellular damage as seen in Figure 2C. This is the rationale for using SI to compare the transcriptional response of β cells between islet preparations.
The amount of premature and mature INS mRNA was not necessary proportional to the size of the islets. Islets, by their nature, have a varying number of beta cells. This natural variation, as well as variations in beta cell viability may be caused by donor factors, or islet isolation itself. Large islets tend to have central necrosis due to limited diffusion of oxygen and nutrients (33, 34), which negatively affects glucose induced INS synthesis. The quality of large and medium sized islets is considered more important in assessing the overall quality of an islet preparation, as the factors to convert islet number to IEQ favor larger islets (35). Our assay was sensitive enough to detect a difference in Ct of the housekeeping gene ACTB among large, medium, and small islet samples (Fig. 3). Overall, small to medium size islets responded better than large islets in our study which agrees with results previously described by Lehmann et al.(32). However, we found that size-dependent glucose-induced INS mRNA synthesis was not consistent from one islet preparation to another. Even if large islets do not respond to high glucose, when the number of large islets in the preparation is small, it will have less influence on the overall islet quality and function. In two islet preparations shown in Figure 3, the adjusted SIs were similar to the SI of medium size of the corresponding islets, since more than 50% of total IEQ were from medium size islets in both cases. The size distribution similar to these two cases was confirmed in additional 10 islet preparations (data not shown). Therefore, it is appropriate to use medium size islets for this assay as representative islets in most of islet preparations.
It is reasonable to speculate that islets losing glucose-induced INS mRNA transcription capacity also fail to secrete insulin over a long period, thus depleting the available insulin. Interestingly, insulin secretion by short-term glucose stimulation also correlated to 16 hours premature INS mRNA synthesis. Glucose induced premature INS mRNA synthesis and insulin released in 16-hour high glucose stimulation were shown to be good indicators to predict islet transplant outcome in diabetic mice. Negative and positive predictive value for SI_INS mRNA and SI_16 hr insulin are to be examined after the results of more cases are collected. Since mice were not treated with exogenous insulin after transplantation, the ability of insulin production by transplanted islets under hyperglycemic condition is a key factor to reverse diabetes. However, clinical islet transplantation recipients are treated with exogenous insulin to control blood glucose levels to avoid the exposure of islets to high glucose. We also plan to determine if a correlation exists between premature INS synthesis in islets and clinical transplantation outcome.
In summary, the measurement of glucose induced premature INS mRNA normalized by mature INS mRNA can be analyzed using a set of single islets. The combination of SI_INS mRNA and SI_16h insulin from the same set of islets significantly predicted human islet function transplanted into diabetic NODscid mice. These two new factors were shown to be more predictable than the results obtained from conventional islet quality assessment methods. This microassay may be a useful method to predict islet function after transplantation in patients with type 1 diabetes.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Nora Eccles Treadwell Foundation (Y.M.), U42 RR16607 NIH grant (F.K.), and a grant from Hitachi Chemical Research Center, Inc. (M.M.).
K.O. participated in research design, performed the research, analyzed the data and wrote the paper. No conflict of Interest.
M.M. participated in research design, contributed the analytic tool, performed the research, and edited the paper. Employee of Hitachi Chemical Research Center Inc.
I.T. performed the research. No conflict of Interest.
J.R. performed the research and edited the paper. No conflict of Interest.
K.D.S. participated in data analysis. No conflict of Interest.
F.K. reviewed the paper. P.I. of the grant founded by NIH (U42 RR16607). No conflict of interest.
Y.M. participated in research design, edited and reviewed the paper. P.I. of the grant founded by Nora Eccles Tread well Foundation. No conflict of interest.
