Abstract
Inhibitory mechanisms are critically involved in goal-directed behaviors. To gain further insight into how such mechanisms shape motor representations during response preparation, motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) and H-reflexes were recorded from left hand muscles during choice reaction time tasks. The imperative signal, which indicated the required response, was always preceded by a preparatory cue. During the postcue delay period, left MEPs were suppressed when the left hand had been cued for the forthcoming response, suggestive of a form of inhibition specifically directed at selected response representations. H-reflexes were also suppressed on these trials, indicating that the effects of this inhibition extend to spinal circuits. In addition, left MEPs were suppressed when the right hand was cued, but only when left hand movements were a possible response option before the onset of the cue. Notably, left hand H-reflexes were not modulated on these trials, consistent with a cortical locus of inhibition that lowers the activation of task-relevant, but nonselected responses. These results suggest the concurrent operation of two inhibitory mechanisms during response preparation: one decreases the activation of selected responses at the spinal level, helping to control when selected movements should be initiated by preventing their premature release; a second, upstream mechanism helps to determine what response to make during a competitive selection process.
Introduction
Inhibition is viewed as an important control process, helping to support goal-oriented behavior (Aron, 2007; Chambers et al., 2009; Zanto and Gazzaley, 2009). The aim of the present study was to explore how inhibition may contribute to response selection and preparation.
A large set of neurophysiological studies in humans have shown suppression of selected movement representations during response preparation (Hasbroucq et al., 1997, 1999; Davranche et al., 2007; van Elswijk et al., 2007). This inhibition has been termed “impulse-control” (Duque and Ivry, 2009), reflecting the hypothesis that it helps to prevent actions from being emitted prematurely. This effect is especially pronounced when the task requires withholding a selected action until the onset of an imperative signal (Boulinguez et al., 2008). As such, this form of inhibition helps to control when a selected response is executed. While the neural generators of such signals remain unclear, the effects of this form of inhibition are manifest at the spinal level (Touge et al., 1998; Prut and Fetz, 1999).
Other lines of evidence suggest that inhibitory mechanisms might also contribute to response selection (Burle et al., 2004; Ridderinkhof et al., 2004). Models of decision making generally postulate a competitive process in which multiple actions are activated in parallel. In many variants of such models, the accumulation of evidence for each potential response is accompanied by inhibitory interactions between their representations, helping to ensure that inappropriate responses fail to reach the selection threshold (Coles et al., 1985; Usher and McClelland, 2004) (but see also Brown and Heathcote, 2005). As such, this inhibition for “competition-resolution” would help to specify what response is required in a given context. These models are supported by behavioral observations (Wijnen and Ridderinkhof, 2007), neural recordings in nonhuman primates (Munoz and Everling, 2004), and physiological studies in humans (Goghari and MacDonald, 2009). Importantly, this form of inhibition is thought to be limited to cortical representations (Duque et al., 2005), although this hypothesis has not been directly investigated.
In the present study, we tested the hypothesis that these two forms of inhibitory control operate concurrently during motor preparation. We measured motor evoked potentials (MEPs) and H-reflexes in a left hand muscle during choice reaction-time tasks. An imperative signal was preceded by a preparatory cue that provided varying degrees of information regarding the forthcoming response. With this procedure, we tested three predictions. First, based on the “impulse-control” hypothesis, MEPs and H-reflexes should be suppressed following the preparatory cue when the probed muscle is a selected respondent, preventing movement initiation until the onset of the imperative signal. Second, MEPs, but not H-reflexes, should be suppressed in a nonselected respondent, reflecting a cortical locus of inhibition related to “competition-resolution.” Third, MEPs should remain unchanged when the probed muscle is task irrelevant; indeed, in this condition, responses involving this muscle do not take part in a competitive process, nor do they require suppression to prevent premature initiation.
Materials and Methods
Participants
A total of 30 right-handed healthy subjects (20 women, 10 men) participated in one of three experiments, except for one subject who participated in both experiments 2 and 3. Handedness was determined via a condensed version of the Edinburgh Handedness Inventory (Oldfield, 1971). Participants were naive to the purpose of the study and financially compensated for their participation; all gave written informed consent. The protocol was approved by the institutional review boards of the University of California, Berkeley and the Université catholique de Louvain, Brussels.
Stimulation procedures
Corticospinal (CS) excitability was assessed by measuring MEPs recorded from the left first dorsal interosseous (FDI, Exps. 1 and 2) or left flexor carpi radialis (FCR, Exp. 3) in response to single pulse TMS applied over the right primary motor cortex (M1). We focused on left hand muscles because CS suppression during movement preparation is generally more pronounced in the nondominant hand (Leocani et al., 2000; Duque et al., 2007). A figure-of-eight coil (diameter of wings 70 mm) connected to a Magstim 200 magnetic stimulator was placed tangentially on the scalp; the handle was oriented toward the back of the head and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus.
After fitting the participant with a tight electroencephalography (EEG) cap, we identified the optimal spot for eliciting MEPs in the left FDI (Exps. 1 and 2) or FCR (Exp. 3). This location was marked on the EEG cap to provide a reference point throughout the experimental session. The resting motor threshold (rMT) was defined as the minimal TMS intensity required to evoke MEPs of ∼50 μV peak-to-peak in the targeted muscle on 5 out of 10 consecutive trials. In experiment 1, the mean left FDI rMT corresponded to 60% (SE = 2.9) of maximum stimulator output (MSO). In experiment 2, it corresponded to 41% (SE = 2.2) of the MSO. The different rMT values were due to the use of different stimulators in the two studies, with the rapid Magstim used in experiment 1 generating a less powerful current (biphasic pulse) for a given output setting compared to the standard Magstim (monophasic pulse) used in experiment 2. In experiment 3, the rMT for eliciting MEPs with TMS in the left FCR corresponded to 45% (SE = 2.6) of the MSO (standard Magstim). The intensity of TMS for the experimental sessions was always 115% of rMT, set on an individual basis.
For the H-reflex measurements in experiment 3, the left median nerve was stimulated (0.5 ms rectangular pulses) through bipolar electrodes placed just above the elbow. The stimulus intensity was adjusted to a level that consistently elicited an H-reflex. M waves, induced by direct stimulation of the axons of the motoneurons, were continuously monitored to check for the stability of nerve stimulation.
EMG recording
EMG activity was recorded from surface electrodes placed over the left and right FDI and abductor digiti minimi (ADM) muscles (Exps. 1 and 2), or FCR muscle (Exp. 3). EMG data were collected for 3 s on each trial, starting at least 200 ms before the TMS pulse. The EMG signals were amplified and bandpass filtered on-line [UC Berkeley: 50–2000 Hz (Delsys); University of Louvain: 10–500 Hz (Neurolog; Digitimer)], and digitized at 2000 Hz for off-line analysis. The EMG signals were used to determine the reaction times (RTs) and to measure peak-to-peak amplitudes of the MEPs. Trials with background EMG activity >100 μV in the 200 ms window preceding the TMS pulse were excluded from the analysis. This was done to prevent contamination of the MEP measurements by significant fluctuations in background EMG (Duque et al., 2005, 2007; Duque and Ivry, 2009).
