Abstract
Recent adult studies suggest that the subgenual anterior cingulate cortex (sgACC) is involved in fundamental mental operations such as affective processing and inhibitory control. However, little is known about inhibition-associated sgACC function in adolescents, and there are no published data regarding whether personality characteristics are related to inhibition-associated sgACC brain activity in adolescents. This study examined the relationship between personality and inhibition-associated sgACC response in healthy adolescents. Seventeen adolescents of 13–17 years of age underwent functional magnetic resonance imaging while performing a parametric stop-signal task. Greater harm avoidance levels were significantly associated with increased inhibition-related sgACC activity. These results establish, for the first time, a link between personality and differential sgACC activation in adolescents.
Keywords: adolescents, emotion, functional MRI, harm avoidance, personality, subgenual anterior cingulate, temperament and character inventory
Introduction
Functional neuroimaging studies of the subgenual anterior cingulate cortex have shown it to be involved with affective processing in both healthy adult and patient populations. The activation of this region of the cingulate has been specifically identified as a correlate of sad mood [1] and greater blood flow to this region has been associated with increasing sadness in healthy adults [2]. In the latter study [2], the authors also examined clinically depressed adults using the same methods and found that with recovery from depression, the patients showed decreased blood flow to the subgenual cingulate area.
In an earlier functional magnetic resonance imaging (fMRI) study of healthy adults [3], we used a parametric motor inhibition task, referred to as the ‘stop’ task [4], to examine the neural systems involved in stopping already initiated motor responses. We reported that specific subregions of the anterior cingulate cortex were differentially activated during errors committed during the easy versus hard trials. Specifically, we observed that the subgenual anterior cingulate cortex was more active during the easy trials. On the basis of these findings, we hypothesized that errors made during the easy trials may have been interpreted as avoidable and therefore attributed to an internal or ‘self’ source, because accurate and fast responses were perceived as attainable by the individual. Consequently, such errors made during the easy trials may have been implicitly processed by the individual as more emotionally negative and evocative. If our hypothesis was correct, it suggested that a personality more consistent with the attribution of errors to an internal source would be associated with greater activation of the subgenual anterior cingulate cortex. In our earlier study [3], however, we did not assess personality in our participants; thus, we were unable to examine how personality might be associated with differential brain activity in the subgenual anterior cingulate cortex.
In this study, we used the temperament and character inventory (TCI) [5] to assess personality just before scanning. The TCI is a well-established questionnaire that has been widely used and published in studies of both healthy and patient populations [6-8]. Temperament in the TCI pertains to biases in automatic reactions to emotional stimuli and is to a degree heritable and stable throughout life regardless of social learning or culture. The TCI distinguishes four independently inherited dimensions of temperament: harm avoidance, reward dependence, novelty seeking, and persistence.
To our knowledge, no fMRI studies have been published using the stop task to examine how inhibition-associated subgenual anterior cingulate cortex activity might relate to personality in healthy adolescents. Thus, the primary goal of this study was to determine whether the degree of inhibition-associated subgenual anterior cingulate cortex activity was related to personality differences as assessed by the TCI in normal adolescents. On the basis of our earlier published findings [3] and the reviewed literature [1,2,6-9], we hypothesized that: (i) greater activation of the subgenual anterior cingulate cortex would be observed as the level of inhibitory difficulty decreased; and (ii) adolescents with a personality more consistent with attributing errors to an internal source were more likely to demonstrate greater subgenual anterior cingulate cortex activation.
Methods
Participants
This study was approved by the University of California at San Diego and Rady Children's Hospital of San Diego Institutional Review Boards. All participants provided written assent, and their parent/legal guardians provided written informed consent to participate. All participants were financially compensated.
Seventeen healthy, right-handed female adolescent participants (ages 13–17 years; mean age 16.54 ± 1.25 years) were recruited from all regions of San Diego through the use of posted flyers and the Internet.
