Abstract
Defects in cardiac valvulogenesis are a common cause of congenital heart disease, and the study of this process promises to provide mechanistic insights and lead to novel therapeutics. Normal valve development involves multiple signaling pathways, and recently roles have been identified for extracellular matrix components, including glycosaminoglycans. We therefore explored the role of the glycosaminoglycan chondroitin sulfate during zebrafish cardiac development. Beginning at 33 hours, there is a distinct zone of chondroitin sulfate expression in the atrioventricular (AV) boundary, in the cardiac jelly between the endocardium and myocardium. This expression is both spatially and temporally restricted, and is undetectable after 48 hours. Chemical as well as genetic inhibition of chondroitin synthesis results in AV canal defects, including loss of the atrioventricular constriction, blood regurgitation, and failure of circulation. Lack of chondroitin disrupts a marker of cell migration, results in a loss of myocardial and endothelial markers of valvulogenesis, and misregulates BMP expression, supporting an early role in AV canal development. In summary, we have defined a requirement for chondroitin sulfate expression in the normal patterning of the AV boundary, suggesting that this component of the cardiac jelly provides a necessary signal in this critical transition in vertebrate cardiogenesis.
Keywords: Cardiac Valve, Cardiac Development, Chondroitin Sulfate, Extracellular Matrix, Cardiac Jelly, Epithelial to mesenchymal transition, Zebrafish
INTRODUCTION
Cardiac valves are complex structures that allow the heart to control blood circulation and pressure in a dynamic fashion (Armstrong and Bischoff, 2004). Defects in valve formation are one of the most common heart defects observed in humans. In the United States alone over 20,000 deaths per year are attributed to cardiac valve diseases, while approximately 99,000 valve surgeries are performed (Rosamond et al., 2007). Valve disease is predicted to become an increasingly serious problem for the aging population both in terms of health care costs and increased morbidity (Nkomo et al., 2006). Therefore the study of cardiac valve development promises to aid in the development of therapeutics for treatment of cardiac valve diseases.
Development of the cardiac valves occurs in the setting of ongoing changes in cardiac morphology and after the onset of cardiac function. The primitive heart is a tube of cardiomyocytes lined internally by a layer of endocardial cells. During cardiac looping, a gelatinous matrix, termed cardiac jelly, is secreted which forms swellings at the levels of the atrioventricular (AV) junction and the outflow tract. The overlying endothelial cells are then activated and undergo an epithelial to mesenchymal transition and migration into the cardiac jelly. Cellular and matrix expansion within the cardiac jelly eventually gives rise to the endocardial cushions from which the cardiac valves are derived (Butcher and Markwald, 2007). The cardiac jelly itself is known to be rich in the extracellular matrix components glycosaminoglycans and proteoglycans. Glycosaminoglycans (GAGs) are long chain sulfated sugar polymers, of which there are three major classes: chondroitin sulfate (CS), heparan sulfate (HS), and hyaluronic acid (HA). Proteoglycans (PG) are the result of covalent attachment of glycosaminoglycans to core proteins. While the proteoglycan may be regarded as a single unit, in many cases the core proteins exhibit distinct functions from the glycosaminoglycans (Rapraeger, 2001).
While both HS and HA have been implicated in vertebrate cardiac development (Yost, 1990; Camenisch et al., 2000; Yue et al., 2004), the role of CS remains largely uncharacterized. Chondroitin sulfate has been shown to be a component of adult human heart valves, although its role in valvular development remains unknown (Grande-Allen et al., 2004). Versican, a putative core protein for chondroitin sulfate is expressed in the developing AV ring and its mutation in the mouse results in loss of endocardial cushions and the absence of valve leaflets (Yamamura et al., 1997; Mjaatvedt et al., 1998). However, it is unclear if the versican knockout mouse phenotype is due to loss of an essential core protein function, or to loss of the chondroitin sulfate side chain. Indeed most studies have failed to determine if the versican isoform under examination has a CS chain attached.
Zebrafish hearts, though two-chambered, undergo many of the same developmental processes as the four-chambered hearts of other vertebrates. This includes the formation of the primitive heart tube, looping, and the development of an AV valve (Stainier, 2002; Hurlstone et al., 2003; Bartman et al., 2004; Chang et al., 2004). The zebrafish mutant jekyll carries a mutation in the UDP-glucose dehydrogenase (ugdh) gene and displays defects in AV ring development (Walsh and Stainier, 2001). The UGDH enzyme is required to generate the glycans UDP-glucuronic acid and UDP-xylose (Perozich et al., 1995), which are building blocks for glycosaminoglycans.