Classification of the functional role of targeted muscles
In the choice RT tasks described below, a muscle was considered as task relevant when it was part of the response set for a given block. Within this category, the muscle was a selected respondent when the preparatory cue or imperative signal indicated that the muscle would be the agonist for the forthcoming response. It was designated a nonselected respondent when the cue or imperative signal indicated that the muscle would not be used for the forthcoming response. When the cue did not specify the response, the muscle was considered as a potential respondent before the onset of the informative imperative signal. Finally, the muscle was task irrelevant when it was never included in the response set for the entire block of trials.
Experiment 1
Experimental procedure.
In experiment 1 (n = 13; 22 ± 0.9 years old), we used a set of choice RT tasks to examine CS excitability changes occurring during response preparation and movement initiation. Participants sat in front of a computer screen with both hands resting on a pillow, palms down, with the arms semiflexed. They had to make a speeded response with one of two fingers, either from the same hand (index or pinky abduction; unileft and uniright choice conditions) or from different hands (left or right index finger abduction; bilateral choice condition).
The three conditions (unileft, uniright, bilateral) were tested in separate blocks. The task was similar to that used in Duque and Ivry (2009) (see Fig. 1A). Briefly, participants were informed that they would play a virtual “soccer game” in which the required response was indicated by the position of a “ball” on the computer screen. The instructions emphasized that the participant should imagine shooting the ball into the appropriate goal. In the unileft and uniright conditions, the participant was told to imagine that the designated hand (left or right) was positioned in the middle of the screen and the goals appeared on the sides of the screen (two brackets opening toward the center). In the bilateral condition, the left and right hands were imagined to be positioned on the left and right sides of the screen and the goals appeared at the center of the screen (two brackets opening to the sides).
Figure 1.
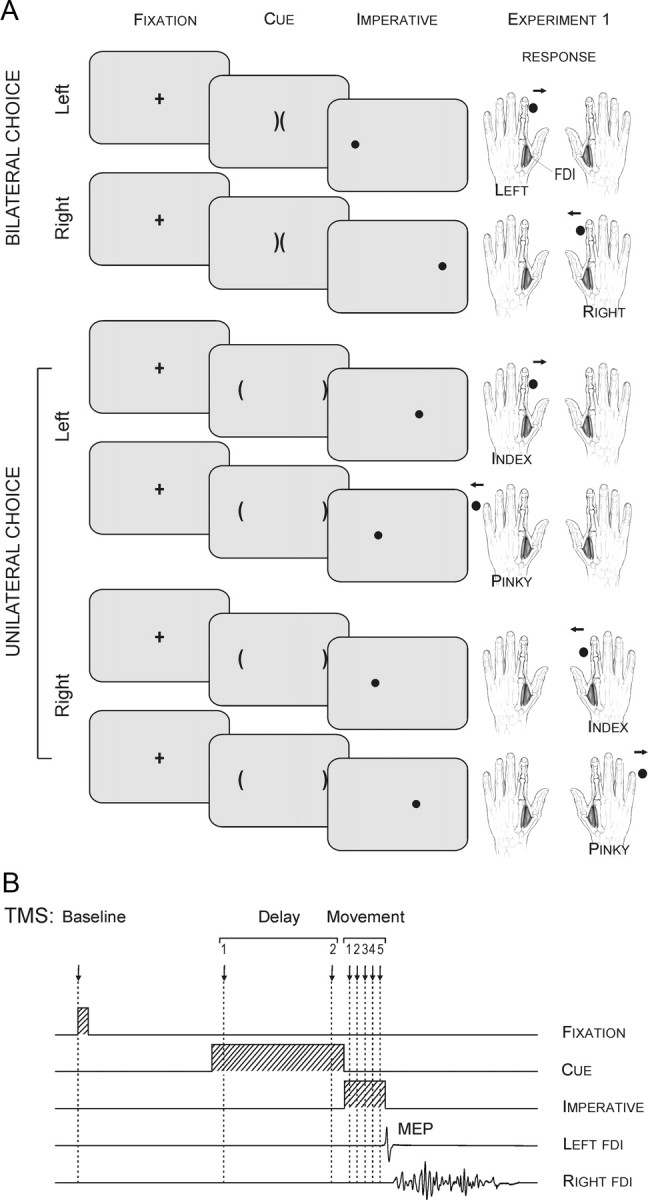
A, The preparatory cue consisted of two brackets, the “soccer goals.” The imperative, a circle or “soccer ball,” indicated the required response in all tasks. Participants were asked to shoot the ball in the appropriate goal by making an abduction movement of the corresponding index finger or pinky. Note that in the unileft and uniright tasks, subjects were asked to imagine they had the left or right hand, respectively, in the middle of the screen; in the bilateral task, the hands were imagined on the sides of the screen. B, Sequence and timing of events in experiment 1: A fixation marker (100 ms) was followed 900 ms later by a preparatory cue that lasted for a fixed delay (900 ms). An imperative signal then appeared (300 ms), indicating that the response should be initiated. A single TMS pulse was applied over the right M1 at eight possible timings during one of three epochs (baseline, delay, movement).
Each trial began with the brief presentation (100 ms) of a fixation marker (cross) at the center of the screen. After a blank screen of 900 ms, a preparatory cue was presented. This cue consisted of two brackets oriented toward the center of the screen in the unilateral conditions and two adjacent central brackets oriented toward the sides of the screen in the bilateral condition. This preparatory cue was always uninformative and remained visible for a fixed interval of 900 ms. It was then replaced by an imperative signal that remained visible for 300 ms. The imperative signal was a filled circle (the “ball”), positioned on the left or right side of the display. The participant was instructed to respond as quickly as possible following the imperative signal. Because of this emphasis, we assume that the participants engaged in some level of preparation of all potential movements for that trial following the onset of the uninformative cue.
Following a short familiarization period with the required movements in the three choice conditions, the main phase of the experiment began. The order of the three conditions was counterbalanced across participants. For each condition, there were four blocks, an initial block without TMS and three blocks with TMS. The no-TMS block consisted of 40 trials (20 per response) and was included to determine the participant's mean RT in the absence of TMS. RT was defined as the time interval between the onset of the imperative signal and a movement-related increase in EMG activity of the FDI (index abductor) or the ADM (pinky abductor). The three TMS blocks consisted of 84 trials each (42/response) and lasted ∼6 min.
Only one TMS pulse was applied in each trial, with eight possible timings (see Fig. 1B). To establish a “baseline,” TMS was applied at the onset of the fixation cross (24 MEPs for each condition). For two of the timings, the TMS pulse was applied during the “delay period,” either 100 (TMSd-early) or 800 ms (TMSd-late) after the onset of the uninformative cue (24 MEPs for each time point in each choice condition). Note that although the present experiment was designed in a way that would enable us to capture inhibitory changes during the delay period (Hasbroucq et al., 1997), it is important to keep in mind that facilitatory effects also occur in parallel during this period (Mars et al., 2007; van den Hurk et al., 2007), consistent with the view that both excitatory and inhibitory mechanisms operate during such a phase of advanced motor preparation. For the five remaining time points, the TMS pulse was applied after the onset of the imperative signal (50, 100, 150, 200, or 250 ms), or what we refer to as the “movement period.” There were 18 trials for each TMS timing condition in each of the three choice conditions. To analyze changes specifically related to response selection, we performed a post hoc analysis in which we pooled trials in which the TMS pulse occurred between 120 ms and 20 ms before EMG onset (TMSselection). To analyze changes occurring during movement execution, we pooled trials in which the TMS pulse occurred between 20 ms before EMG onset and 150 ms following EMG onset (TMSexecution).