Each participant was administered the following items: (i) Computerized Diagnostic Interview Schedule for Children version 4.0 [10] and the Diagnostic Predictive Scale (DPS) [11] to assess for the presence of any axis I diagnoses, (ii) standard snellen eye chart, (iii) Ishihara Color Plates Test (8 plate, 2005 edition), and (iv) Edinburgh handedness inventory [12]. Each participant completed the following self-administered questionnaires: (i) TCI [13], (ii) demographics questionnaire, and (iii) medical and developmental history form. The exclusion criteria were: (i) any current or lifetime Diagnostic and Statistical Manual-IV axis I psychiatric disorder, (ii) color blindness, (iii) less than 20/40 corrected vision, (iv) history of a serious medical, developmental, or neurological disorder, (v) history of loss of consciousness for more than 2 min, (vi) left handedness, and (vii) MRI contraindications (ferrometallic implants, braces, pregnancy).
Experimental task
The visual stimuli, an ‘X’ or an ‘O’, in white capital letters appeared on a black background back-projected to the MRI room subtending a visual angle of approximately 6°. Participants were told to press the right button as quickly as possible whenever an ‘O’ appeared and the left button when an ‘X’ appeared. In addition, participants were told that when they heard a tone delivered through headphones during a trial, they were not to press either button. Stimuli appeared at the beginning of each of the trials. Each trial lasted 1300 ms or until the participant responded. Trials were separated by 200 ms interstimulus intervals (blank screen). The individual response latency was used to denote the period of inhibitory processing and provided a naturally jittered reference function. Participants performed 72 total stop trials, which were pseudo-randomized throughout the task and counter-balanced. Six blocks were performed, each containing 48 total trials (12 stop and 36 nonstop trials in each block). Task instructions were presented for 12 s between blocks.
Just before scanning, participants performed the stop task in a behavioral testing session to determine their mean reaction time (RT). On the basis of this data, six different trial types were designed based on the amount of time after the beginning of the trial (when an ‘X’ or ‘O’ stimuli first appeared) when the stop signal was delivered: those when the stop signal was delivered at the participant's mean RT, and those when the stop signal was delivered at 100 (RT-100), 200 (RT-200), 300 (RT-300), 400 (RT-400), or 500 ms (RT-500) less than the mean RT after the beginning of the trial. Behavioral response data were collected on all participants during the scan. Behavioral data for five participants were corrupted and therefore not used in the behavioral data analysis.
To examine the neural circuitry involved in processing less versus more difficult trials, we compared the brain activation pattern related to performance of ‘easy’ trials (both correct and incorrect) with the brain activation pattern related to performance of ‘hard’ trials (both correct and incorrect). During easy inhibit trials, the time from the beginning of the tone to the appearance of the next visual stimulus was 400 or 500 ms less than the participant's mean RT from behavioral testing. During hard inhibit trials, the time from the beginning of the tone to the appearance of the next visual stimulus was equal to or 100 ms less than the participant's mean RT, calculated from performance of the task in the behavioral testing session.
Image acquisition
A fast event-related fMRI design was used. Images were acquired on a 3-T GE scanner (General Electric, Milwaukee, Wisconsin, USA) with twin-speed gradients using a GE 8-channel head coil. Each session consisted of a three-plane scout scan, high-resolution anatomical scan, T2*-weighted echo-planar imaging (EPI) scan to measure the blood-oxygen-level-dependent response, and EPI-based field maps to correct for susceptibility-induced geometric distortions. Functional scans, covering the entire brain, were acquired parallel to the anterior and posterior commissure (T2*−weighted EPI, repetition time = 2000 ms, echo time = 32 ms, field of view = 23 × 23 cm, 64 × 64 matrix, 30 2.6 mm oblique slices parallel with the ac-pc axial plane with a 1.4 mm gap, 256 repetitions, 512 s). During the same experimental session, a T1−weighted image with an inversion time of TI = 450 ms to null the cerebro-spinal fluid (fast spoiled gradient-echo, repetition time = 8.0 ms, echo time = 3.1 ms, flip angle = 12°, field of view = 25 cm, matrix = 256 × 256, 0.98 × 0.98 1.0 mm3 voxels) was collected in the sagittal plane for anatomical reference.