Because of the paucity of knowledge in the function of CS during cardiac development, and the circumstantial evidence that this GAG may be important in zebrafish valvulogenesis, we decided to examine the specific role of CS during cardiac development.
RESULTS
Chondroitin sulfate is expressed in the zebrafish heart during early development
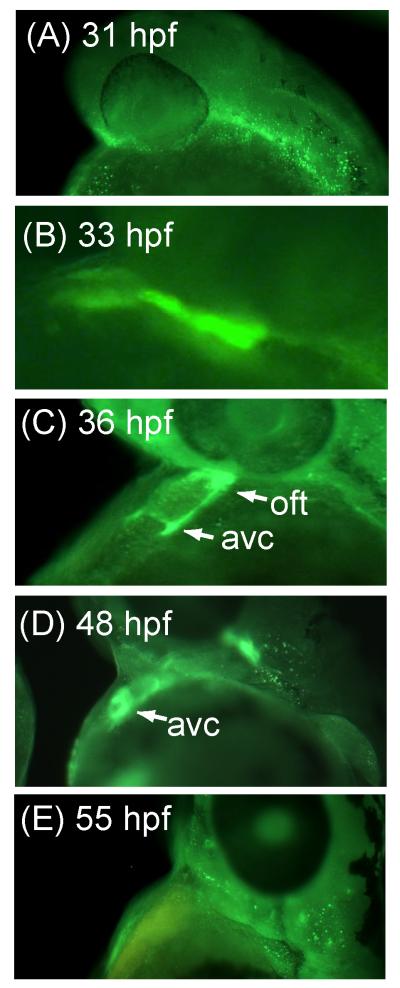
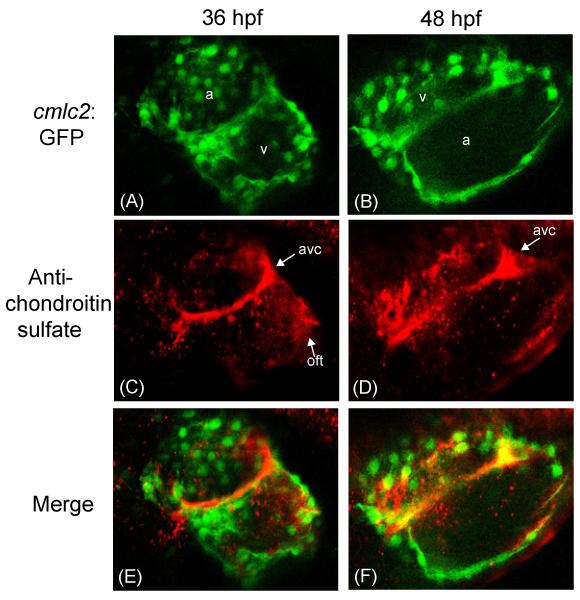
We first sought to characterize the cardiac expression patterns of CS during normal zebrafish development. Surprisingly, CS is expressed in the developing heart only between 33-48 hours post fertilization (hpf) (Fig. 1B-1D, Fig. 2), a time window known to be critical for subsequent cardiac valve formation (Armstrong and Bischoff, 2004). Before 33 hpf, CS is observed primarily in the cranial region and the primitive vascular region of the tail (Fig. 1A and Fig. S1 in supplemental data). At 33 hpf we observe diffuse CS staining throughout the primitive heart tube (Fig. 1B), along with strong staining distal to the forming outflow tract. At 36-48 hpf CS expression becomes restricted to the AV ring and outflow tract (Fig. 1C and 1D). Confocal microscopy images provide greater clarity of this distribution pattern, as we observe a clearly defined circumferential ring of CS midway through the heart tube at 36 hpf (Fig. 2A, 2C, and 2E). At 48 hpf, the ring is not as clearly defined, and CS is more strongly expressed at opposite sides of the ring (Fig. 2B, 2D, and 2F). We hypothesize that these are the forming endocardial cushions After 48 hpf, CS cardiac staining is no longer observed (Fig. 1E), compared to the positive control staining observed with the anti-myosin antibody S46 (Fig. S2 in supplemental data). The spatial and temporal pattern of CS expression suggests that it may have an important function in cardiac AV canal development. Further, CS localization appears to be an early marker of the AV canal.