Statistical analysis.
Given that TMS may have both specific and nonspecific effects on RT (Davare et al., 2007; Duque and Ivry, 2009), we only used trials from the no-TMS blocks in the RT analysis. We further limited the RT analysis to index finger responses since this allowed for a comparison of identical movements in the unilateral and bilateral conditions. RT effects were analyzed with a two-way repeated-measure ANOVA (ANOVARM) with the factors condition (bilateral, unilateral) and hand (left, right).
To improve the normality of MEP amplitude distribution across the different TMS timings, a logarithmic transformation of the MEP data was performed before the statistical tests. To examine the overall dynamics of CS excitability, the transformed left FDI MEP data were analyzed by means of a one-way ANOVARM for each of the three choice conditions (factor TMS timing: TMSbaseline, TMSd-early, TMSd-late, TMSselect_1, TMSselect_2, TMSexecut_1, TMSexecut_2). The numbers “1” and “2” refer to the two potential responses in each condition. These correspond to the left and right index finger responses in the bilateral condition, respectively, and to the index finger and pinky responses in the unilateral conditions. Post hoc comparisons were conducted using the Fisher's least significant difference (LSD) procedure. All of the data are expressed as mean ± SE.
Experiment 2
In experiment 1, the preparatory cue was always uninformative and the responding hand was defined in advance for each unimanual condition. Given this design, left FDI was always either task relevant (unileft and bilateral) or irrelevant (uniright) for the entire block of trials. To investigate a possible dynamic interaction between inhibitory mechanisms during response preparation, we performed a second experiment (n = 7; 25 ± 1.3 years old) in which we included conditions with partially informative cues. For these conditions, the preparatory cue indicated the response hand (left or right) for the forthcoming response, but the actual response (index or pinky) was only specified by the imperative signal (Fig. 2A). In the “fixed” conditions, a block was composed of either left or right hand trials only, similar to the unilateral blocks of experiment 1. As a consequence, the preparatory cue was invariant in these blocks. In the “flexible” condition, the cue specified the selected hand for the forthcoming trial, and by inference, the nonselected hand.
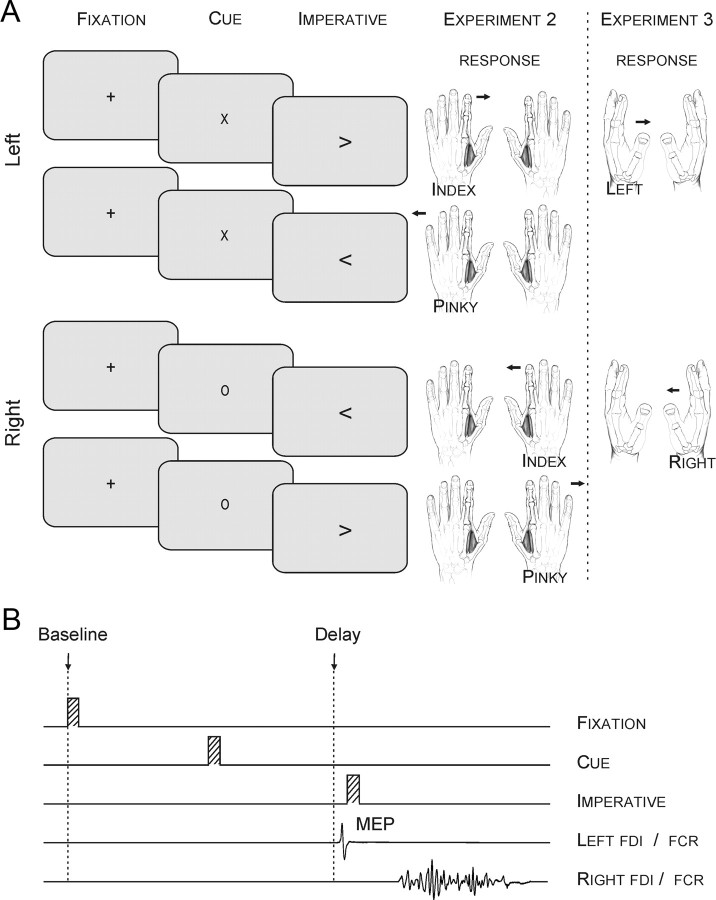
Figure 2.
A, The preparatory cue consisted of a letter: “x” indicated a forthcoming left hand movement and “o” indicated a right hand movement. The imperative signal was an arrow presented at the screen center. B, Sequence and timing of events in experiments 2 and 3: A fixation marker (100 ms) was followed 900 ms later, by a preparatory cue that lasted for a fixed delay (100 ms). An imperative signal indicating the required response appeared after a delay (Exp. 2: 900 ms; Exp. 3: 900–1200 ms). A single TMS or electrical pulse was applied over the right M1 or the left median nerve, respectively, during one of two epochs (baseline, delay). In this example, a TMS pulse was applied during the delay period of a right index finger abduction (Exp. 2) or wrist flexion (Exp. 3) movement.
This procedure provided two key comparisons. First, we can compare left FDI MEPs during the delay period between conditions in which this muscle remains a potential respondent (fixleft and flexleft) to conditions in which the cue has eliminated it as a potential respondent (fixright and flexright). Second, we can compare trials in which left FDI was task relevant, but has been dynamically excluded from the response set by the cue (flexright) to trials in which it was task irrelevant for the entire block of trials (fixright).
Experimental procedure.
Each trial began with the brief presentation (100 ms) of a fixation cross at the screen center (Fig. 2A). After a blank screen of 900 ms, a preparatory cue was presented for 100 ms. The cue was either the letter “x,” indicating a forthcoming left hand movement, or the letter “o,” indicating a forthcoming right hand movement. After a fixed delay of 900 ms, an imperative signal appeared for 100 ms (< or >), indicating if the response should be made with the index or pinky of the cued hand. The participant was instructed to perform the specified finger movement as quickly as possible following the imperative signal. The preparatory cue was always valid and participants were instructed to use this information to reduce their RTs. Note that in the fixed condition, the same cue was presented on every trial (e.g., “x” if left hand block).
Each subject performed one fixed block for each hand (fixleft and fixright, 66 trials each) and two flexible blocks (56 trials each). Because we did not observe increases in inhibition following the imperative signal in experiment 1 (see also (Duque and Ivry, 2009), we focused on excitability changes during the delay period (Fig. 2B). TMS pulses were applied at baseline (fixation onset) or at TMSdelay, 800 ms after the cue. There were 30 trials for each of the two timings in the fixed blocks and 32 trials for each of the two timings in the flexible blocks. No-TMS trials were also included in each block (6 and 8 in the fixed and flexible blocks, respectively).
Statistical analysis.
For the RT analysis, we limited the dataset to those trials in which the TMS pulse was coincident with the fixation onset (TMSbaseline). RTs were analyzed by means of a two-way ANOVARM with the factors hand (left, right) and condition (fixed, flexible). The data from the index finger and pinky movements were pooled together to increase the number of observations per condition.