Statistical analysis of imaging data
All functional and structural image processing and analyses were conducted with the Analysis of Functional Neuro-Images software (Bethesda, Maryland, USA) [14]. Details of the image analysis pathway have been published elsewhere [3,15]. To minimize motion artifact, an Analysis of Functional NeuroImages 3D-coregistration algorithm (3dvolreg) was used to realign all echoplanar images. Data were time-corrected for slice acquisition order, and the time series data for each individual were analyzed using a multiple regression model (3dDeconvolve).
For the analysis of task difficulty, three task regressors of interest: ‘hard’ [MRT (mean reaction time) and MRT-100 ms], ‘medium’ (MRT-200 ms and MRT-300 ms), and ‘easy’ (MRT-400 ms and MRT-500 ms), and five nuisance regressors: roll, pitch, yaw (to account for residual motion), baseline (which included block instructions), and linear trend (to eliminate slow signal drifts) were entered into a linear multiple regression. Before inclusion in the regression model, the task-related regressors were convolved with a modified γ variate function to account for the hemodynamic delay and the slow rise and fall of the hemodynamic response. Activation in each voxel during each specific task condition was divided by the baseline activation to obtain the percentage of signal difference for each task condition.
A 4 mm full-width at half-maximum Gaussian filter was applied to the voxel-wise percentage of signal difference data to account for individual variations in anatomical landmarks. After smoothing, each participant's data were normalized to Talairach coordinates and a whole-brain mask screened out nonbrain voxels. To test our a priori hypothesis regarding the subgenual anterior cingulate cortex activity, a region-of-interest (ROI) analysis was performed using an anatomical mask that was defined by the Talairach Daemon Atlas [16]. Using the same method, a control brain ROI analysis was performed on the left and right visual cortices (Brodmann areas 17, 18, 19). The regressors of interest were combined to measure task difficulty, and one-sample t-tests were subsequently performed to determine where in the subgenual anterior cingulate cortex the blood-oxygen-level-dependent response contrast inhibitory load significantly differed from zero.
A threshold/cluster method was then applied. This threshold adjustment method was based on Monte-Carlo simulations and was utilized to guard against identifying false positive areas of activation using a 4 mm full-width at half-maximum Gaussian filter [17]. On the basis of these simulations, it was determined that for the ROI analysis of the subgenual anterior cingulate cortex a minimum volume of 192 μl and a connectivity radius of 4.0 mm was necessary to ensure that a voxel-wise a priori probability of 0.05 would result in a corrected cluster-wise activation probability of 0.05.
Statistical analyses of behavioral and temperament and character inventory data
All behavioral and statistical analyses were carried out with SPSS 14.0 (Chicago, Illinois, USA) [18]. Correlational analyses examined the relationship between four independent TCI dimensions of temperament (harm avoidance, novelty seeking, reward dependence, and persistence) and the percentage of signal change in the subgenual anterior cingulate cortex during the performance of stop task for the easy condition relative to the hard condition.
Results
Behavioral data
Participants responded with the correct button press in 94% of the nonstop trials. A repeated-measures analysis of variance revealed that participants made significantly more errors during the hard inhibit trials than during the easy inhibit trials [F(5,7) = 6.61, P = 0.014].
Brain activation
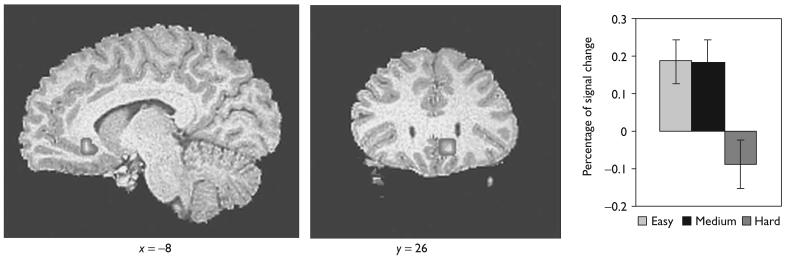
Using the anatomical mask, significant activation was found in the subgenual anterior cingulate cortex [x = −8.4, y = 26.2, z = − 6.4; t (16) = 3.32]. Furthermore, the subgenual anterior cingulate cortex was incrementally activated as the task level of difficulty decreased for the easy minus hard contrast (Fig. 1).