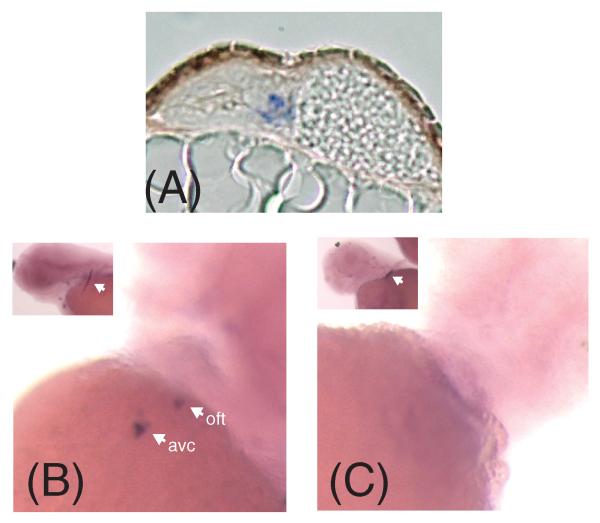
Figure 1.
Whole mount immunofluorescence images of Danio rerio hearts stained with an anti-chondroitin sulfate antibody at the indicated hours post fertilization (hpf).
Figure 2.
Confocal images of Danio rerio hearts stained with an anti-chondroitin sulfate antibody at 36 and 48 hours post fertilization (hpf). (A,B) Confocal microscopy of Danio rerio containing the myocardium specific cmlc2:GFP marker (green). (C,D) Confocal microscopy of the same Danio rerio images stained with a CS-specific antibody (red). (E,F) Merged images. a= atrium; v= ventricle; avc = atrioventricular canal forming region; oft = outflow tract.
Chondroitin sulfate is expressed between the endocardium and myocardium
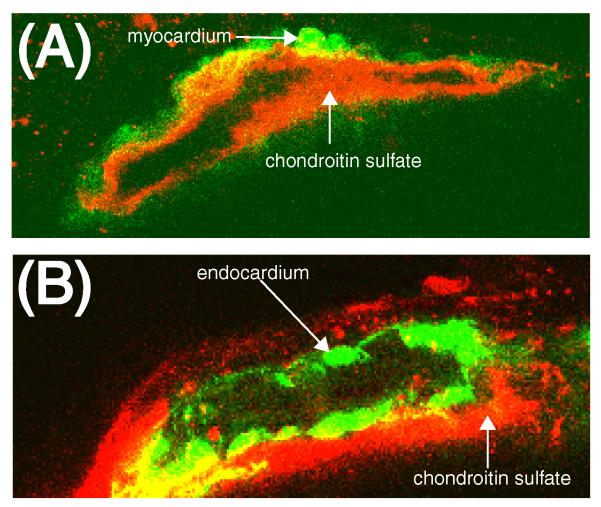
We used confocal microscopy to examine the specific localization of CS. During early development, the vertebrate heart is comprised of two cell layers, the outer layer consists of myocardium, while the inner layer is endocardium, the two layers separated by extracellular matrix. We used a transgenic line which marks the myocardial cell layer with GFP (cmlc2:GFP) and counterstained with an antibody to CS (Fig. 3A). A circumferential cross-section through the 36hpf heart tube shows that CS is localized lumenal to the myocardial cell layer. We next examined CS expression in a transgenic line which marks the endothelial cell layer (flk:EGFP)(Fig. 3B) and found that CS surrounds the endocardial cell layer on the external, or ablumenal side. Together, these results indicate that CS is located in a layer between the myocardium and the endocardium, a region referred to as cardiac jelly. The cardiac jelly has been implicated in the process by which endothelial cells transition to mesenchymal cells, an important step in the formation of cardiac valves (Butcher and Markwald, 2007).
Figure 3.
Chondroitin sulfate is localized to the cardiac jelly between the myocardium and endocardium. (A) Confocal microscopy of showing a circumferential cross section of 36 hpf Danio rerio containing the myocardium specific cmlc2:GFP marker (green) and stained with a CS-specific antibody (red). (B) Confocal microscope image of Danio rerio containing the endocardium specific flk:EGFP marker (green) and stained with a CS-specific antibody (red).