A similar two-way ANOVARM was used to examine CS excitability changes during the delay period. For this analysis, we expressed left FDI MEP amplitudes at TMSdelay with respect to MEPs at TMSbaseline. In addition, we performed a secondary analysis with the data from the flexible condition to look at sequential effects, assessing whether left FDI MEPs were influenced by whether or not that muscle had been used in the preceding trial. For this analysis, we used a three-way ANOVARM with the factors current condition (flexbaseline, flexleft, flexright), preceding hand (left, right), and preceding finger (index, pinky). We restricted this analysis to the flexible condition given that the same hand was always used in the fixed condition. Post hoc comparisons were conducted using the Fisher's LSD procedure. All of the data are expressed as mean ± SE.
Experiment 3
A third experiment (n = 11; 29 ± 2.3 years old) was performed to determine changes in spinal excitability during response preparation. To this end, we measured MEPs and H-reflexes at baseline and at the end of the delay period (∼TMSdelay). The H-reflex is a physiological response primarily mediated by a monosynaptic pathway. This response has been used as a probe of spinal cord excitability during a variety of motor tasks (Pierrot-Deseilligny and Mazevet, 2000). One limitation with this measure is that it is difficult to obtain consistent H-reflexes from intrinsic hand muscles (Mazzocchio et al., 1995). Thus, participants were required to perform wrist movements rather than finger movements in experiment 3. We measured H-reflexes in the left FCR, a wrist flexor muscle.
Experimental procedure.
The participants sat in front of a computer screen with the arms semiflexed and the forearms resting on a pillow with the thumbs positioned upward. The basic procedure was identical to that used in experiment 2 (see Fig. 2A,B). The one exception was that the delay between the preparatory cue and imperative signal varied between 900 and 1200 ms. Following the imperative signal, the participant made a wrist flexion response with either the left or right hand.
Since there is only one response associated with each hand in experiment 3 (i.e., wrist flexion), the cue allowed the participant to fully prepare the forthcoming response. Thus, unlike experiments 1 and 2, response selection could be completed before the imperative signal. This procedure allowed us to assess CS suppression in a muscle that was either a selected respondent (left cue) or a nonselected respondent (right cue) following a fully informative cue. Two procedural changes were introduced to minimize anticipatory responses. First, as described above, the duration of the delay period was variable. Second, we included catch trials in which the preparatory cue was not followed by an imperative signal. On these trials, the participants were instructed to not respond.
Each participant performed two TMS blocks followed by two H-reflex blocks, with 36 trials per block. Each block included 18 left and 18 right cues, selected in a random order. Four of the trials were catch trials. Stimulation (TMS or electrical) was applied on each trial, resulting in a total of 24 stimulation trials at baseline (fixation onset) and 24 stimulation trials at TMSdelay (800 ms after the cue) for both left and right hand trials. Note that stimulation was also given on catch trials.
Statistical analysis.
To examine changes in CS and spinal excitability, the amplitude of left FCR MEPs and left FCR H-reflexes were measured at baseline and during the delay period. A logarithmic transformation was first applied to the data. Then, the transformed MEPs and H-reflexes were analyzed by means of separate one-way ANOVARM with the factor TMS condition (baseline, delayleft, delayright). Given our previous results showing an MEP reduction in the selected hand following an informative cue (Duque and Ivry, 2009), one-tailed paired t tests were used for post hoc comparisons of left FCR MEPs. The Fisher's LSD procedure was used for post hoc comparisons of the left FCR H-reflexes. All of the data are expressed as mean ± SE.
Results
Experiment 1
Reaction time
The mean RT for left index finger responses measured in the no-TMS blocks was 184 ms (SE = 13.9, n = 13) and 191 ms (SE = 7.3) in the bilateral and unilateral choice conditions, respectively. The mean RT for right index finger responses was 187 ms (SE = 13.1) and 210 ms (SE = 8.2) in the corresponding conditions. Left index finger responses were faster than right index finger responses (F = 9.0, p = 0.01). The difference between the two choice conditions was not significant (F = 1.6, p = 0.22).
These visual RTs are quite fast. Moreover, when compared to previous studies involving partial cueing, the mean values are similar to conditions in which participants can prepare a subset of the responses in advance of an imperative signal (Goodman and Kelso, 1980; Rosenbaum, 1980). As such, although we did not include a condition in which there was no preparatory signal, we infer that the participants followed the instructions and used the preparatory cues to minimize their RTs. Note that using a similar task we included a condition in which the imperative signal was presented without a preparatory cue. Under this condition, RTs were ∼100 ms longer than those observed in the current study (unpublished observations).
CS excitability
During the “baseline period,” the mean amplitude of the left FDI MEPs was 1.26 mV (SE = 0.16, n = 13), 1.39 mV (SE = 0.16), and 1.25 mV (SE = 0.15) for the bilateral, unileft, and uniright conditions, respectively. These baseline MEP amplitudes were not significantly different from one another (F < 1).
In the bilateral choice condition (Fig. 3A), CS excitability of left FDI was modulated over the course of the trial (factor TMS timing: F = 26.6, p < 0.0001). Following the imperative signal, this modulation was influenced by whether the response was made with the left or right index finger. MEPs evoked just after the uninformative cue (TMSd-early) were unchanged with respect to baseline. However, the MEPs were strongly inhibited by the end of the delay period (TMSd-late; 36% reduction, post hoc test p = 0.004). Following an imperative signal indicating a left index finger movement, an increase in MEP amplitude was observed in left FDI, the agonist for the response (TMSselection and TMSexecution; both p < 0.001). When the imperative signal indicated a right hand response, left FDI MEP amplitudes were unchanged during the selection phase, remaining below baseline. Left FDI MEPs increased when the right index finger movement was initiated (TMSexecution, p < 0.001). The overall pattern of results from this bilateral condition is consistent with the “impulse-control” hypothesis (Duque and Ivry, 2009). Left FDI MEPs showed marked inhibition before the imperative signal, a time when the left index finger was always a potential respondent.
Figure 3.