ABBREVIATIONS
- INS
Human insulin gene
- SI
Stimulation index
- ACTB
Human beta actin gene
- Ct
Cycle threshold
- IEQ
Islet equivalents
- ELISA
Enzyme-linked ImmunoSorbent Assay
- TUNEL
Terminal uridine deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Berney T, Ferrari-Lacraz S, Buhler L, et al. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am J Transplant. 2009;9 (2):419. doi: 10.1111/j.1600-6143.2008.02481.x. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54 (7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355 (13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.Markmann JF, Deng S, Desai NM, et al. The use of non-heart-beating donors for isolated pancreatic islet transplantation. Transplantation. 2003;75 (9):1423. doi: 10.1097/01.TP.0000061119.32575.F4. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Okitsu T, Iwanaga Y, et al. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;82 (4):460. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- 6.Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52 (12):2935. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283 (5742):100. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- 8.Wicksteed B, Alarcon C, Briaud I, Lingohr MK, Rhodes CJ. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. J Biol Chem. 2003;278 (43):42080. doi: 10.1074/jbc.M303509200. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes CJ. Diabetes Mellitus. Lippincott Williams and Wilkins; Philidelphia, PA: 2000. [Google Scholar]
- 10.Giddings SJ, Chirgwin J, Permutt MA. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982;31 (7):624. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- 11.Welsh M, Nielsen DA, MacKrell AJ, Steiner DF. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985;260 (25):13590. [PubMed] [Google Scholar]
- 12.Giddings SJ, Chirgwin J, Permutt MA. The effects of fasting and feeding on preproinsulin messenger RNA in rats. J Clin Invest. 1981;67 (4):952. doi: 10.1172/JCI110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell GI, Pictet RL, Rutter WJ, Cordell B, Tischer E, Goodman HM. Sequence of the human insulin gene. Nature. 1980;284 (5751):26. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- 14.Steiner DF, Chan SJ, Welsh JM, Kwok SC. Structure and evolution of the insulin gene. Annu Rev Genet. 1985;19:463. doi: 10.1146/annurev.ge.19.120185.002335. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Shen L, Najafi H, et al. Regulation of insulin preRNA splicing by glucose. Proc Natl Acad Sci U S A. 1997;94 (9):4360. doi: 10.1073/pnas.94.9.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans-Molina C, Garmey JC, Ketchum R, Brayman KL, Deng S, Mirmira RG. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes. 2007;56 (3):827. doi: 10.2337/db06-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuhashi M, Tomozawa S, Endo K, Shinagawa A. Quantification of mRNA in whole blood by assessing recovery of RNA and efficiency of cDNA synthesis. Clin Chem. 2006;52 (4):634. doi: 10.1373/clinchem.2005.048983. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuhashi M, Keller C, Akitaya T. Gene manipulation on plastic plates. Nature. 1992;357 (6378):519. doi: 10.1038/357519a0. [DOI] [PubMed] [Google Scholar]
- 19.Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24 (6):954. [PubMed] [Google Scholar]
- 20.Ito T, Omori K, Rawson J, et al. Improvement of canine islet yield by donor pancreas infusion with a p38MAPK inhibitor. Transplantation. 2008;86 (2):321. doi: 10.1097/TP.0b013e31817ef6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bank HL. Assessment of islet cell viability using fluorescent dyes. Diabetologia. 1987;30 (10):812. doi: 10.1007/BF00275748. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto M, Morimoto Y, Nozawa Y, Balamurugan AN, Xu B, Inoue K. Establishment of fluorescein diacetate and ethidium bromide (FDAEB) assay for quality assessment of isolated islets. Cell Transplant. 2000;9 (5):681. doi: 10.1177/096368970000900514. [DOI] [PubMed] [Google Scholar]
- 23.Zawalich WS, Yamazaki H, Zawalich KC. Biphasic insulin secretion from freshly isolated or cultured, perifused rodent islets: comparative studies with rats and mice. Metabolism. 2008;57 (1):30. doi: 10.1016/j.metabol.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55 (12):3470. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 25.Sweet IR, Gilbert M, Scott S, et al. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am J Transplant. 2008;8 (1):183. doi: 10.1111/j.1600-6143.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- 26.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7 (3):707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7 (1):38. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5 (7):1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 29.Todorov I, Al-Abdullah IH, Nair I, Umedai C, Iglesias-Meza I, Kandeel F. Comparison of cell composition of while vs. dissociated human islets using laser scanning cytometry. Xenotransplantation. 2007;14 (5):500. [Google Scholar]
- 30.Hanson MS, Steffen A, Danobeitia JS, Ludwig B, Fernandez LA. Flow cytometric quantification of glucose-stimulated beta-cell metabolic flux can reveal impaired islet functional potency. Cell Transplant. 2008;17 (12):1337. doi: 10.3727/096368908787648038. [DOI] [PubMed] [Google Scholar]
- 31.Leibiger B, Moede T, Schwarz T, et al. Short-term regulation of insulin gene transcription by glucose. Proc Natl Acad Sci U S A. 1998;95 (16):9307. doi: 10.1073/pnas.95.16.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann R, Zuellig RA, Kugelmeier P, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56 (3):594. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani M, Moritz W, Bodmer E, et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14 (1):67. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 34.Kauri LM, Jung SK, Kennedy RT. Direct measurement of glucose gradients and mass transport within islets of Langerhans. Biochem Biophys Res Commun. 2003;304 (2):371. doi: 10.1016/s0006-291x(03)00595-3. [DOI] [PubMed] [Google Scholar]
- 35.Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27 (3):185. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.