Chemical and genetic downregulation of chondroitin sulfate results in AVC defects
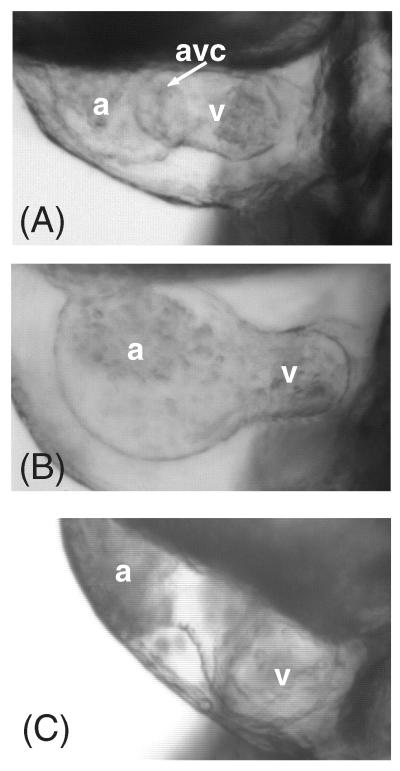
Based on the specific, early expression pattern of CS in the valve rings we suspected that CS might play an important role in cardiac chamber segmentation. In order to inhibit CS synthesis we employed the compound cis/trans-decahydro-2-napthol-ß-D-xyloside (DX) (Fritz et al., 1994). DX is known to inhibit CS proteoglycan biosynthesis by incorporation and chain termination. At very high concentrations, heparan sulfate biosynthesis can also be affected (Fritz et al., 1994). We found that treatment of developing embryos with DX resulted in reduction of cardiac CS staining (Fig. S3) but no reduction in the related glycosaminoglycan heparan sulfate (Supplemental Fig. S4). The DX phenotype includes loss of the AV constriction, AV valve regurgitation, and lack of circulation in 100% (n=150) of the embryos affected (Fig. 4B). These differences are clearly visible upon comparison of videos of a wild type beating heart (supplemental video SV1), to the DX-treated beating heart (supplemental video SV2). Treated animals also have shortened, curled tails, and smaller heads (data not shown). The use of a chemical inhibitor afforded temporal control over the inhibition of CS synthesis. Time course exposures revealed a critical window of DX exposure that extended from 7 hpf through 48 hpf, significantly longer, but overlapping with the time when CS is highly expressed within the heart (Fig. S5). While shorter exposures (i.e. 24-48 hpf) can result in the same cardiac defects, a smaller number of embryos are affected. There are several potential explanations for this broad time window, including issues of DX bioavailability, and possible redistribution of CS produced in early development. Prior to 36 hpf, heart function and rate appears normal in DX-treated fish.
Figure 4.
Chemical and genetic knockdown of chondroitin sulfate prevents the proper formation of the AV canal in zebrafish. (A) Wild type Danio rerio heart at 55 hpf. (B) 55 hpf animals treated from 7-48 hpf with DX, an inhibitor of CS formation. (C) 55 hpf animals injected with a morpholino to chondroitin synthase-1 (chys-1). at = atrium; v = ventricle; av = atrioventricular canal forming region.
As further confirmation of the role of CS, we reasoned that genetic knockdown of chondroitin synthase should phenocopy DX treatment. Injection of a previously characterized morpholino antisense oligonucleotide targeting the chondroitin synthase gene chys-1 (Zhang et al., 2004), resulted in loss of chondroitin expression in the heart (Fig. S2 in supplemental material) and failure of the development of the AV canal. Injected embryos (14/48, 29%) developed AV regurgitation, loss of circulation and lack of AV constriction, similar to the phenotypes observed in the DX-treated animals (Fig. 4C). These animals also had axis defects similar to DX-treated fish, although not as severe (data not shown). Similarities in phenotypes are highlighted when comparing video of chys-1 MO beating hearts (supplemental video SV3) to DX-treated hearts (supplemental video SV2). Due to the low penetrance and high lethality of chys-1 morphants, all subsequent experiments were performed using DX to inhibit CS.
Downregulation of chondroitin sulfate disrupts the cell migration marker spp1
Because we observed CS to be a component of the cardiac jelly which is thought to be important in AV canal patterning and cardiac cushion formation, we hypothesized that the cardiac defects observed during CS depletion result from a perturbation of these events. Cushion formation is a complex multi-step process in which endothelial cells become activated, break away from their epithelial cell layer, and migrate into the cardiac jelly. They then transform into mesenchymal cells, forming cardiac cushions that give rise to the valves. Because of the early expression of CS during AV canal patterning we decided to examine one of the early critical steps: cell migration.