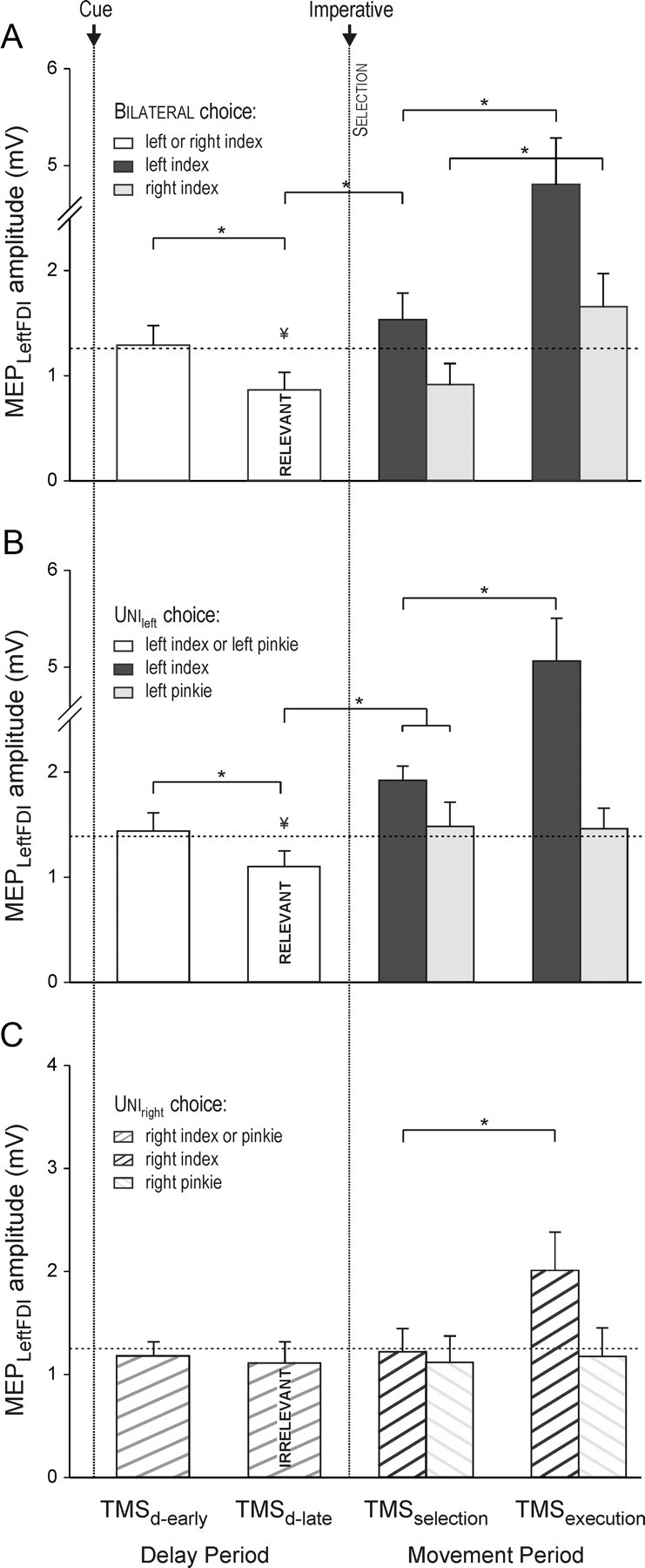
Amplitude (in millivolts) of MEPs recorded from left FDI following right M1 TMS in the bilateral (A), unileft (B), and uniright (C) tasks in experiment 1. MEP amplitudes are shown for the delay (following cue) and movement (following imperative) periods. The horizontal dashed line represents the baseline MEP amplitude. Left FDI MEP amplitudes were lower at the end of the delay period (TMSd-late) when the index finger was a potential respondent (bilateral and unileft) but not when it was task irrelevant (uniright). MEP amplitudes increased when the imperative signaled a left index finger response (TMSselection and TMSexecution). *p < 0.05. ¥Significant difference between MEPs at TMSd-late and TMSbaseline.
Our main interest in this experiment was the two unilateral conditions (Fig. 3B,C), where we compared modulation of left FDI MEPs in choice conditions when this muscle was a potential respondent (task relevant: unileft) (Fig. 3B) or was never used in the block (task irrelevant: uniright) (Fig. 3C). In the former, the pattern was very similar to that observed in the bilateral condition (factor TMS timing: F = 21.4, p < 0.0001) (see Fig. 3B). Left FDI MEPs were suppressed at the end of the delay period (22% decrease; p = 0.02) and rose over the movement period when the imperative stimulus signaled a left index finger response (TMSselection, p < 0.0001; TMSexecution, p < 0.0001). When the imperative stimulus signaled a left pinky response, left FDI MEPs also increased (TMSselection, p = 0.01), but this increase was less pronounced compared to when FDI was the agonist (p = 0.05). There was no additional increase in left FDI MEPs during the execution of a pinky movement (TMSexecution, p = 0.5).
In the uniright condition (Fig. 3C), there was also a significant effect of factor TMS timing on left FDI MEPs (F = 6.0, p < 0.0001). However, this effect was restricted to a large increase in MEP amplitude between the TMSselection and TMSexecution epochs, and only when the right FDI was the agonist for the response (p < 0.0001). Notably, left FDI MEPs were not reliably suppressed at the end of the delay period (TMSd-late, p = 0.3).
In summary, the results of experiment 1 demonstrate the specificity of inhibitory control mechanisms during response preparation and selection. MEPs were inhibited when the targeted muscle was a potential respondent for the forthcoming movement, even when the exact response remained to be specified. We assume this inhibition reflects an “impulse-control” mechanism, one that targets all viable response possibilities during the delay period. This form of control is likely necessary in the current study given that our instructions emphasized fast responses. Notably, MEP suppression in left FDI was not enhanced when the imperative signal indicated that the response for that trial would not involve this muscle. Inhibition was also absent during response preparation and selection when the targeted muscle was task irrelevant. In this condition, a facilitatory effect was observed in left FDI around the time the participant began to execute a right index finger movement. This could reflect the default activation of homologous muscles during response execution (Swinnen, 2002; Carson et al., 2008).
Experiment 2
In the unilateral conditions of experiment 1, the involvement of a given hand was fixed for the entire block of trials. Under these conditions, no change was observed in left FDI following a noninformative preparatory cue in right hand blocks where the muscle was task irrelevant. In experiment 2, we examined whether the absence of inhibition would also be observed in right hand trials when hand involvement is defined by a preparatory cue that varies in a dynamic manner from trial to trial.
To address this issue, we compared performance between two types of choice conditions. In the fixed condition, the task-relevant hand was the same for an entire block of trials. The other hand was task irrelevant. In the flexible condition, both hands were task relevant in a given block of trials but a partially informative cue indicated the selected hand for the current trial. By inference, the cue also indicated which hand was nonselected. In all conditions, the onset of the imperative signal indicated the finger to be used for the movement on that trial.
Reaction time
RTs were computed from trials in which the TMS pulse was coincident with the fixation onset and averaged for the two responding fingers of each hand (i.e., index and pinky). The mean RT for left hand movements was 329 ms (SE = 26.1, n = 7) and 339 ms (SE = 32.0) in the fixleft and flexleft tasks, respectively. The mean RT for right hand movements was 333 ms (SE = 34.3) and 312 ms (SE = 14.2) in the fixright and flexright conditions, respectively. There was no difference between these values (no effect of hand or condition). The longer RTs in this experiment compared to experiment 1 are likely due to differences in the format of the imperative signals.
CS excitability
During the baseline period, left FDI MEPs averaged 1.4 mV (SE = 0.58, n = 7) and 1.1 mV (SE = 0.48) in the fixleft and fixright blocks, respectively, and 1.3 mV (SE = 0.60) in the flexible blocks. There was no difference between these values.
We examined the modulation of CS excitability following the cue by calculating the percentage change in the MEPs elicited late in the delay period (TMSdelay) as a function of the baseline MEPs. The main effect of hand was significant (F = 13.5, p = 0.01) as was the hand × condition interaction (F = 10.9, p = 0.02) (Fig. 4). The results from the fixed conditions replicate those observed in experiment 1: left FDI MEPs were significantly inhibited when the left index finger was a potential respondent (task relevant), but not when this finger was task irrelevant (fixleft vs fixright task, p = 0.003).