Fig. 1.
Sagittal (x = − 8) and coronal (y = 26) slices showing increased activity for the subgenual anterior cingulate cortex as the inhibitory level of difficulty decreased in the ROI analysis. The bar graphs on the right show the percentage of signal change for the easy, medium, and hard inhibit conditions. Error bars indicate standard error.
Correlation results
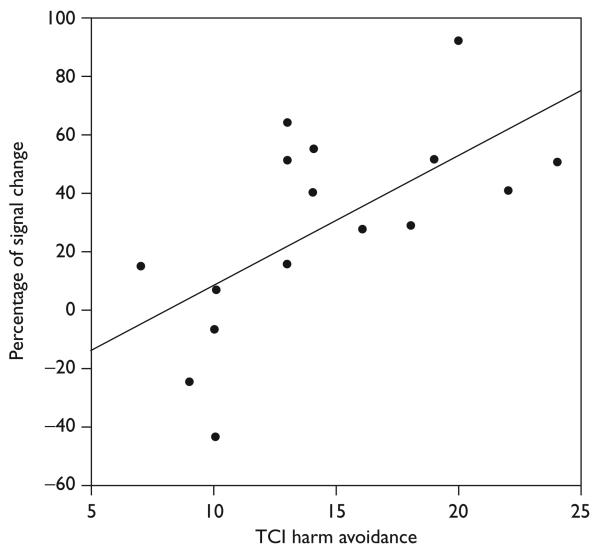
TCI harm avoidance scores correlated positively with subgenual anterior cingulate cortex percentage of signal change for the easy minus hard condition in the 17 adolescents (Spearman's ρ = 0.71, P = 0.001, two-tailed). See Fig. 2 for scatter plot. The correlations between the subgenual anterior cingulate cortex activation for the easy minus hard condition and TCI scores for novelty seeking (Spearman's ρ = 0.021, P = 0.94), reward dependence (Spearman's ρ = 0.087, P = 0.74), and persistence (Spearman's ρ = − 0.36, P = 0.15) were not statistically significant. The correlations between the percentage of signal change of the control brain regions for the easy minus hard condition and TCI scores for harm avoidance were not statistically significant (left visual cortex: Spearman's ρ = 0.13, P = 0.63; right visual cortex: Spearman's ρ = 0.024, P = 0.93).
Fig. 2.
Scatterplot showing the percentage of signal change in the subgenual anterior cingulate cortex and temperament and character inventory (TCI) harm avoidance scores for 17 healthy adolescents.
Discussion
This study is the first one to use a fast, event-related, parametric, individualized stop task design to examine the subgenual anterior cingulate cortex and how inhibition-associated subgenual anterior cingulate cortex activity might relate to personality in healthy adolescents. Two main results were obtained: (i) greater activation of the subgenual anterior cingulate cortex was observed as the inhibitory level of difficulty decreased, and (ii) greater levels of harm avoidance as measured by the TCI were significantly associated with increased subgenual anterior cingulate cortex activity.
The ROI analysis showed that greater activation of the subgenual anterior cingulate cortex occurred as the inhibitory level of difficulty decreased. To our knowledge, this finding demonstrates the differential activation of the subgenual anterior cingulate cortex in adolescents using a fast, event-related, parametric stop task for the first time. In addition, this finding is consistent with our published results in normal adults [3] and extends our findings to include healthy adolescents of 13–17 years of age as well as adults.
The correlation results between subgenual anterior cingulate cortex activation and personality demonstrated that greater levels of harm avoidance were significantly associated with increased subgenual anterior cingulate cortex activity in healthy adolescents. This finding is consistent with our previously published hypothesis [3] that errors made during the easy trials may have been attributed to an internal source because of more available time for response outcome processing. In our earlier fMRI study [3], we hypothesized that participants may have attributed their failure on the easy trials to themselves, whereas for the more difficult inhibitory trials, they may have attributed their failure to the increased level of task difficulty. On the basis of this hypothesis, we suggested that errors made during the easy trials may have been implicitly processed as more emotionally negative and evocative by the participants. At the time of our earlier study, we wrote that we did not have any available data to support our hypothesis.