Osteopontin or secreted phosphoprotein 1 (spp1) is upregulated in migrating cells (Katagiri et al., 1999; Alonso et al., 2007; Saika et al., 2007). Zebrafish spp1 was independently identified in an expression screen for genes preferentially expressed in cardiac tissue during development (data not shown). In situ hybridization demonstrated expression of spp1 in the endocardium of the AVC and OFT at 72 hpf (Fig. 5A and 5B). Treatment with DX prevents spp1 expression (Fig. 5D) in the heart, but not in the pectoral fin (20/20, 100% embryos). Thus, CS depletion and the failure of the AV canal to develop are associated with disruption of this marker of cell migration.
Figure 5.
Depletion of chondroitin sulfate leads to changes in the cell migration marker spp1. (A) In situ hybridization section demonstrating that the zebrafish osteopontin marker spp1 is localized to the atrioventricular canal endocardium in zebrafish. (B) spp1 is normally expressed in the atrioventricular canal (avc) and outflow tract (oft). (C) DX-treatment from 7 to 48 hpf results in the disappearance of spp1 in the AVC and OFT at 72 hpf. Note that while spp1 expression is absent in DX treated embryos, the presence of a stripe of spp1 expression at the base of the pectoral fin demonstrating the success of the in situ protocol (white arrow in inset of B and C).
Downregulation of chondroitin sulfate leads to changes in AV canal markers
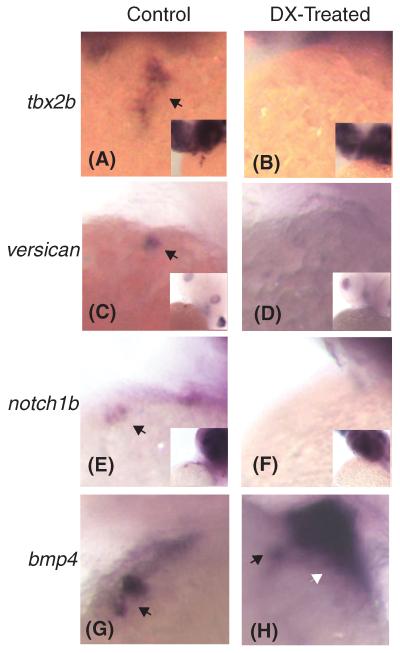
Multiple signaling pathways have been implicated in the formation of cardiac cushions and valves. We therefore examined several of these pathways using the well-characterized expression markers tbx2b, versican, notch1b, and bmp4. All of these genes are known to be upregulated in the AV canal at 48 hpf; bmp4, tbx2b, and versican are expressed in the myocardium, while notch1b is an endocardial marker (Chi et al., 2008).
Treatment of embryos with the CS inhibitor DX resulted in loss of tbx2b, versican, and notch1b expression in the AV ring at 48hpf (Fig. 6, A-F). Conversely, after DX treatment, bmp4 is still expressed in the AVC at 48hpf (Fig. 6H, black arrow) and is also found throughout the ventricle (Fig. 6H, white arrow).
Figure 6.
Depletion of chondroitin sulfate results in disruption of multiple signaling pathways. Tbx2b, versican, and notch1b are normally expressed within the atrioventricular canal at 48 hpf (black arrows in (A), (C), and (E)). However, treatment with the compound DX results in the severe downregulation of expression ((B), (D), and (F)). Bmp4 expression remains within the AVC region when treated with DX (black arrow in (G) and (H)), but ventricular expression is upregulated (white arrow in (H)). For those genes that are downregulated, we have included an image of the entire upper body, as all three of these genes stain the head. This is to demonstrate the absence of heart expression is not due to failure of the in situ probe.
DISCUSSION
Chondrotin sulfate proteoglycans have previously been shown to regulate several diverse biological processes, including neuronal development, inflammation, leukocyte trafficking, and neural regeneration (Sugahara et al., 2003). Proteoglycans are composed of a core protein and an attached glycosaminoglycan. Of interest, two putative CS core proteins are expressed in the vertebrate heart: versican and aggrecan (Zanin et al., 1999). Versican knockout mice display defects in endocardial cushions and the absence of AV valve leaflets (Yamamura et al., 1997; Mjaatvedt et al., 1998). However, such studies can not distinguish between effects that are due to loss of the core protein versican versus those due to loss of the glycosaminoglycan chondroitin sulfate. Furthermore, versican has numerous splice isoforms, some with predicted CS attachment sites, and at least one that does not (Rahmani et al., 2006). Our results, which specifically target the glycosaminoglycan component of the proteoglycan(s) identify a specific role for CS, independent from the core protein, as a necessary component of the cardiac jelly in the AV canal forming process.