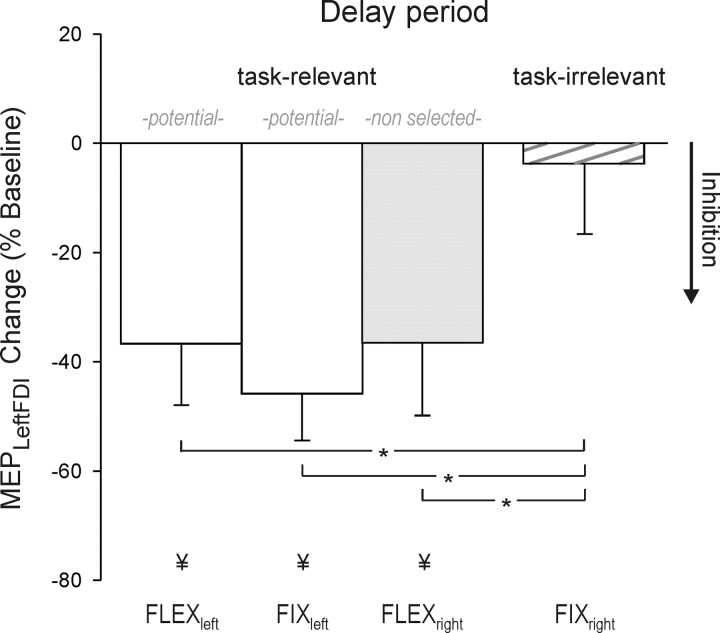
Figure 4.
Percentage change, relative to baseline, in the amplitude of left FDI MEPs in experiment 2, recorded just before the imperative signal (TMSdelay). The “fixed” condition defines either left or right hand trials only. The “flexible” condition mixes left and right hand trials. Left FDI MEP suppression was evident when the left index finger was a potential respondent (fixleft, flexleft) and after a right hand cue in the flexible condition (flexright).
In contrast, this difference was not apparent during the flexible blocks. Here, suppression of left FDI MEPs was similar in trials in which the cue specified a left or right hand response (p = 0.98). The differential effect of the fixed and flexible conditions was highlighted in an analysis limited to right hand trials. Suppression of left FDI MEPsdelay was stronger when the left hand was task relevant but not selected (flexright) compared to when the hand was irrelevant for the entire block of trials (fixright; p = 0.01) (see Fig. 4). These results are consistent with the hypothesis that inhibitory mechanisms target task-relevant but not task-irrelevant response representations.
Thus, inhibition of left FDI was not only found following a partially informative cue indicating that this muscle might be selected, but it was also observed when the cue indicated that the left FDI would not be selected (flexright). It is possible that the difference here is another manifestation of “impulse-control” inhibition. Inhibition might be targeted at a task-relevant muscle even when it is nonselected. Alternatively, it might arise because of carry-over effects across trials in the flexible condition. Given that the left hand is relevant on half of the trials, a simple control mechanism might target both hands or be biased to act in a similar manner from trial to trial.
An alternative hypothesis is that MEP suppression of the nonselected muscle reflects a second form of inhibition, one which is specifically directed at nonselected response representations. By this view, inhibition of left FDI would be reflective of a “competition-resolution” mechanism, one that would help reduce the activation associated with what had been a potential respondent before the onset of the preparatory cue. This mechanism would help ensure that only right hand representations are strongly activated at the onset of the imperative signal.
To evaluate these hypotheses, we performed a secondary analysis of sequential effects across trials in the flexible condition. Consistent with the analysis above, the factor current condition was significant (F = 4.0, p < 0.05), confirming the suppression of left FDI MEPs in flexleft and flexright trials with respect to baseline (both p < 0.04). Most interesting, this analysis revealed a significant main effect of factor preceding hand on left FDI MEPs (F = 11.6, p < 0.02) in the absence of any interaction with current condition or preceding finger (both F<2.5, p > 0.1). Left MEPs were smaller following right (0.69 mV on average) than left hand (0.80 mV on average; p < 0.02) responses. The absence of an interaction and the fact that left hand suppression was attenuated when the left hand was cued on successive trials are at odds with the hypothesis of an “impulse-control” carry-over effect. Rather, the sequential effect is more consistent with the hypothesis that inhibition directed at nonselected responses is part of a “competition-resolution” process. However, it is important to note here that this argument is based on a null result. We turn to a more direct test in the next experiment.
Experiment 3
To distinguish further between two possible sources of inhibition in selected and nonselected response representations, we measured H-reflexes in a third experiment. Previous physiological studies in primates have shown that inhibition of a planned movement in a delayed response task is evident in the activity of spinal interneurons (Prut and Fetz, 1999), indicating parallel and early processing of critical motor control strategies at spinal level. In contrast, inhibition of nonselected responses, presumably related to “competition-resolution,” has been attributed to cortical interactions (Munoz and Everling, 2004). Here we examine whether a similar dissociation between these two forms of inhibition is observed in humans.
Participants performed wrist flexion movements. In this experiment, the imperative signal was preceded by a fully informative cue, with left FCR MEPs and H-reflexes measured at baseline and during the delay period (Fig. 5A). During the baseline period, left FCR MEPs were smaller than those typically obtained in intrinsic hand muscles with a mean value of 0.5 mV (SE = 0.07, n = 11) when evoked at 115% of rMT. Relative to baseline, the MEPs were suppressed following cues indicating either a left (33% suppression; one-tailed t = 4.0, p < 0.002 with respect to baseline) or right (24% suppression, one-tailed t = 3.6, p < 0.003) hand response (factor TMS condition: F = 13.6, p < 0.001) (Fig. 5A). This suppression was more pronounced following a left hand cue compared to a right hand cue (one-tailed t = 2.5, p < 0.02), similar to what we observed in a previous study in which we measured MEPs in intrinsic hand muscles (Duque and Ivry, 2009).
Figure 5.
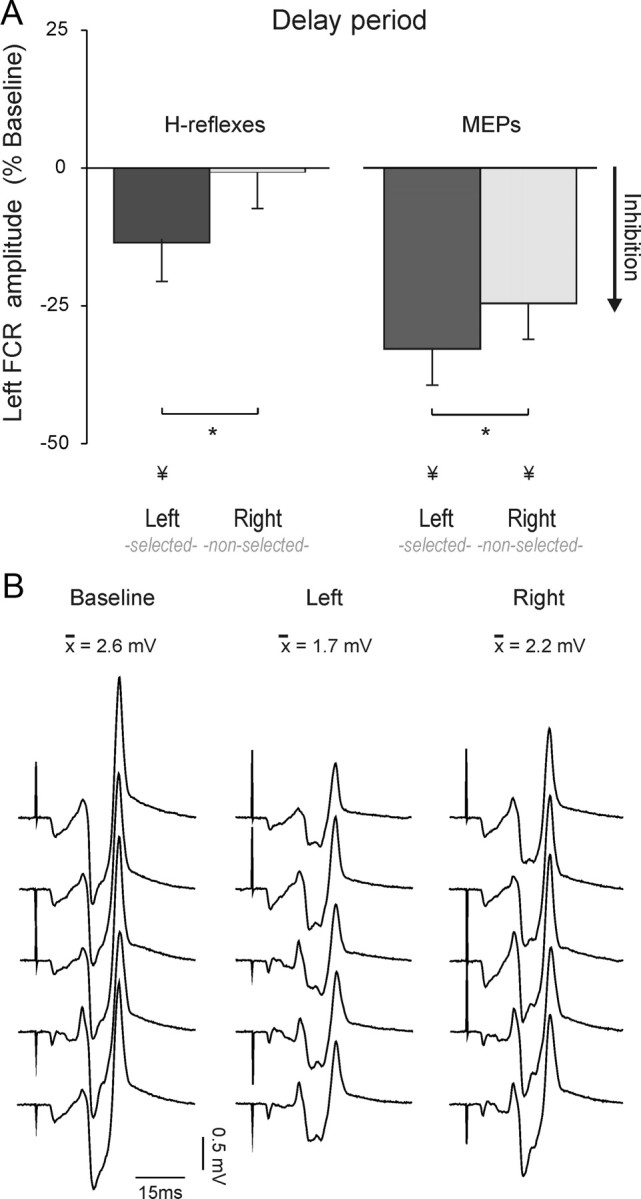
A, Percentage change in H-reflex (left histograms, n = 11) or MEP (right histograms, n = 11) amplitude recorded from the left FCR (flexor carpi radialis) at the end of the delay period. Data are expressed as a function of baseline values. *p < 0.05. ¥Significant difference with respect to baseline. B, Individual H-reflexes recorded from the left FCR in experiment 3 from a representative subject during the baseline epoch or delay period following a left or right hand cue.