In this study, we used a standardized and widely published tool, the TCI, to measure the personality of each of our participants just before scanning. Three studies using the TCI that is relevant to our results are available. In one study, the authors examined personality changes associated with depression recovery. By using the TCI to assess the patients at different times, they found that a favorable outcome of depression was associated with a decrease in harm avoidance [8]. Another study looking at depressed patients receiving successful treatment with antidepressants found a reduction in harm avoidance [6]. To determine whether assessing personality using the TCI might allow the prediction of future mood changes, researchers followed healthy adults over 1 year. They discovered that baseline levels and changes in harm avoidance explained the majority of the variance in the change in depression at the 1-year evaluation [7].
Functional neuroimaging studies have demonstrated that increased blood flow in the subgenual cingulate area is associated with increased sadness in both healthy [1] and depressed participants [2], and that decreased blood flow to the subgenual cingulate is associated with recovery from depression [2]. In addition, a common cognitive distortion of depressed patients is to attribute the cause of failure to themselves rather than to an external source even when they are clearly not to blame [19]. Taken together, the results of the published TCI, functional neuroimaging, and depression studies are consistent with both our current finding of a significant correlation between harm avoidance and subgenual anterior cingu-late cortex activation and our previously published study [3]. A limitation of this study was the inclusion of only adolescents. Future studies should include both adolescents and adults to compare the two groups for possible differences in brain activation patterns during the processing of social and emotional signals.
Conclusion
By using a fast, event-related, parametric, individualized stop task design to examine how inhibition-associated subgenual anterior cingulate cortex activity might relate to personality in healthy adolescents, we demonstrated, for the first time, that increased activation of the subgenual anterior cingulate cortex occurred as the inhibitory level of difficulty decreased and that higher levels of harm avoidance were associated with significantly increased subgenual anterior cingulate cortex activity in healthy adolescents. These results establish a link between personality and differential subgenual anterior cingulate cortex activation in normal adolescents for the first time.
Acknowledgements
The authors acknowledge the invaluable help of Dr. Rebecca Theilmann, Dr. Scott Roesch, Dr. Don Slymen, Kevin Hahn, and Sarah Jurick. This work was supported by grants from NIMH (K23MH70791) and NARSAD Foundation to TTY.
References
- 1.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 3.Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. NeuroReport. 2005;16:755–760. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- 4.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 5.Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 6.Chien AJ, Dunner DL. The Tridimensional Personality Questionnaire in depression: state versus trait issues. J Psychiatr Res. 1996;30:21–27. doi: 10.1016/0022-3956(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Cloninger CR, Svrakic DM, Przybeck TR. Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. J Affect Disord. 2006;92:35–44. doi: 10.1016/j.jad.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Corruble E, Duret C, Pelissolo A, Falissard B, Guelfi JD. Early and delayed personality changes associated with depression recovery? A one-year follow-up study. Psychiatry Res. 2002;109:17–25. doi: 10.1016/s0165-1781(01)00366-3. [DOI] [PubMed] [Google Scholar]
- 9.Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Brain Res Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lucas C, Zhang H, Mroczek D. The DISC predictive scales: efficiently predicting DISC diagnoses; 44th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Toronto. 1997. [DOI] [PubMed] [Google Scholar]
- 12.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 13.Cloninger C, Przybeck T, Svarkic D, Wetzel R. The temperament and character inventory (TCI): A guide to its development and use. Center for Psychobiology of Personality, Washington University; St. Louis, MO: 1994. [Google Scholar]
- 14.Cox RW. Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 15.Yang TT, Simmons AN, Matthews SC, Tapert SF, Bischoff-Grethe A, Frank GK, et al. Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci Lett. 2007;428:109–114. doi: 10.1016/j.neulet.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey LH, et al. Automated Talairach atlas for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 18.Norusis MJ. SPSS Base System User's Guide. SPSS Inc.; Chicago: 1990. [Google Scholar]
- 19.Kuiper NA. Depression and causal attributions for success and failure. J Pers Soc Psychol. 1978;36:236–246. doi: 10.1037//0022-3514.36.3.236. [DOI] [PubMed] [Google Scholar]