The localization of CS within the cardiac jelly may provide clues to the mechanism for impaired AV canal formation. This intercellular layer is critical for the migration of endothelial cells into the forming endocardial cushions. One interesting hypothesis is that lack of CS in the cardiac jelly may inhibit steps in this epithelial to mesenchymal transition thus leading to the defective AV canal specification. Indeed, our data suggest that cell migration may be impaired, as demonstrated by the loss of spp1expression. Spp1 is known to bind to the cell surface receptor CD44 during cell migration (Weber et al., 1996; Katagiri et al., 1999) and CD44 itself is known to have both an attached CS sidechain, as well as a CS-binding motif (Jackson et al., 1995; Sugahara et al., 2008). Thus chondroitin sulfate, CD44, and spp1 may all work together in a common pathway to regulate cell migration during AV canal formation.
We also find that CS depletion results in lack of expression of three AV canal markers: notch1b, versican, and tbx2b. Our hypothesis is that the CS normally acts upstream of these signaling pathways, and that lack of CS prevents their expression. Interestingly, CS depletion causes expansion of bmp4 expression into the ventricle. These data are similar to those of Rutenberg et al, who observe bmp4 expansion in zebrafish hey2 mutants (Rutenberg et al., 2006). Hey2 transcriptional repressors lie downstream of Notch signaling, and the absence of notch1b expression in CS depleted animals may lead to the downregulation of the repressor hey2 and subsequent expansion of bmp4 into the ventricle.
The in situ expression patterns we observed in CS knockdown embryos and those reported in jekyll mutants are not identical (Walsh and Stainier, 2001). Such differences might be due to the fact that the jekyll mutation should result in the loss of hyaluronic acid and heparan sulfate as well as with chondroitin sulfate. Each GAG is known to affect multiple signaling pathways, and the combination of knocking down all three may result in complex or synergistic signaling effects (Esko and Selleck, 2002; McDonald and Camenisch, 2003; Sugahara et al., 2003). In contrast, our approach was designed to determine the specific contribution of CS in cardiac development.
Our results also imply that versican may not be the cognate core protein for CS during zebrafish valvulogenesis. While both CS and versican show similar expression patterns before 48 hpf (Walsh and Stainier, 2001), CS is absent in the heart after 48 hpf, while versican mRNA expression persists up to 72 hpf (Lee et al., 2007). Instead, aggrecan may be the relevant core protein, as it is known to have CS side chains, and has also been implicated in vertebrate valvulogenesis (Lincoln et al., 2006; Shelton and Yutzey, 2007). The role of aggrecan during cardiac development has yet to be characterized in zebrafish. Importantly, in these experiments we have isolated the role of the CS side chain, distinct from the core protein.
Our results may also provide clues to mechanisms of human diseases. Mitral valve prolapse is a condition in which heart valves exhibit gross changes in valve shape and rigidity. These valves have an increase in chondroitin sulfate, but it is unknown whether this change is causative (Grande-Allen et al., 2003). Our data support a potential signaling role for CS during AV canal and subsequent valve development, and perturbation of this signal may result in the defects observed in human valve diseases such as mitral valve prolapse.
There is increasing recognition of the importance of extracellular matrix elements in biologic processes. Here we describe that depletion of a specific glycosaminoglycan, chondroitin sulfate, leads to early and specific defects in formation of the atrioventricular canal. Further, we characterize the zebrafish cardiac jelly as a CS-rich milieu, and show the absence of CS within the jelly results in disruption of an early endothelial cell migration marker. Finally, we indicate that CS may be acting in part through the BMP signaling pathway. We believe that these observations shed light on a newly defined role of CS in heart development, and open new lines of investigation into the biology of cardiac development and disease.
EXPERIMENTAL PROCEDURES
Zebrafish Lines
All experiments were performed in either wild-type zebrafish (Tubingen AB) or the transgenic strains Tg(cmlc2:GFP) (Burns et al., 2005), and Tg(flk1:EGFP)s843 (Beis et al., 2005). Zebrafish were maintained using standard methods.