During the baseline period, left FCR H-reflexes averaged 1.5 mV (SE = 0.4, n = 11). Figure 5B shows H-reflex traces in a representative subject. Similarly to what we observed for the MEPs, left H-reflex amplitudes were attenuated during the delay period (factor TMS condition: F = 3.6, p < 0.05) (Fig. 5A,B). Most striking, this effect was only observed after a left hand cue (p = 0.02) and not after a right hand cue (p = 0.7). When examined on an individual basis, two subjects showed an increase in H-reflexes at the end of the delay period of left hand trials. However, even in these two subjects, the H-reflex amplitude was smaller in left FCR when the cue indicated that this muscle was the agonist for the forthcoming response, compared to when the cue indicated that this muscle was excluded from the forthcoming response (p = 0.05).
Together, the results point to a dissociation between inhibitory effects as measured in MEPs and H-reflexes. MEPs were suppressed for both selected and nonselected muscles, with the effect more pronounced for selected muscles. In contrast, H-reflexes were only attenuated for selected muscles. This dissociation is consistent with the hypothesis that “impulse-control” inhibition targets selected muscle representations and is manifest at the spinal level. Suppression of nonselected responses is limited to changes in cortical excitability, presumably resulting from a separate form of inhibition related to “competition-resolution.”
Discussion
The present set of experiments explored the physiological changes that arise during a simple decision-making process in which subjects select and prepare manual responses. A variety of physiological signals, including population vectors (Cisek and Kalaska, 2005) and lateralized readiness potentials (Taylor et al., 2007), have been used to highlight the emergence of cortical representations of forthcoming actions. Less attention has been given to the role of inhibitory mechanisms in shaping the response topography, despite the abundance of evidence indicating a fundamental role of inhibition in cognitive control (Aron, 2007; Zanto and Gazzaley, 2009). By manipulating the degree of information conveyed by a preparatory cue, we examined the modulation of excitability in the CS pathways associated with both selected and nonselected responses. The results provide evidence for two distinct forms of inhibition, one associated with regulating when a selected response is initiated and a second helping to determine what response is selected.
Inhibition for “impulse-control” in selected respondents
Consistent with previous findings, we observed a strong inhibition of potential response representations before the onset of an imperative signal. In fact, left FDI MEPs were systematically suppressed during the delay period when the left index finger might be required for a speeded response. We had previously observed this form of inhibition following fully informative cues (Duque and Ivry, 2009). With such cues, this inhibition is much stronger in a selected muscle compared to when that muscle is not selected for the forthcoming action. This surprising result led us to term this form of inhibition, “impulse-control,” reflecting the idea that it prevents the premature initiation of a planned response. In the current study, we observed a similar effect when the cue was either uninformative (experiment 1) or partially informative (experiment 2), indicating the hand, but not the finger, for the forthcoming response. Moreover, left hand MEP suppression was larger following a left hand cue than a right hand cue when the forthcoming movement was fully specified by an informative cue (experiment 3).
These results are in agreement with the hypothesis that “impulse-control” processes target the representation of potential respondents for the forthcoming movement. According to this view, activation of potential respondents progressively increases following the onset of the cue given the emphasis on speeded responses. We hypothesize that when a response representation is recruited, an inhibitory command is coactivated, providing a safeguard against premature response initiation during the accrual process (see also Sinclair and Hammond, 2009). The risk of premature responses may be especially large before the imperative signal given that increasing preparatory activity is observed in multiple brain regions (Houk and Wise, 1995; Horwitz et al., 2000; Calton et al., 2002; Bastian et al., 2003; Hoshi and Tanji, 2006; Beurze et al., 2007). Indeed, using a paired-pulse TMS procedure, we have shown that the marked suppression of task-relevant MEPs observed during response preparation is accompanied by a cortical “signature” of increased activation for the selected response (Duque and Ivry, 2009). Other studies have also reported a parallel decrease in CS excitability during a delay period when M1 activity is increased (Davranche et al., 2007; Sinclair and Hammond, 2008).
The H-reflex measurements (Exp. 3) provide an important insight into the target of this form of inhibition. The amplitude of left FCR H-reflexes was smaller when participants prepared a left wrist response compared to when they prepared a right wrist response. Indeed, we did not observe any change in the H-reflex in the latter condition. Thus, the effects of “impulse-control” inhibition are manifest in the selective reduction of excitability at the spinal level for selected muscle representations (Touge et al., 1998; Hasbroucq et al., 1999). Single-unit recordings in monkeys have also revealed inhibition-related changes in the activity of spinal interneurons preceding an instructed movement (Prut and Fetz, 1999). This activity was postulated to reflect a “general braking mechanism.” However, our results challenge the notion of a generic inhibitory signal given that the modulation of the H-reflex was limited to the selected muscle.
While these findings suggest that the effects of “impulse-control” are also manifest at the spinal level, the source of these signals is unknown. One likely candidate is dorsal premotor cortex (Sawaguchi et al., 1996). This region is engaged during response preparation (Wise et al., 1992; Terao et al., 2007), exhibits robust delay-related activity (Cisek and Kalaska, 2005), and sends direct projections to the spinal cord (Dum and Strick, 1991). Dorsal premotor cortex, as part of its contribution to preparing a selected response, may send corollary inhibitory signals to spinal interneurons. Of course such signals might also arise from other cortical, or even subcortical, areas. However, the critical point we wish to emphasize here is that “impulse-control” is expressed at the spinal cord level, presumably allowing preparation to proceed without premature movement.
Inhibition of nonselected respondents: to facilitate “competition-resolution”?
Left hand MEPs were also suppressed during the delay period when the cue indicated that the forthcoming response would exclusively involve the right hand. Notably, this inhibition was only evident when the left index finger was task relevant. Left FDI MEPs were not suppressed when the left hand was not relevant for the entire block of trials. However, when the left index was a potential respondent at the start of the trial, suppression was observed even when the cue indicated that the forthcoming response would be limited to the right hand. Thus, inhibition is observed when a cue indicates that a task-relevant muscle should not be selected.