Microscopy
Immunofluorescent images were taken using a Zeiss Discovery.V8 Stereo microscope with an AxioCam MRc camera and AxioVision 4.5 software. Confocal images were taken using a Zeiss LSM 5 Pascal confocal microscope with accompanying software. Video imaging of live hearts were captured using a Zeiss Axioplan with Photron FASTCAM Viewer software. In situ hybridization images were taken using a Leica Wild M10 with Windows Spot Basic v4.0.8 software.
Injection of morpholino oligonucleotide for chys-1
Morpholino oligonucleotides (MOs, Gene Tools, LLC) for chys-1 have previously been described (Zhang et al., 2004). MOs were re-suspended in distilled water, and 1.5 ng was injected into single cell Tubingen AB embryos.
Whole mount immunohistochemistry
Embryos were stained for chondroitin sulfate using standard methodology. Briefly, Tubingen AB strain embryos were collected at the indicated stage and fixed with 4% PFA overnight at 4°C. Embryos were permeabilized with −20°C acetone and 1% Tween-20 in PBS, then blocked for at least 1 hour (0.2% BSA, 5% sheep serum, 0.1% Tween-20 in PBS). This was followed by overnight incubation at 4°C with mouse α-chondroitin sulfate (1:200, Sigma, Cat. # C-8035). To examine heparan sulfate reactivity, we used the same protocol with the anti-heparan sulfate 10E4 (1:50, USBiological, Cat. # H1890). As a positive control, we used the myosin specific antibody S46 (1:10, Developmental Studies Hybridoma Bank). Embryos were rinsed four times in block, then incubated overnight at 4°C with either Alexa-Fluor 555 conjugated goat α-mouse IgG (1:200, Invitrogen, Cat. # A21424) or Alexa-Fluor 488 conjugated goat α-mouse IgG (1:200, Invitrogen, Cat. # A11029). Embryos were rinsed in block four times, and visualized as described.
Treatment of embryos with DX
Dried cis/trans-decahydro-2-napthol-β-D-xyloside (DX), (gift of J. Esko and J. Brown) was resuspended with 14% DMSO in E3 water to get a DX stock concentration of 17 mM and stored at −20°C until use. Stock was diluted in E3 to a final concentration of 2 mM for most experiments unless otherwise noted. Controls consisted of DMSO in E3 at relevant concentrations. Embryos were incubated for up to 48 hours, transferred to fresh E3 water, and their hearts were visualized by video microscopy.
In situ hybridization
In situ hybridization was performed as previously described (Thisse et al., 1993). Probes used were versican (Thisse and Thisse, 1999), bmp4 (Nikaido et al., 1997), notch1b (Westin and Lardelli, 1997), tbx2b (Gross and Dowling, 2005), and spp1 (Kawasaki et al., 2004).
Supplementary Material
Supplementary video SV1: Video of wild type Danio rerio heart beating.
Supplementary video SV2: Video of wild type animal heart beating after being treated with DX, an inhibitor of CS formation.
Supplementary video SV3: Video of wild type animal heart beating after being injected with a morpholino to chondroitin synthase-1 (chys-1).
ACKNOWLEDGEMENTS
We wish to thank Jeff Esko, Jillian Brown (UC-San Diego), Caroline Burns, and Peter Schlueter for reagents, Randy Peterson for discussions, and Burcu Guner, Heather Kolpa and Janet Mosley for technical assistance. This work was funded by a Grant in Aid from the American Heart Association to DM and by grant 07CVD04 from the Fondation Leducq, Paris, France, for the Transatlantic MITRAL Network of Excellence to DSP and DM.
REFERENCES
- Alonso SR, Tracey L, Ortiz P, Perez-Gomez B, Palacios J, Pollan M, Linares J, Serrano S, Saez-Castillo AI, Sanchez L, Pajares R, Sanchez-Aguilera A, Artiga MJ, Piris MA, Rodriguez-Peralto JL. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007;67:3450–3460. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T, Walsh EC, Wen K-K, McKane M, Ren J, Alexander J, Rubenstein PA, Stainier DYR. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:673–681. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin S-W, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen J-N, Stainier DYR, Jungblut B. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Markwald RR. Valvulogenesis: the moving target. Phil Trans R. Soc. B. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-P, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Chi N, Shaw R, De Val S, Kang G, Jan L, Black B, Stainier DYR. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:706–710. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Fritz TA, Lugemwa FN, Sarkar AK, Esko JD. Biosynthesis of heparan sulfate on beta-D-xylosides depends on aglycone structure. J. Biol. Chem. 1994;269:300–307. [PubMed] [Google Scholar]
- Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–633. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. Journal of the American College of Cardiology. 2003;42:271–277. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- Gross JM, Dowling JE. Tbx2b is essential for neuronal differentiation along the dorsal/ventral axis of the zebrafish retina. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4371–4376. doi: 10.1073/pnas.0501061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone AFL, Haramis A-PG, Wienholds E, Begthel H, Korving J, van Eeden F, Cuppen E, Zivkovic D, Plasterk RHA, Clevers H. The Wnt/β-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Jackson DG, Bell JI, Dickinson R, Timans J, Shields J, Whittle N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J. Cell Biol. 1995;128:673–685. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44s cooperate with β1-containing Integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- Kawasaki K, Suzuki T, Weiss KM. Genetic basis for the evolution of vertebrate mineralized tissue. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11356–11361. doi: 10.1073/pnas.0404279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C, Tsai J-N, Liao P-Y, Tsai W-Y, Lin K-Y, Chuang C-C, Sun C-K, Chang W-C, Tsai H-J. Glycogen synthase kinase 3alpha and 3beta have distinct functions during cardiogenesis of zebrafish embryo. BMC Developmental Biology. 2007;7:93. doi: 10.1186/1471-213X-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Bio. 2006;292:290–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Camenisch TD. Hyaluronan: Genetic insights into the complex biology of a simple polysaccharide. Glycoconjugate J. 2003;19:331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capeheart AA, Turner D, Markwald R. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardiac cushion formation. Dev Bio. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Saji T, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mechanisms of Development. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- Perozich J, Leksana A, Hemple J. UDP-glucose dehydrogenase. Structural characteristics. Adv Exp Med Biol. 1995:79–84. [PubMed] [Google Scholar]
- Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Versican: signaling to transcriptional control pathways. Can. J. Physiol. Pharmacol. 2006;84:77–92. doi: 10.1139/y05-154. [DOI] [PubMed] [Google Scholar]
- Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol. 2001;12:107–116. doi: 10.1006/scdb.2000.0239. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, for the American Heart Association Statistics Committee and Stroke Statistics S Heart Disease and Stroke Statistics--2007 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Shirai K, Yamanaka O, Miyazaki K-i, Okada Y, Kitano A, Flanders KC, Kon S, Uede T, Kao WW-Y, Rittling SR, Denhardt DT, Ohnishi Y. Loss of osteopontin perturbs the epithelial-mesenchymal transition in an injured mouse lens epithelium. Lab Invest. 2007;87:130–138. doi: 10.1038/labinvest.3700508. [DOI] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Developmental Biology. 2007;302:376–388. doi: 10.1016/j.ydbio.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DYR. Zebrafish genetics and vertebrate heart formation. Nat Rev Genetics. 2002;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struc Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sugahara KN, Hirata T, Tanaka T, Ogino S, Takeda M, Terasawa H, Shimada I, Tamura J-i, ten Dam GB, van Kuppevelt TH, Miyasaka M. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 2008;68:7191–7199. doi: 10.1158/0008-5472.CAN-07-6198. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DYR. UDP-Glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Westin J, Lardelli M. Three novel Notch genes in zebrafish: implications for vertebrate Notch gene evolution and function. Dev Genes Evol. 1997;207:51–63. doi: 10.1007/s004270050091. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mouse. Dev Bio. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yost HJ. Inhibition of proteoglycan synthesis eliminates left-right asymmetry in Xenopus laevis cardiac looping. Development. 1990;110:865–874. doi: 10.1242/dev.110.3.865. [DOI] [PubMed] [Google Scholar]
- Yue X, Schultheiss TM, McKenzie EA, Rosenberg RD. Role of heparan sulfate in dextral heart looping in chick. Glycobiology. 2004;14:745–755. doi: 10.1093/glycob/cwh083. [DOI] [PubMed] [Google Scholar]
- Zanin MKB, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. The Anatomical Record. 1999;256:366–380. doi: 10.1002/(SICI)1097-0185(19991201)256:4<366::AID-AR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lefebvre JL, Zhao S, Granato M. Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat Neurosci. 2004;7:1303–1309. doi: 10.1038/nn1350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video SV1: Video of wild type Danio rerio heart beating.
Supplementary video SV2: Video of wild type animal heart beating after being treated with DX, an inhibitor of CS formation.
Supplementary video SV3: Video of wild type animal heart beating after being injected with a morpholino to chondroitin synthase-1 (chys-1).