Models of choice RT tasks generally assume a parallel activation of all task-relevant responses with a competitive process determining the final selection (Cisek, 2007). As information accumulates, activation becomes stronger for responses that remain viable candidates. While some models posit the competition as a “race” between independent response alternatives (Brown and Heathcote, 2005), other models posit, at least implicitly, inhibitory processes between these alternatives (e.g., Ridderinkhof et al., 2005). That is, each candidate not only accrues supporting evidence, but also inhibits the other options (Coles et al., 1985). We propose that the inhibition observed in nonselected, but task-relevant, muscles reflects the operation of a control mechanism to facilitate “competition-resolution.”
Inhibition associated with “competition-resolution” may arise from lateral connections between the alternative response representations, or through top-down signals, possibly originating from the prefrontal cortex. Indeed prefrontal cortex has been shown to ensure the monitoring and implementation of cognitive control processes related to conflict resolution (MacDonald et al., 2000; Botvinick et al., 2001; Fassbender et al., 2009), a function consistent with the presumed role of an inhibitory component involved in “competition-resolution” (Bunge, 2004; Heekeren et al., 2006). The neural overlap of inhibitory mechanisms engaged during response preparation and those associated with other control operations such as inhibiting planned responses (Aron et al., 2004) is a topic for future investigation.
Can the effects we attribute to “competition-resolution” be subsumed by a broader application of the “impulse-control” mechanism? Perhaps the cue automatically triggers activation in all task-relevant respondents, and as proposed above, this process includes the recruitment of inhibitory mechanisms linked to each respondent. The recruitment of “impulse-control” might occur at a shorter time scale than that required to identify the cue and determine the response candidates for the forthcoming trial. While a single mechanism account is more parsimonious, the H-reflex results suggest that inhibition of selected and nonselected responses occurs at different levels of the neural axis. While the MEPs were suppressed for both selected and nonselected responses (although larger for selected), the modulation of the left H-reflex was only observed when the left wrist was selected. As such, the CS suppression observed for task-relevant but nonselected representations is not manifest at the spinal level. By exclusion, we propose that a second inhibitory source is restricted to modulation of cortical excitability, helping to resolve the outcome of a competitive selection process.
We also considered the hypothesis that MEP suppression in nonselected representations might reflect carry-over effects of “impulse-control” inhibition between trials. This idea would be consistent with the fact that we observed MEP suppression of nonselected representations in the flexible condition of experiment 2, even when the cue had indicated a right hand response, but not when the left hand was task irrelevant (fixed condition). However, a post hoc sequential analysis of MEP changes in the flexible condition failed to support this prediction. Indeed, the main sequential effect observed was a reduction in left MEPs at baseline (and during the delay period) on trials following a right hand response. Inhibition for “competition-resolution” may induce more sustained effects than inhibition related to “impulse-control.”
Conclusions
Our results suggest that CS suppression during response preparation reflects two concurrent inhibitory mechanisms. One mechanism, “impulse-control,” exerts a specific influence on selected respondents, resulting in inhibitory changes that are manifest at the spinal level. Such a mechanism helps to regulate when selected responses are initiated, ensuring that premature responses are not implemented as supraspinal response codes are activated. A second mechanism, “competition-resolution” helps to specify what response is required in a given context. This form of inhibition, observed in nonselected response representations, is not evident at the spinal level and is assumed to reflect cortical interactions.
Footnotes
This work was supported by the National Institute of Health (NS040813). J.D. was supported by the Belgian American Educational Foundation and Fulbright program; she is currently a postdoctoral research fellow at the Belgian National Funds for Scientific Research.
References
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schöner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 2003;18:2047–2058. doi: 10.1046/j.1460-9568.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol. 2007;97:188–199. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boulinguez P, Jaffard M, Granjon L, Benraiss A. Warning signals induce automatic EMG activations and proactive volitional inhibition: evidence from analysis of error distribution in simple RT. J Neurophysiol. 2008;99:1572–1578. doi: 10.1152/jn.01198.2007. [DOI] [PubMed] [Google Scholar]
- Brown S, Heathcote A. A ballistic model of choice response time. Psychol Rev. 2005;112:117–128. doi: 10.1037/0033-295X.112.1.117. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- Carson RG, Kennedy NC, Linden MA, Britton L. Muscle-specific variations in use-dependent crossed-facilitation of corticospinal pathways mediated by transcranial direct current (DC) stimulation. Neurosci Lett. 2008;441:153–157. doi: 10.1016/j.neulet.2008.06.041. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362:1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. J Exp Psychol Hum Percept Perform. 1985;11:529–553. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17:353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Prefrontal and midline interactions mediating behavioural control. Eur J Neurosci. 2009;29:181–187. doi: 10.1111/j.1460-9568.2008.06557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW., 3rd The neural basis of cognitive control: response selection and inhibition. Brain Cogn. 2009;71:72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Kelso JA. Are movements prepared in parts? Not under compatible (naturalized) conditions. J Exp Psychol Gen. 1980;109:475–495. doi: 10.1037//0096-3445.109.4.475. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci U S A. 2006;103:10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Deiber MP, Ibáñez V, Sadato N, Hallett M. Correlations between reaction time and cerebral blood flow during motor preparation. Neuroimage. 2000;12:434–441. doi: 10.1006/nimg.2000.0632. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mars RB, Bestmann S, Rothwell JC, Haggard P. Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res. 2007;182:125–129. doi: 10.1007/s00221-007-1055-4. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Rossi A. Distribution of Ia effects onto human hand muscle motoneurones as revealed using an H reflex technique. J Physiol. 1995;489:263–273. doi: 10.1113/jphysiol.1995.sp021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Scheres A, Oosterlaan J, Sergeant JA. Delta plots in the study of individual differences: new tools reveal response inhibition deficits in AD/HD that are eliminated by methylphenidate treatment. J Abnorm Psychol. 2005;114:197–215. doi: 10.1037/0021-843X.114.2.197. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Yamane I, Kubota K. Application of the GABA antagonist bicuculline to the premotor cortex reduces the ability to withhold reaching movements by well-trained monkeys in visually guided reaching task. J Neurophysiol. 1996;75:2150–2156. doi: 10.1152/jn.1996.75.5.2150. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res. 2008;186:385–392. doi: 10.1007/s00221-007-1241-4. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res. 2009;194:103–113. doi: 10.1007/s00221-008-1684-2. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Furubayashi T, Okabe S, Mochizuki H, Arai N, Kobayashi S, Ugawa Y. Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19:1556–1573. doi: 10.1162/jocn.2007.19.9.1556. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. Loss aversion and inhibition in dynamical models of multialternative choice. Psychol Rev. 2004;111:757–769. doi: 10.1037/0033-295X.111.3.757. [DOI] [PubMed] [Google Scholar]
- van den Hurk P, Mars RB, van Elswijk G, Hegeman J, Pasman JW, Bloem BR, Toni I. Online maintenance of sensory and motor representations: effects on corticospinal excitability. J Neurophysiol. 2007;97:1642–1648. doi: 10.1152/jn.01005.2006. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Wijnen JG, Ridderinkhof KR. Response inhibition in motor and oculomotor conflict tasks: different mechanisms, different dynamics? Brain Cogn. 2007;63:260–270. doi: 10.1016/j.bandc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wise SP, Di Pellegrino G, Boussaoud D. Primate premotor cortex: dissociation of visuomotor from sensory signals. J Neurophysiol. 1992;68:969–972. doi: 10.1152/jn.1992.68.3.969. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]